Abstract
In the human placental syncytiotrophoblast, C19 steroids are converted to estrogens by aromatase P450, product of the CYP19 gene. When human cytotrophoblasts, which lack the capacity to express aromatase, are cultured in 20% O2, they spontaneously fuse to form a multinuclear syncytiotrophoblast and CYP19 expression is markedly induced. On the other hand, when cytotrophoblasts are cultured in 2% O2, syncytiotrophoblast differentiation and induction of CYP19 expression are prevented. We previously observed that expression of the transcription factor Mash-2 (mammalian achaete/scute homologue 2), which is elevated in human cytotrophoblasts and maintained at elevated levels by hypoxia, declines with syncytiotrophoblast differentiation. Overexpression of Mash-2 prevents syncytiotrophoblast differentiation and induction of CYP19 expression. In the present study, we observed that unexpectedly immunoreactive Mash-2 protein was localized predominately to the cytoplasm of human cytotrophoblasts. Elevated cytoplasmic levels of Mash-2 were maintained when trophoblasts were cultured in 2% O2 and declined to undetectable levels upon culture in 20% O2. Previously, we found that Mash-2 inhibited CYP19 promoter activity through sequences within a 350-bp region upstream and within placenta-specific exon I.1 containing three E boxes (E1 at −325 bp, 5′-CACTTG-3′; E2 at −58 bp, 5′-CACATG-3′; and E3 at +26 bp, 5′-CACGTG-3′). In this study, we found that trophoblast nuclear protein binding to these E boxes declined with syncytiotrophoblast differentiation in 20% O2 and was induced by hypoxia; however, Mash-2 did not appear to bind to any of these E boxes. On the other hand, the basic helix-loop-helix leucine zipper transcription factors upstream stimulatory factors 1 and 2 (USF1 and USF2) did bind to E2 and E3 but not E1. Nuclear levels of USF1 and USF2 and DNA-binding activity declined with syncytiotrophoblast differentiation and were maintained at elevated levels by hypoxia and overexpression of Mash-2, whereas USF1 mRNA levels were unaffected. Finally, USF1 overexpression in cultured human trophoblasts markedly inhibited endogenous CYP19 expression, differentiation of cultured human trophoblast cells, and CYP19 promoter activity. These findings suggest that increased protein levels and DNA binding of USF1 and USF2 mediate the inhibitory effects of hypoxia and of Mash-2 on CYP19 gene expression in human placenta.
The chorionic villi of the human placenta are comprised of two morphologically and functionally distinct cell types: a core of proliferating mononuclear cytotrophoblasts and an outer layer of multinuclear syncytiotrophoblast. The replication of cytotrophoblasts, which drives placental growth, is regulated by autocrine and paracrine factors (35). As cytotrophoblasts mature, they stop dividing and spontaneously fuse to form the terminally differentiated syncytiotrophoblast layer. The syncytiotrophoblast, which is bathed in maternal blood, functions in nutrient and gas exchange, in the production of steroid and polypeptide hormones required for fetal growth and development, and in the maintenance of uterine quiescence (38).
The human placenta has a remarkable capacity to aromatize C19 steroids, produced by the fetal adrenals, to estrogens. This reaction is catalyzed by aromatase, an enzyme complex of the endoplasmic reticulum that contains a unique form of cytochrome P450 (P450arom, product of the CYP19 gene). In placenta, CYP19 gene expression is restricted to the syncytiotrophoblast layer. Human CYP19 is a single-copy gene that spans ∼130 kb (28). The aromatase protein is encoded by exons II to X. Expression of CYP19 mRNA transcripts in various estrogen-producing tissues (including gonads, brain, adipose tissue, and placenta) is driven by tissue-specific promoters which lie upstream of unique first exons. These alternative first exons are spliced onto a common site just upstream of the translation initiation codon in exon II (28). Interestingly, the placenta-specific first exon (exon I.1) lies ∼100 kb upstream of the start site of translation in exon II.
In studies with transgenic mice, we found that human CYP19I.1−501:hGH fusion genes containing 501 bp of genomic sequence flanking the 5′ end of placenta-specific exon I.1 were highly expressed in a developmental and placenta-specific manner. Furthermore, transgene expression was localized exclusively within the labyrinthine trophoblast (27), which shares many properties with the human syncytiotrophoblast in that it contains syncytial cells, is highly vascularized, and is bathed in maternal blood. We also observed that placental transgene expression was initiated as early as E10.5 (27). Interestingly, the temporal pattern of induction of estrogen biosynthesis by the human placenta (after the 9th week of gestation), as well as the initiation of transgene expression in mouse placenta, coincides with the time that placental vascularization and O2 availability are markedly increased. The molecular mechanisms that promote and maintain syncytiotrophoblast differentiation and mediate increased expression of a number of genes, including CYP19, are not completely understood; however, it is evident that O2 tension plays an important role in trophoblast differentiation and function (3, 13, 15).
In previous studies, we observed that cytotrophoblasts isolated from midterm human placenta have low or undetectable levels of aromatase activity and P450arom mRNA transcripts (26). When cultured in a 20% O2 environment, the cytotrophoblasts fuse to form syncytiotrophoblast and aromatase activity and CYP19 gene expression are markedly increased (26). In contrast, when the cells are cultured in a 2% O2 environment, they fail to fuse and aromatase activity and CYP19 gene expression are not induced (24). We also found that syncytiotrophoblast differentiation and induction of CYP19 gene expression in 20% O2 were associated with a marked decline in expression of the basic helix-loop-helix (bHLH) transcription factor Mash-2 (mammalian achaete/scute homologue 2) (24). When the human trophoblast cells were cultured in a hypoxic (2% O2) environment, Mash-2 mRNA levels remained elevated. Interestingly, we also observed that overexpression of Mash-2 markedly inhibited human trophoblast cell fusion and CYP19 gene expression (24). Mash-2 is highly expressed in early mouse placenta; expression is diminished as trophoblast cells differentiate into giant cells (17). Targeted mutation of the Mash-2 gene in mice results in midgestation lethality associated with complete absence of spongiotrophoblast formation, impaired development of the labyrinthine layer, and increased numbers of giant cells (17). Mash-2 appears to act in a cell-autonomous fashion to maintain the diploid trophoblast cells within the spongiotrophoblast layer, either by stimulating their replication or by inhibiting their differentiation into giant cells (42). The absence of a spongiotrophoblast layer, in turn, results in the impaired development of the labyrinthine trophoblast, a major cause of embryonic lethality (42).
Our previous findings suggest that Mash-2 is a hypoxia-inducible transcription factor that inhibits syncytiotrophoblast differentiation and CYP19 gene expression (24). The objective of the present study was to define the mechanisms whereby hypoxia and elevated expression of Mash-2 prevent trophoblast cell fusion, differentiation, and the induction of CYP19 gene expression. We made the surprising observation that the inhibitory effects of hypoxia and Mash-2 on syncytiotrophoblast differentiation and on CYP19 gene expression are directly mediated by the binding of the bHLH-leucine zipper (bHLH-Zip) transcription factors upstream stimulatory factors 1 and 2 (USF1 and USF2) to two E boxes at −58 and +26 bp upstream and within placenta-specific CYP19 exon I.1.
MATERIALS AND METHODS
Primary culture of human trophoblast cells.
Middle-trimester human placental tissues were obtained in accordance with the Donors Anatomical Gift Act of the State of Texas after obtaining consent in writing. In all cases, consent forms and protocols were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. A placental primary culture system (29) was modified for isolation and culture of cytotrophoblasts from midgestation human placenta (26). Briefly, the placental tissues were washed with Hanks' balanced salt solution, pH 7.4 (GIBCO, Grand Island, N.Y.), and then finely minced and digested with 0.125% trypsin in Hanks' balanced salt solution at 37°C for 20 min. This procedure was repeated twice. At the end of each digestion step, the supernatant was collected, layered over 10 ml of serum, and then briefly centrifuged. The pellet was suspended in Dulbecco's modified Eagle's medium (DMEM; GIBCO), filtered, and layered over a Percoll gradient (70 to 5%). The gradients were centrifuged at 1,200 × g for 20 min at room temperature, and cells in the middle layer (density, 1.045 to 1.062) were collected, washed, and counted. The cells were then resuspended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1.2% antibiotic-antimycotic solution (GIBCO) and plated at a density of 2 × 106 cells per dish in 35-mm-diameter culture dishes or 15 × 106 cells per dish in 100-mm-diameter dishes. They were then incubated at 37°C in a humidified atmosphere of 95% air-5% CO2 (20% O2) or placed in a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, Calif.) in an atmosphere containing 2% O2, 93% N2, and 5% CO2. Cells were cultured overnight, and the medium was then changed to DMEM containing 2% FBS. Cells that were cultured in either 20 or 2% O2 were harvested at ambient O2 tension. To ensure that the findings obtained with cells cultured at 2% O2 were not altered by harvesting in room air, a parallel experiment was performed in which cells cultured in 2% O2 were harvested in 2% O2 by using a glove box.
Cloning of the human ortholog of rat Mash-2 (rMash-2).
Total RNA was isolated from freshly harvested cytotrophoblast cells, as previously described (5, 9). The RNA (5 μg) was reverse transcribed into double-stranded cDNAs with SuperScript II reverse transcriptase and RNase H (GIBCO, Carlsbad, Calif.). The double-stranded cDNAs were then used as a template to amplify human Mash-2 (hMash-2) cDNA by PCR with 5′ primer 5′-CGGGTGGATGCAGGCGCGATGGAC-3′ and 3′ primer 5′-TAGGTCGAGGGCGCTCAGTAGC-3′, which were designed to amplify the complete coding sequence of the human achaete-scute homologue 2 (ASCL2) gene (GenBank accession no. U77629), and Advantage2 GC Taq polymerase (Clontech, Palo Alto, Calif.). PCR products were subcloned into pT-Adv vector (Clontech).
Purification of a GST fusion protein comprised of the N-terminal 51 amino acids of hMash-2 (N51) and generation of hMash-2 antibody.
A 153-bp sequence encoding the N-terminal 51 amino acids of hMash-2 (N51) was amplified by PCR (5′ primer, 5′-GAATTCTAATGGACGGCGGCACACTG-3′; 3′ primer, 5′-AAGCTTGGCCGCTGCGCCGCCTCC-3′) and subcloned directly into pGEX-KG in frame with an N-terminal glutathione S-transferase (GST) tag (GST-N51). The recombinant plasmid (pGEX-KG/GST-N51) was used to transform Escherichia coli that was subsequently treated with IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM; GIBCO) to induce high levels of GST-N51 expression. GST-N51 fusion protein was purified using glutathione-Sepharose 4B (Pharmacia Biotech, Piscataway, N.J.). Purified hMash-2 fusion protein (300 μg) was then injected intradermally into a rabbit with complete Freund's adjuvant for the first immunization and with incomplete Freund's adjuvant at 2-week intervals for a total of three immunizations to generate hMash-2 antiserum. Immune serum was collected, and anti-GST antibodies were removed by adsorption to an immobilized GST column (Pierce, Rockford, Ill.); immunoglobulin (IgG) was purified on an immobilized protein A column (Pharmacia Biotech). The Mash-2 IgG was characterized by immunoblot analysis of primary cultures of human trophoblast cells and of purified GST-N51 fusion protein used to immunize rabbits. In the case of the trophoblast cells, the antibodies reacted with a 32-kDa protein, which is the expected size of Mash-2 (Fig. 1A). Analysis of the purified expressed bacterial proteins revealed that the antibodies reacted with the GST-N51 fusion protein and with a thrombin-released ∼6-kDa fragment (data not shown).
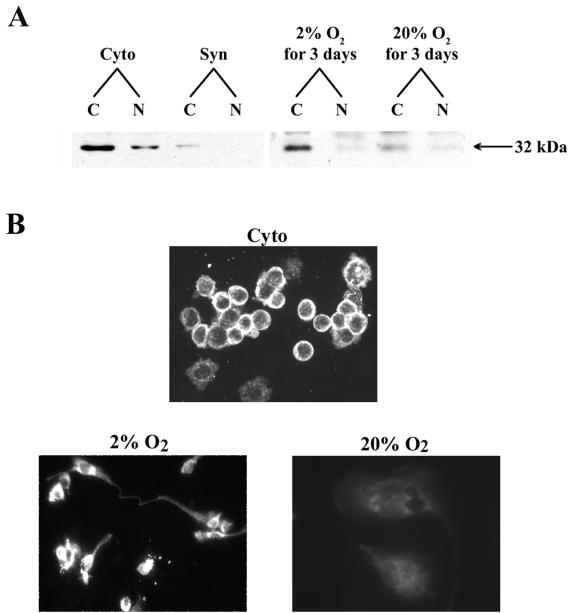
FIG. 1.
Mash-2 protein levels, which decline in human trophoblast cells upon differentiation in a 20% O2 environment and are maintained in trophoblast cells cultured in a hypoxic environment, are primarily localized to the cytoplasm. (A) Immunoblot analysis of hMash-2 protein in nuclear and cytosolic fractions (20 μg/lane) from human cytotrophoblasts before culture (Cyto) and after 3 days of culture in a 20% O2 environment (Syn) (left panel) or in human trophoblasts cultured in either 2 or 20% O2 for 3 days (right panel). C, cytoplasmic; N, nuclear. (B) Immunofluorescence staining of hMash-2 protein in freshly isolated cytotrophoblasts (Cyto) or in trophoblast cells cultured for 3 days in either a 2 or a 20% O2 environment.
Immunoblot analysis.
Nuclear and cytosolic proteins were extracted from freshly isolated cytotrophoblasts or from trophoblast cells that had been cultured in 2 or 20% O2 for 3 days. Briefly, ∼108 trophoblast cells were homogenized in buffer A (10 mM HEPES [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol) containing protease inhibitors (Roche, Mannheim, Germany) and centrifuged at 2,000 rpm (700 × g) for 2 min. The supernatants were retained for analysis of crude cytosolic proteins. Pellets were used for extraction of nuclear proteins as follows: crude nuclei were resuspended in buffer C (20 mM HEPES [pH 7.4], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 20% glycerol) and centrifuged at 60,000 rpm (100,000 × g) for 5 min. The resulting supernatant was retained for analysis of crude nuclear proteins. Nuclear and cytosolic proteins (20 μg) were electrophoresed on sodium dodecyl sulfate-12% polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Midwest Scientific, Valley Park, Mo.). Membranes were blocked with 5% nonfat dry milk in TBS-T (20 mM Tris base, 137 mM NaCl, 0.1% Tween 20, pH 7.6) and incubated for 60 min with hMash-2 antibody (2.5 μg/ml) or with antibodies to either human USF1 (hUSF1) or hUSF2 (1 μg/ml) (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). A donkey anti-rabbit IgG, horseradish peroxidase-linked F(ab′)2 fragment (Amersham Pharmacia Biotech Inc.) was used as secondary antibody. Protein was detected using an ECL kit (Amersham Pharmacia Biotech Inc.) according to the manufacturer's protocol.
Immunofluorescence staining.
Freshly isolated cytotrophoblast cells were either made to directly adhere to glass slides by Cytospin (Miles Scientific) or cultured for 3 days on glass coverslips in DMEM containing 2% FBS in atmospheres of 2 or 20% O2. Cells were fixed with methanol at −20°C for 6 min and then incubated with hMash-2 antibody (10 μg/ml) in phosphate-buffered saline containing 0.5% bovine serum albumin (Sigma, St. Louis, Mo.) for 45 min. Goat anti-rabbit IgG-fluorescein conjugate (Molecular Probes, Eugene, Oreg.) was used as secondary antibody. Slides were examined by immunofluorescence microscopy by using a B-2 filter for fluorescein isothiocyanate (Nikon, Kanagawa, Japan).
Electrophoretic mobility shift assay (EMSA).
Nuclear proteins were purified from freshly isolated cytotrophoblast cells and from cultured trophoblasts (maintained in 2 or 20% O2 for 3 days) as described above. Double-stranded oligonucleotides corresponding to three E-box sequences (E1 at −325 bp, E2 at −58 bp, and E3 at +26 bp) identified within placenta-specific exon I.1 (GenBank accession no. M30795) and its 5′-flanking region were synthesized by GIBCO and IDT (Coralville, Iowa). The upper-strand sequences of the double-stranded oligonucleotides used as probes (E-box sequences are underlined) are as follows: E1, 5′-ACTCCCATGACACTTGCTGAGGTCTT-3′; E2, 5′-TTTGTTCAATCACATGCTTCAGTCAT-3′; and E3, 5′-GAGGGCTGAACACGTGGAGGCAAACA-3′.
For EMSA, the double-stranded oligonucleotides were end labeled with [γ-32P]ATP by using T4 kinase (GIBCO) and incubated with trophoblast nuclear proteins (3 μg) for 30 min at room temperature in binding buffer (20 mM HEPES [pH 7.4], 12% glycerol, 84 mM KCl, 1 mM EDTA, 1 mM dithiothreitol) and 1 μg of poly(dI-dC)-poly(dI-dC) (Pharmacia) as nonspecific competitor. The DNA-protein complexes were resolved on 5% nondenaturing polyacrylamide gels and visualized by autoradiography. For supershift EMSA, the nuclear proteins were incubated for 1 h at 4°C in binding buffer in the absence or presence of IgG (1 μg) for hMash-2, hUSF1, or hUSF2 (Santa Cruz Biotechnology, Inc.). Labeled E1, E2, or E3 oligonucleotides were added to the reaction mixture, and the incubation was continued for another 30 min at room temperature, before separation on 5% native polyacrylamide gels and visualization by autoradiography.
Northern blot analysis of RNA isolated from trophoblast cells infected with recombinant adenoviruses.
Freshly isolated trophoblast cells were infected with recombinant adenoviruses expressing either cytomegalovirus Mash-2 (CMV-Mash-2) (24), CMV-USF1, or CMV β-galactosidase (CMV-β-Gal) (2) at a multiplicity of infection (MOI) ranging from 0.5 to 10. After 72 h of culture, total RNA was isolated as described above and 20 μg was size fractionated on a 7.4% formaldehyde-0.9% agarose gel. The RNA was transferred to Zeta-Probe blotting membrane (Bio-Rad Laboratories, Inc., Hercules, Calif.) and hybridized overnight at 65°C to radiolabeled cDNA probes encoding human aromatase (11) and hUSF1 (kindly provided by Michele Sawadogo, M. D. Anderson Cancer Center, Houston, Tex.). The cDNAs were 32P labeled with a Prime-It RmT random primer labeling kit (Stratagene, La Jolla, Calif.). After washing, the relative levels of mRNA were assessed by autoradiography. The Northern blots were stripped and reprobed using a radiolabeled β-actin cDNA (American Type Culture Collection, Manassas, Va.) to assess loading and transfer of RNA.
Morphological analysis.
Cytotrophoblasts cultured on glass coverslips in DMEM containing 2% FBS were infected with adenoviruses expressing either CMV-USF1 (MOI = 5 or 10) or CMV-β-Gal (MOI = 10). After culture for 72 h in 20% O2, cells were rinsed with phosphate-buffered saline and fixed in 75% ethanol. Hematoxylin and eosin Y were used to stain nuclei and cytoplasm, respectively. Morphology was analyzed by light microscopy.
RESULTS
Mash-2 protein is primarily localized in the cytoplasm of freshly isolated cytotrophoblasts and of trophoblasts cultured under hypoxic conditions.
We previously observed that Mash-2 is expressed in human cytotrophoblasts and that expression declines during syncytiotrophoblast differentiation (24). When trophoblasts were cultured under hypoxic conditions, Mash-2 expression was maintained at high levels and syncytiotrophoblast differentiation and the induction of CYP19 gene expression were blocked. Furthermore, overexpression of rMash-2 inhibited syncytiotrophoblast differentiation and the associated induction of hCYP19 gene expression (24). In the present study, we raised antibodies against hMash-2 to study protein levels and subcellular localization during syncytiotrophoblast differentiation. We first cloned a full-length cDNA encoding hMash-2 by reverse transcriptase PCR by using total RNA from freshly isolated human cytotrophoblasts and primers that corresponded to the 5′ and 3′ untranslated regions. To determine whether hMash-2 has the same inhibitory action as does rMash-2, we subcloned hMash-2 cDNA into the expression vector pCMV5 and cotransfected BeWo choriocarcinoma cells with the pCMV5:hMash-2 plasmid together with a reporter gene construct containing 501 bp of DNA flanking the 5′ end of placenta-specific exon I.1 of the hCYP19 gene (CYP19I.1−501:hGH). pCMV5 vector was cotransfected as a control. hMash-2 manifested the same inhibitory action on CYP19I.1−501:hGH fusion gene expression as did rMash-2 (data not shown).
To investigate hMash-2 protein levels in human trophoblast cells before and after culture in different oxygen environments, polyclonal antibodies were raised in rabbits against a bacterially expressed GST fusion protein containing the N-terminal 51 amino acids of hMash-2. As shown in the immunoblot in Fig. 1A (left panel), an immunoreactive band (32,000 Da) comparable in size to the deduced Mr of hMash-2 protein was present at relatively high levels in cytoplasmic fractions of freshly isolated cytotrophoblasts and only at relatively low levels in nuclear fractions. Mash-2 protein levels were greatly reduced in both cytoplasmic and nuclear fractions upon syncytiotrophoblast differentiation after 3 days of culture in 20% O2. When the trophoblast cells were cultured in 2% O2 for 3 days, Mash-2 protein was maintained at elevated levels compared to cells cultured in 20% O2 and again was primarily localized to the cytoplasm (Fig. 1A, right panel). Similar results were obtained by immunofluorescence staining (Fig. 1B). In freshly isolated cytotrophoblasts, intense immunostaining for hMash-2 was evident in the cytoplasm of the majority of the cells (Fig. 1B, top panel). In a small population of cells, hMash-2 was detectable at low levels in nuclei. When trophoblasts were cultured in 2% O2 for 3 days, immunoreactive hMash-2 protein remained highly expressed in the cytoplasm and again was detectable at lower levels in nuclei (Fig. 1B, bottom left panel). Immunostaining was essentially undetectable in multinuclear syncytiotrophoblast that had been cultured in 20% O2 for 3 days (Fig. 1B, bottom right panel). In other experiments, trophoblast cells were both cultured and harvested under hypoxic (2% O2) conditions with a glove box. Under these conditions, Mash-2 was still primarily localized to the cytoplasm (data not shown).
Binding activity of trophoblast nuclear proteins for three E boxes upstream of placenta-specific exon I.1 of the hCYP19 gene is elevated in freshly isolated cytotrophoblasts and is reduced upon syncytiotrophoblast differentiation.
Mash-2 is a bHLH transcription factor that forms a heterodimer with ubiquitously expressed E proteins (e.g., E12) and is reported to bind to a MyoD response element (E box), such as that found in the muscle creatine kinase promoter (25). We examined a 501-bp region flanking the 5′ end of the placenta-specific exon I.1 of the hCYP19 gene that we previously found to be required for placenta-specific expression (27) and identified three potential E boxes (E1, −325 bp; E2, −58 bp; and E3, +26 bp within placenta-specific exon I.1) (Fig. 2A). By EMSA, using the E1, E2, and E3 boxes as probes, we compared DNA-binding activities of nuclear extracts from freshly isolated cytotrophoblasts with those of syncytiotrophoblast that had been cultured for 3 days in a 20% O2 environment. As can be seen in Fig. 2B, binding activity for all three E boxes was readily detectable in nuclear extracts from cytotrophoblasts but was markedly reduced when cells differentiated to form syncytiotrophoblast. Binding was effectively competed by a 500-fold molar excess of the corresponding nonradiolabeled E box but not by a comparable amount of a nonradiolabeled unrelated oligonucleotide.
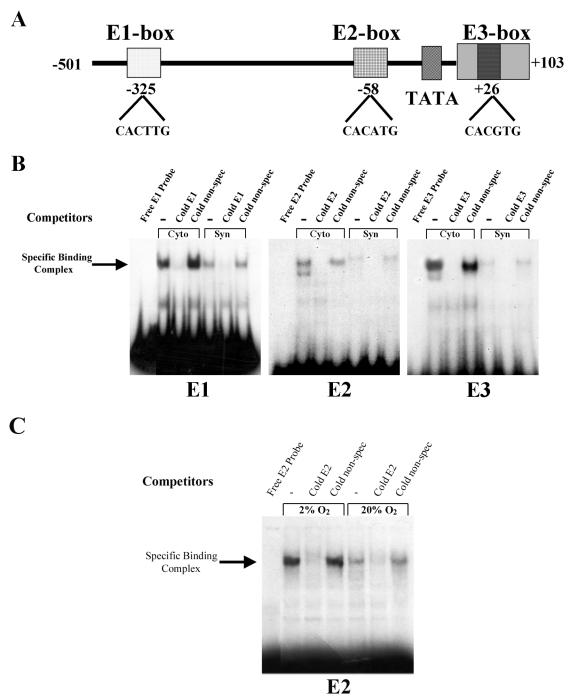
FIG. 2.
Binding activity of human trophoblast nuclear proteins for the E1, E2, and E3 boxes upstream and within hCYP19 placenta-specific exon I.1 is elevated in cytotrophoblasts, declines with syncytiotrophoblast differentiation in a 20% O2 environment, and is maintained at elevated levels by hypoxia (2% O2). (A) Schematic of three E-box consensus sequences at −325 bp (E1), −58 bp (E2) upstream, and +26 bp (E3) within hCYP19 placenta-specific exon I.1. (B) EMSA of binding of nuclear proteins from freshly isolated human cytotrophoblasts (Cyto) and from syncytiotrophoblast (Syn) after 3 days of culture in a 20% O2 environment to 32P-labeled oligonucleotides containing the E1, E2, and E3 boxes in the absence (−) or presence of a 500-fold excess of nonradiolabeled (Cold) E1, E2, or E3 or nonrelated (non-spec) oligonucleotides. (C) EMSA of binding of nuclear proteins from human trophoblast cells after 3 days of culture in a 2 or 20% O2 environment to a 32P-labeled oligonucleotide containing the E2 box in the absence (−) or presence of a 500-fold excess of nonradiolabeled (Cold) E2 or nonrelated (non-spec) oligonucleotides. Binding of nuclear proteins to the E1 and E3 boxes was not affected by hypoxia (data not shown).
Binding activity of nuclear proteins from human trophoblast cells is increased by hypoxia.
The effects of hypoxia on binding activity of nuclear proteins for the E2 box were analyzed in trophoblast cells cultured for 3 days in either a 2 or a 20% O2 environment. As can be seen in Fig. 2C, binding activity for the E2 box was markedly increased in nuclear proteins from trophoblasts that were cultured in 2% O2 for 3 days, compared to cells that were cultured for the same period in 20% O2. Binding activity of trophoblast nuclear proteins for E1 and E3 boxes was not affected by hypoxia (data not shown). These findings suggest that different complexes of proteins bind to these elements and that only those that bind to the E2 box are induced by hypoxia.
To determine whether Mash-2 protein has the capacity to bind directly to the E1, E2, or E3 boxes, we implemented hMash-2 antibody-mediated supershift EMSA by using nuclear proteins from freshly isolated cytotrophoblasts and from trophoblast cells cultured for 3 days in 2 or 20% O2. Mash-2 antibody was unable to supershift the protein complexes bound to the three E boxes (data not shown), suggesting that Mash-2 may be incapable of binding to these sites. This could also be due to the inability of the antibodies to supershift the protein-DNA complexes or to the relatively small amounts of Mash-2 protein present within the nucleus, which could possibly be caused by its association with another protein that blocks nuclear translocation and/or its ability to bind to DNA.
In this regard, the repressor protein I-mfa, which is highly expressed in extraembryonic lineages in the mouse, has been reported to interact with Mash-2 and inhibit its nuclear localization, DNA-binding, and transcriptional activities (31). In preliminary studies, we found that I-mfa mRNA and protein levels in human trophoblasts appeared to be unaffected during differentiation to syncytiotrophoblast or by changes in O2 tension (B. Jiang and C. R. Mendelson, unpublished observations) (I-mfa cDNA and antibodies were generously provided by D. N. Kraus, Boehringer Ingelheim Pharma KG, Ingelheim, Germany). Despite the findings that I-mfa expression was not altered by trophoblast differentiation, changes in O2 tension, or Mash-2 overexpression, we cannot discount the possibility that increased expression levels of I-mfa in human trophoblast cells may retain the majority of Mash-2 in the cytoplasm. On the other hand, in a transient-transfection assay of BeWo cells, overexpression of I-mfa increased expression of a CYP19I.1−501:hGH fusion gene, whereas overexpression of Mash-2 prevented this stimulatory action of I-mfa (data not shown). These findings suggest that I-mfa may have the capacity to antagonize the inhibitory effects of endogenous Mash-2 on CYP19I.1 promoter activity.
USF1 and USF2 protein levels decline during syncytiotrophoblast differentiation and are kept elevated by hypoxia and by overexpression of Mash-2.
In examining the E boxes upstream of hCYP19 exon I.1, it was apparent that the core sequence of the E2 and E3 boxes contains a consensus binding site for a subfamily of bHLH transcription factors that contain a leucine zipper (bHLH-Zip) motif. These include USF1 and USF2, Myc, Max, and Mad (8). As can be seen in Fig. 3A (left panel), USF1 and USF2 proteins were expressed at relatively high levels in cytotrophoblast nuclei but declined markedly in syncytiotrophoblast after culture for 3 days in a 20% O2 environment. By contrast, when trophoblasts were cultured in 2% O2, nuclear protein levels of USF1 and USF2 were maintained at elevated levels compared to those of cells maintained in 20% O2 (Fig. 3A, right panel). In contrast to the predominately cytoplasmic localization of Mash-2, USF1 and USF2 were strictly localized to trophoblast nuclei. Furthermore, protein levels of USF1 appeared to be more abundant than those of USF2 in the human trophoblasts before or after culture. Together these findings suggest that USF1 and USF2 decline with trophoblast differentiation and are maintained by hypoxia.
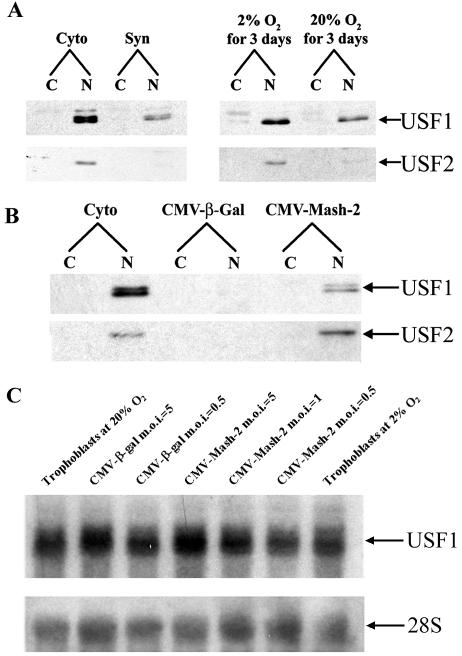
FIG. 3.
Nuclear levels of USF1 and USF2 proteins decline with syncytiotrophoblast differentiation and are maintained at elevated levels by hypoxia and Mash-2 overexpression, while USF1 mRNA levels are unaffected. (A) Nuclear (N) and cytoplasmic (C) fractions were prepared from human trophoblasts before (Cyto) and after (Syn) culture for 3 days in 20% O2 or in an independent experiment after 3 days of culture in an atmosphere of 2 or 20% O2. Twenty micrograms of protein was analyzed for USF1 (43 kDa) and USF2 (44 kDa) by immunoblotting. (B) Nuclear (N) and cytoplasmic (C) fractions were prepared from human trophoblasts before culture (Cyto) or from trophoblasts that were infected with recombinant adenovirus expressing either Mash-2 (CMV-Mash-2; MOI = 5) or β-Gal (CMV-β-Gal; MOI = 5) and analyzed for USF1 and USF2 by immunoblotting (20 μg of protein/lane). (C) Total RNA isolated from human trophoblast cells cultured for 72 h in an atmosphere of 2 or 20% O2 or from trophoblast cells cultured in 20% O2 and infected with recombinant adenoviruses containing CMV-β-Gal or CMV-Mash-2 at an MOI of 0.5 to 5.0 was analyzed for hUSF1 mRNA levels by Northern blotting.
It was of interest to determine whether the stimulatory effects of hypoxia and Mash-2 overexpression on USF protein levels are mediated by effects on USF gene expression. Therefore, RNA isolated from human trophoblast cells cultured for 72 h in an atmosphere of 2 or 20% O2 or from trophoblast cells cultured in 20% O2 and infected with recombinant adenoviruses containing CMV-β-Gal or CMV-Mash-2 at an MOI of 0.5 to 5.0 was analyzed for hUSF1 mRNA levels by Northern blotting. Surprisingly, neither hypoxia nor Mash-2 overexpression had an effect on USF1 mRNA levels (Fig. 3C). This suggests that Mash-2 may enhance USF1 protein levels through effects on protein stability and/or mRNA translatability.
Since the regulation of expression of USF1 and USF2 mimics that of Mash-2, it was of interest to determine whether expression of USFs is regulated by Mash-2. Freshly isolated cytotrophoblasts were infected immediately after plating with recombinant adenoviruses expressing either rMash-2 (CMV-rMash-2) or β-Gal (CMV-β-Gal) at an MOI of 5. The cells were cultured in a 20% O2 environment for 3 days, and the levels of USF1 and USF2 were analyzed by immunoblotting. We previously observed that overexpression of Mash-2 blocks syncytiotrophoblast differentiation and the induction of CYP19 gene expression (24). As can be seen in Fig. 3B, overexpression of rMash-2 caused a marked increase in the levels of immunoreactive USF1 and USF2 compared to cells infected with the recombinant adenovirus containing CMV-β-Gal. Since the antibodies raised against the N-terminal 51 amino acids of hMash-2 do not cross-react with rMash-2 protein, we were unable to ascertain whether the endogenous hMash-2 and transfected rMash-2 proteins colocalized to the same subcellular compartments. Nonetheless, it should be noted that the effects of overexpressed rMash-2 are highly similar to those in cells where endogenous hMash-2 was upregulated by hypoxia. In the latter case, Mash-2 protein was predominantly localized to the cytoplasm.
USF1 and USF2 bind directly to the E2 and E3 boxes.
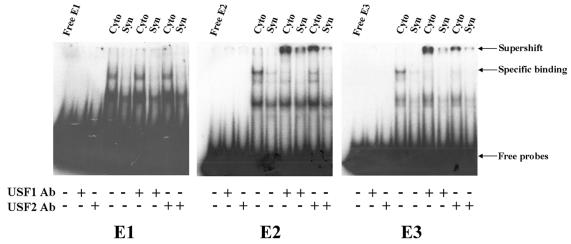
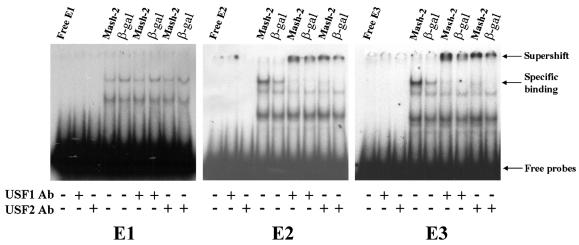
We next wanted to determine whether USF1 and USF2 bind to the E1, E2, and E3 boxes present in the 5′-flanking region and in exon I.1 of the hCYP19 gene. Radiolabeled E1, E2, and E3 oligonucleotides were incubated with nuclear extracts from freshly isolated cytotrophoblasts or syncytiotrophoblast after culture for 3 days in 20% O2, in the absence or presence of antibodies to hUSF1 or hUSF2. Nuclear extracts from cytotrophoblasts manifested much stronger binding activity for all three probes than did those from syncytiotrophoblast (Fig. 4). Interestingly, antibodies to USF1 and USF2 caused a strong supershift of the lowest-mobility complex binding to the E2 and E3 probes; however, no supershift was observed with either antibody when the E1 box was used as a probe (Fig. 4). USF1 and USF2 antibodies did not bind directly to any of the probes. Thus, USF1 and USF2 bind to E2 and E3 boxes and binding activity declines with syncytiotrophoblast differentiation. This suggests that USFs may play an inhibitory role in the induction of CYP19 gene expression that occurs with syncytiotrophoblast differentiation. Furthermore, these results suggest that cytotrophoblast nuclear proteins other than USF1 and USF2 with potentially inhibitory transcriptional activity bind to the E1 box.
FIG. 4.
USF1 and USF2 in trophoblast nuclear extracts bind to the E2 and E3 boxes upstream and within hCYP19 exon I.1, respectively, but not to the E1 box; endogenous USF binding activity declines with syncytiotrophoblast differentiation. Nuclear extracts from human cytotrophoblasts before (Cyto) and after 3 days of culture in a 20% O2 environment (Syn) were incubated with 32P-labeled oligonucleotides containing the E1, E2, and E3 boxes in the absence (−) or presence (+) of antibodies specific for USF1 and USF2 and analyzed by EMSA.
Overexpression of Mash-2 increases binding activity of trophoblast nuclear proteins for E2 and E3 boxes.
Freshly isolated cytotrophoblasts were infected with recombinant adenoviruses carrying CMV-rMash-2 or CMV-β-Gal (MOI = 5) and cultured for 3 days in a 20% O2 environment. Nuclear extracts from the infected cells were incubated with E1, E2, and E3 probes in the absence or presence of antibodies to hUSF1 and hUSF2 and analyzed by EMSA. Overexpression of Mash-2 caused a marked increase in binding activity of nuclear proteins for the E2 and E3 boxes, compared to nuclear extracts from cells infected with CMV-β-Gal; no effect of Mash-2 overexpression on binding for the E1 box was observed (Fig. 5). The lowest-mobility complex binding to E2 and E3 was supershifted by USF1 and USF2 antibodies. These findings suggest that Mash-2 exerts its inhibitory effects on CYP19 gene expression by increasing expression of USF1 and USF2, which bind directly to the E2 and E3 boxes.
FIG. 5.
Mash-2 overexpression increases binding activities of USF1 and USF2 proteins for the E2 and E3 boxes upstream and within hCYP19 exon I.1. Freshly isolated cytotrophoblasts were infected with recombinant adenoviruses expressing either Mash-2 (CMV-Mash-2; MOI = 5) or β-Gal (CMV-β-Gal; MOI = 5) and cultured for 3 days in a 20% O2 environment. Nuclear proteins isolated from the cultured cells were incubated with 32P-labeled oligonucleotides containing the E1, E2, and E3 boxes in the absence (−) or presence (+) of antibodies specific for USF1 and USF2 and analyzed by EMSA.
USF1 overexpression inhibits endogenous CYP19 expression.
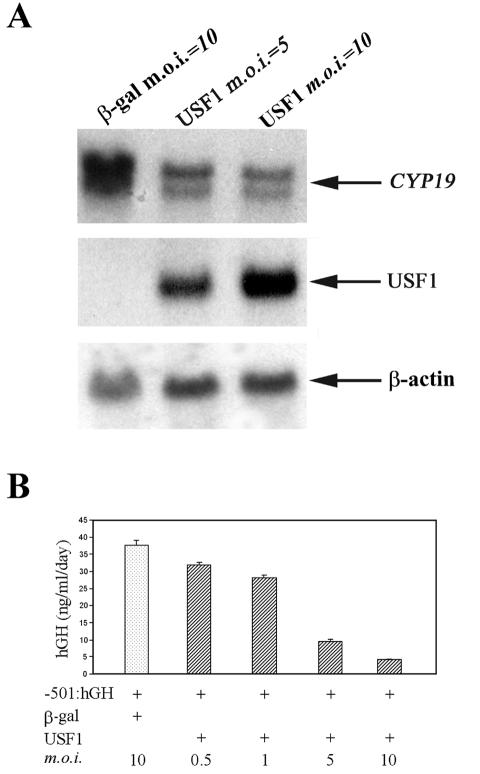
Since USF1 and USF2 binding to E2 and E3 boxes was increased by Mash-2 overexpression, which we previously found to inhibit CYP19 gene expression (24), it was of interest to determine whether USF overexpression inhibits endogenous CYP19 gene expression, as well as promoter activity in cultured human trophoblast cells. Freshly isolated human cytotrophoblasts were infected with adenoviruses expressing either USF1 (MOI = 5 or 10) or β-Gal (MOI = 10) and cultured for 3 days in a 20% O2 environment. As can be seen in Fig. 6A, USF1 overexpression (middle panel) caused a pronounced inhibition of CYP19 mRNA levels compared to cells infected with control adenoviruses (top panel). These findings suggest that USF1 mediates hypoxia and Mash-2 inhibition of CYP19 gene expression in human trophoblast cells.
FIG. 6.
USF1 overexpression prevents the induction of endogenous CYP19 gene expression in cultured human trophoblast cells and causes a dose-dependent inhibition of expression of a transfected CYP19I.1−501:hGH fusion gene. (A) Northern blot analysis of endogenous CYP19 mRNA levels in cultured human trophoblast cells infected with recombinant adenoviruses expressing either USF1 (MOI = 5 or 10) or β-Gal (MOI = 10). The cDNA probes used were specific for hCYP19, hUSF1, and β-actin mRNAs. (B) hCYP19 promoter I.1 activity in cultured human trophoblasts coinfected with a recombinant adenovirus containing a CYP19I.1−501:hGH fusion gene and increasing MOI of a recombinant adenovirus expressing hUSF1 (MOI = 0.5, 1, 5, or 10), or β-Gal (MOI = 10). Data are the means ± standard errors of the means of the hGH secreted into the medium between 48 and 72 h of incubation (n = 3).
To analyze the effects of USF1 overexpression on CYP19I.1 promoter activity, freshly isolated cytotrophoblasts were coinfected with a USF1-expressing recombinant adenovirus (MOI = 0.5, 1, 5, or 10) and with a recombinant adenovirus containing a fusion gene comprised of 501 bp of DNA flanking the 5′ end of placenta-specific CYP19 exon I.1 linked to the hGH structural gene, as reporter (CYP19I.1−501:hGH; MOI = 0.5) (26). CMV-β-Gal adenovirus (MOI = 10) was coinfected as a control. As can be seen in Fig. 6B, overexpression of USF1 markedly inhibited fusion gene expression in a dose-dependent manner. This suggests that USF1 overexpression inhibits endogenous CYP19 gene expression through the inhibition of CYP19 promoter activity.
USF1 overexpression inhibits syncytiotrophoblast differentiation.
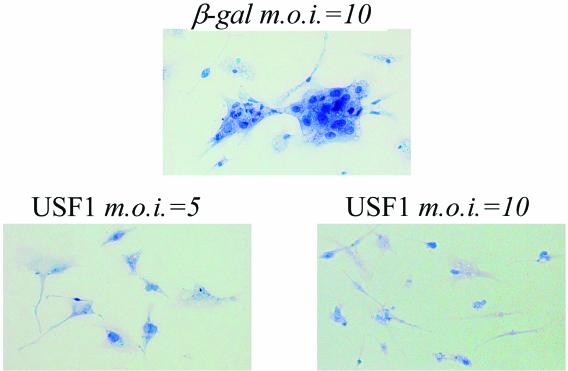
To investigate the effects of USF1 overexpression on trophoblast differentiation, human cytotrophoblasts were plated onto glass coverslips and infected with recombinant adenoviruses expressing USF1 (MOI = 5 or 10) or β-Gal (MOI = 10), as a control. After 3 days of culture in a 20% O2 environment, there was clear evidence of syncytium formation in the cells infected with recombinant adenovirus containing CMV-β-Gal (Fig. 7). By contrast, when the cells were incubated with adenovirus expressing USF1 (MOI = 5 or 10), syncytium formation was markedly inhibited (Fig. 7). These results suggest that USF1 mediates hypoxia and Mash-2 inhibition of human trophoblast differentiation.
FIG. 7.
USF1 overexpression prevents trophoblast cell fusion and differentiation. Freshly isolated cytotropholast cells were cultured on coverslips and infected with different amounts of recombinant adenovirus expressing either USF1 (MOI = 5 or 10) or β-Gal (MOI = 10). After 72 h, the cells on the coverslips were stained with hematoxylin and eosin. Magnification, ×200.
DISCUSSION
The human placenta has a remarkable capacity to produce estrogens by aromatization of C19-steroid precursors secreted by the fetal adrenals. Estrogen biosynthesis by the human placenta increases markedly after the 9th week of gestation (38). This coincides with the time that cytotrophoblast invasion, remodeling, and enlargement of uterine arterioles are initiated, resulting in a pronounced rise in placental O2 tension and induction of antioxidant enzymes (23). Oxygen has been suggested to play an important role in the regulation of trophoblast differentiation. When cytotrophoblasts from midgestation human placenta are placed in monolayer culture in a 20% O2 environment, they rapidly fuse to form syncytiotrophoblast (24, 26, 29). This is associated with the induction of CYP19 gene expression and of aromatase activity (24, 26). On the other hand, when these cells are cultured in a hypoxic (2% O2) environment, cell fusion and the induction of aromatase mRNA levels and activity are prevented.
In previous studies, we observed that the effects of hypoxia on syncytiotrophoblast differentiation and on CYP19 gene expression in human trophoblast cells in primary culture were mediated by the bHLH transcription factor Mash-2 (24). We found that Mash-2 mRNA levels were relatively high in cytotrophoblasts and declined as a function of syncytiotrophoblast differentiation in a 20% O2 environment. On the other hand, when human trophoblast cells were maintained in a hypoxic environment, Mash-2 mRNA levels remained elevated. Furthermore, Mash-2 overexpression in cultured human trophoblast cells inhibited cell fusion and the induction of CYP19 gene expression. In transfection studies of human trophoblast cells, we observed that the inhibitory effect of Mash-2 on CYP19I.1 promoter activity appeared to be mediated by sequences within 125 bp upstream of the transcription start site in exon I.1 (24).
Mash-2 was previously reported to bind as a heterodimer with E12 to an E-box sequence in the muscle creatine kinase promoter that is known to serve as a binding site for MyoD (25). In the present study, we identified three E boxes at −325 bp (E1), −58 bp (E2), and +26 bp (E3) upstream and within placenta-specific CYP19 exon I.1. Using antibodies raised against hMash-2, we observed that hMash-2 protein mimicked Mash-2 mRNA in that protein levels were relatively high in freshly isolated cytotrophoblasts and declined upon syncytiotrophoblast differentiation in a 20% O2 environment. When the cells were cultured in 2% O2, the levels of hMash-2 protein remained elevated. The finding that binding activity for these E boxes was higher in nuclear extracts from cytotrophoblasts than in extracts from syncytiotrophoblast and was greatly increased by hypoxia suggested that one or more of the E boxes mediate the inhibitory actions of Mash-2 protein on CYP19 promoter I.1 activity. However, in immunocytochemical studies, we made the surprising observation that hMash-2 protein was predominately localized to the cytoplasm of cytotrophoblasts before and after culture in 2% O2, although some nuclear staining was evident. Furthermore, in antibody-mediated supershift EMSA, we were unable to detect binding of hMash-2 to the three E boxes. These findings suggested that hypoxia and Mash-2 inhibition of CYP19 gene expression in human placental cells might be mediated by Mash-2 regulation of another protein.
We noted that the E2 and E3 boxes at −58 and +26 bp contained the consensus binding motifs for the bHLH-leucine zipper (bHLH-Zip) transcription factors USF1 and USF2 (CACATG and CACGTG, respectively). The sequence of the E1 box predicted that it would not likely serve as a USF-binding site. Interestingly, like Mash-2, USF1 and USF2 protein levels were found to be elevated in cytotrophoblasts, declined upon syncytiotrophoblast differentiation, and remained elevated under hypoxic conditions. However, unlike Mash-2, USF1 and USF2 were exclusively nuclear in their cellular localization. Furthermore, in antibody-mediated supershift EMSA, USF1 and USF2 in human cytotrophoblast nuclear extracts were found to bind specifically to the E2 and E3 boxes; USF1-USF2 did not appear to bind to the E1 box. Additionally, Mash-2 overexpression in primary cultures of human trophoblast cells markedly increased nuclear levels of USF1 and USF2 proteins and their binding to the E2 and E3 boxes. The finding that USF1 overexpression inhibited trophoblast cell fusion, induction of aromatase expression, and CYP19 promoter I.1 activity in primary cultures of human trophoblast cells suggests that USFs directly mediate the inhibitory effects of Mash-2 and hypoxia on syncytiotrophoblast differentiation and CYP19 gene expression.
USF1 and USF2 are structurally related bHLH-Zip transcription factors of 43 and 44 kDa, respectively (12, 22, 34). Although USF1 and USF2 are ubiquitously expressed (12, 40), they appear to be involved in the regulation of developmental and tissue-specific expression of a number of genes, including those for surfactant protein A (12), steroidogenic factor 1 (18), and carboxyl ester lipase (4). The finding that embryonic lethality occurred in mice homozygous for targeted deletion in the usf2 gene and either homozygous or heterozygous for a mutation in the usf1 gene suggests that USF proteins are essential for embryonic development (39). The cause of embryonic death in these mutant mice was not reported. On the other hand, USFs have been reported to serve as repressors of a number of target genes, including the ABCA1 transporter (45) and Xenopus MyoD (32). USF1 was found to inhibit transcription of the rabbit CYP19A1 gene by competing with the aryl hydrocarbon receptor (AhR)-AhR nuclear translocator (ARNT) for binding to the promoter (41).
Although USF1 and USF2 have not previously been implicated in the hypoxic response, a number of bHLH transcription factors that contain a PAS (Per-ARNT-Sim) domain are upregulated by hypoxia and mediate enhanced expression of target genes, including those encoding glycolytic enzymes, glucose transporters, and growth factors that induce erythropoiesis and angiogenesis (6, 10, 37). These bHLH-PAS domain transcription factors, which bind to E boxes, include hypoxia-inducible factor 1α (HIF-1α), human endothelial PAS domain protein 1 (EPAS-1/HIF-2α), and their common heterodimeric partner ARNT/HIF-1β. HIF-1α and EPAS-1 protein levels decline under normoxic conditions (37, 44) because of increased proteasomal degradation (44). This occurs as a result of increased ubiquitination upon O2-mediated hydroxylation of a proline residue in HIF-1α and EPAS-1 proteins, which promotes recruitment of von Hippel-Lindau tumor-suppressor protein (VHL; recognition component of an E3 ubiquitin-protein ligase) (19, 21). HIF-1α protein and mRNA levels were reported to be elevated in first-trimester human placenta (5 to 8 weeks) and to decline markedly at later stages of gestation (7). When human trophoblast villous explants were cultured under hypoxic conditions, there was upregulated expression of HIF-1α (7), VHL, and EPAS-1 (14), whereas these proteins were downregulated when explants were cultured in 20% O2.
Interestingly, mice that are homozygous for targeted deletion of Vhl die because of a failure in placental development (16). These mice appeared to develop normally until E9.5 to 10.5 when there was a failure of placental vasculogenesis. Arnt−/− embryos also die by E10.5 because of a complete failure in placental vascularization (30, 33) with markedly reduced labyrinthine and spongiotrophoblast layers and increased numbers of giant cells (1). It is possible that these defects resulted from impaired function of HIF-1α or EPAS-1, which requires heterodimerization with ARNT for transcriptional responses to hypoxia. However, Hif-1α−/− embryos, which are developmentally arrested at E9.0 because of enhanced mesenchymal cell death and defects in vascular development, manifest no apparent defects in placental development (20, 36). Furthermore, Epas-1−/− mice die after E12.5, presumably due to a defect in catecholamine homeostasis; no morphological defects in placental development were evident (43). In the present study, we observed that EPAS-1 levels declined during differentiation of human trophoblasts cultured in a 20% O2 environment and were increased by hypoxia and by Mash-2 overexpression (data not shown). This suggests that, like USFs, EPAS-1 may also be a target of Mash-2. On the other hand, we were unable to detect EPAS-1 binding to the E2 and E3 boxes surrounding the hCYP19 placenta-specific promoter, although this could have been due to the properties of antibodies that were utilized. We also have assessed the binding of ARNT to the E2 and E3 boxes using antibody-mediated supershift assays. However, antibodies to ARNT only modestly lightened the complex of proteins binding to these E boxes without causing a detectable supershift (Jiang and Mendelson, unpublished). This suggests that ARNT likely does not bind to this site.
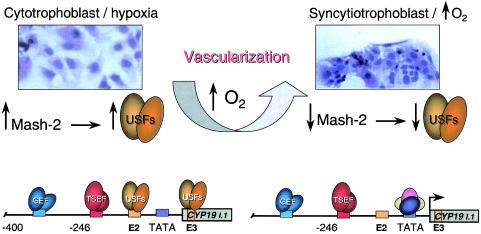
Although the detailed mechanisms for O2 regulation of human trophoblast differentiation and CYP19 gene expression remain to be determined, the present findings suggest a novel signal transduction cascade that culminates in trophoblast differentiation and the induction of CYP19 gene expression (Fig. 8). During the first trimester of gestation, the villous cytotrophoblast stem cells exist in a relatively hypoxic environment, resulting in increased expression of Mash-2 and an associated increase in cytotrophoblast proliferation and inhibition of cell fusion and differentiation. The increased levels of Mash-2 cause enhanced expression of USF1 and USF2, which bind to the E2 and E3 boxes upstream and within placenta-specific exon I.1 of the hCYP19 gene, resulting in repression of hCYP19 gene expression. We suggest that this possibly may be caused by USF recruitment of corepressors, which prevent hCYP19 promoter activation by trophoblast-specific and general enhancer factors. The rapidly proliferating cytotrophoblasts aggregate to form columns and invade the decidua and part of the myometrium, resulting in remodeling and enlargement of the spiral arterioles. This, in turn, leads to a marked increase in O2 tension within the placenta, causing a pronounced decline in Mash-2 and USF1 and USF2 expression with associated syncytiotrophoblast differentiation. The decreased binding of USF1-USF2 heterodimers to the E2 and E3 boxes also results in activation of hCYP19 gene expression, possibly by recruitment to the hCYP19 promoter of activating transcription factors and coactivators. Studies are in progress to decipher the interrelationships between the different families of bHLH transcription factors in placental differentiation and induction of CYP19 gene expression and the mechanisms whereby changes in O2 tension effect their expression and activation.
FIG. 8.
Possible mechanism for oxygen-mediated induction of human trophoblast differentiation and CYP19 gene expression. During the first trimester of human gestation the placenta exists in a relatively hypoxic environment, resulting in the maintenance of elevated expression of Mash-2 and consequent induction of USF1 and USF2 gene expression. USF1 and USF2, in turn, bind as heterodimers to the E2 and E3 boxes upstream and within hCYP19 placenta-specific exon I.1, respectively, preventing stable assembly of the basal transcription complex and inhibition of CYP19I.1 promoter activity. The elevated levels of USF1 and USF2 also increase cytotrophoblast proliferation and prevent their fusion to form syncytiotrophoblast. Upon cytotrophoblast invasion of the spiral arterioles and the resulting increase in oxygenation, the levels of Mash-2 decline, resulting in decreased USF1 and USF2 expression with increased syncytiotrophoblast differentiation. Decreased binding of USF1 and USF2 to the E2 and E3 boxes, in turn, may facilitate stable assembly of the basal transcription complex and activation of CYP19 gene expression by tissue-specific and general enhancer factors (TSEF and GEF, respectively).
Acknowledgments
WE thank Vickey Chau for isolation of cytotrophoblast cells and Alan Varley for assistance in editing the manuscript.
This work is supported by Public Health Service grant R01 DK-31206 from the National Institute of Diabetes and Digestive and Kidney Diseases (C.R.M.).
REFERENCES
- 1.Adelman, D. M., M. Gertsenstein, A. Nagy, M. C. Simon, and E. Maltepe. 2000. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcorn, J. L., M. E. Smith, J. F. Smith, L. R. Margraf, and C. R. Mendelson. 1997. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am. J. Respir. Cell Mol. Biol. 17:672-682. [DOI] [PubMed] [Google Scholar]
- 3.Alsat, E., P. Wyplosz, A. Malassine, J. Guibourdenche, D. Porquet, C. Nessmann, and D. Evain-Brion. 1996. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J. Cell. Physiol. 168:346-353. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson, S. H., K. Madeyski-Bengtson, J. Nilsson, and G. Bjursell. 2002. Transcriptional regulation of the human carboxyl ester lipase gene in THP-1 monocytes: an E-box required for activation binds upstream stimulatory factors 1 and 2. Biochem. J. 365:481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggaram, V., K. Qing, and C. R. Mendelson. 1988. The major apoprotein of rabbit pulmonary surfactant. Elucidation of primary sequence and cyclic AMP and developmental regulation. J. Biol. Chem. 263:2939-2947. [PubMed] [Google Scholar]
- 6.Bruick, R. K., and S. L. McKnight. 2002. Oxygen sensing gets a second wind. Science 295:807-808. [DOI] [PubMed] [Google Scholar]
- 7.Caniggia, I., H. Mostachfi, J. Winter, M. Gassmann, S. J. Lye, M. Kuliszewski, and M. Post. 2000. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J. Clin. Investig. 105:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carthew, R. W., L. A. Chodosh, and P. A. Sharp. 1987. The major late transcription factor binds to and activates the mouse metallothionein I promoter. Genes Dev. 1:973-980. [DOI] [PubMed] [Google Scholar]
- 9.Chirgwin, J. M., A. E. Przybyla, R. J. MacDonald, and W. J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294-5299. [DOI] [PubMed] [Google Scholar]
- 10.Ema, M., K. Hirota, J. Mimura, H. Abe, J. Yodoi, K. Sogawa, L. Poellinger, and Y. Fujii-Kuriyama. 1999. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 18:1905-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, C. T., D. B. Ledesma, T. Z. Schulz, E. R. Simpson, and C. R. Mendelson. 1986. Isolation and characterization of a complementary DNA specific for human aromatase-system cytochrome P-450 mRNA. Proc. Natl. Acad. Sci. USA 83:6387-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao, E., Y. Wang, J. L. Alcorn, and C. R. Mendelson. 1997. The basic helix-loop-helix-zipper transcription factor USF1 regulates expression of the surfactant protein-A gene. J. Biol. Chem. 272:23398-23406. [DOI] [PubMed] [Google Scholar]
- 13.Genbacev, O., R. Joslin, C. H. Damsky, B. M. Polliotti, and S. J. Fisher. 1996. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Investig. 97:540-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genbacev, O., A. Krtolica, W. Kaelin, and S. J. Fisher. 2001. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev. Biol. 233:526-536. [DOI] [PubMed] [Google Scholar]
- 15.Genbacev, O., Y. Zhou, J. W. Ludlow, and S. J. Fisher. 1997. Regulation of human placental development by oxygen tension. Science 277:1669-1672. [DOI] [PubMed] [Google Scholar]
- 16.Gnarra, J. R., J. M. Ward, F. D. Porter, J. R. Wagner, D. E. Devor, A. Grinberg, M. R. Emmert-Buck, H. Westphal, R. D. Klausner, and W. M. Linehan. 1997. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl. Acad. Sci. USA 94:9102-9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillemot, F., A. Nagy, A. Auerbach, J. Rossant, and A. L. Joyner. 1994. Essential role of Mash-2 in extraembryonic development. Nature 371:333-336. [DOI] [PubMed] [Google Scholar]
- 18.Harris, A. N., and P. L. Mellon. 1998. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol. Endocrinol. 12:714-726. [DOI] [PubMed] [Google Scholar]
- 19.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 20.Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal, A. S., and S. Narayan. 2001. Upstream stimulating factor-1 (USF1) and USF2 bind to and activate the promoter of the adenomatous polyposis coli (APC) tumor suppressor gene. J. Cell. Biochem. 81:262-277. [DOI] [PubMed] [Google Scholar]
- 23.Jauniaux, E., A. L. Watson, J. Hempstock, Y. P. Bao, J. N. Skepper, and G. J. Burton. 2000. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 157:2111-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, B., A. Kamat, and C. R. Mendelson. 2000. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol. Endocrinol. 14:1661-1673. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. E., S. J. Birren, T. Saito, and D. J. Anderson. 1992. DNA binding and transcriptional regulatory activity of mammalian achaete-scute homologous (MASH) proteins revealed by interaction with a muscle-specific enhancer. Proc. Natl. Acad. Sci. USA 89:3596-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamat, A., J. L. Alcorn, C. Kunczt, and C. R. Mendelson. 1998. Characterization of the regulatory regions of the human aromatase (P450arom) gene involved in placenta-specific expression. Mol. Endocrinol. 12:1764-1777. [DOI] [PubMed] [Google Scholar]
- 27.Kamat, A., K. H. Graves, M. E. Smith, J. A. Richardson, and C. R. Mendelson. 1999. A 500-bp region, approximately 40 kb upstream of the human CYP19 (aromatase) gene, mediates placenta-specific expression in transgenic mice. Proc. Natl. Acad. Sci. USA 96:4575-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat, A., M. M. Hinshelwood, B. A. Murry, and C. R. Mendelson. 2002. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol. Metab. 13:122-128. [DOI] [PubMed] [Google Scholar]
- 29.Kliman, H. J., J. E. Nestler, E. Sermasi, J. M. Sanger, and J. F. Strauss III. 1986. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567-1582. [DOI] [PubMed] [Google Scholar]
- 30.Kozak, K. R., B. Abbott, and O. Hankinson. 1997. ARNT-deficient mice and placental differentiation. Dev. Biol. 191:297-305. [DOI] [PubMed] [Google Scholar]
- 31.Kraut, N., L. Snider, C. M. A. Chen, S. J. Tapscott, and M. Groudine. 1998. Requirement of the mouse I-mfa gene for placental development and skeletal patterning. EMBO J. 17:6276-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lun, Y., M. Sawadogo, and M. Perry. 1997. Autoactivation of Xenopus MyoD transcription and its inhibition by USF. Cell Growth Differ. 8:275-282. [PubMed] [Google Scholar]
- 33.Maltepe, E., J. V. Schmidt, D. Baunoch, C. A. Bradfield, and M. C. Simon. 1997. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386:403-407. [DOI] [PubMed] [Google Scholar]
- 34.Meier, J. L., X. Luo, M. Sawadogo, and S. E. Straus. 1994. The cellular transcription factor USF cooperates with varicella-zoster virus immediate-early protein 62 to symmetrically activate a bidirectional viral promoter. Mol. Cell. Biol. 14:6896-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringler, G. E., and J. F. Strauss III. 1990. In vitro systems for the study of human placental endocrine function. Endocr. Rev. 11:105-123. [DOI] [PubMed] [Google Scholar]
- 36.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1 α is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenza, G. L. 2001. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1-3. [DOI] [PubMed] [Google Scholar]
- 38.Simpson, E. R., and P. C. MacDonald. 1981. Endocrine physiology of the placenta. Annu. Rev. Physiol. 43:163-188. [DOI] [PubMed] [Google Scholar]
- 39.Sirito, M., Q. Lin, J. M. Deng, R. R. Behringer, and M. Sawadogo. 1998. Overlapping roles and asymmetrical cross-regulation of the USF proteins in mice. Proc. Natl. Acad. Sci. USA 95:3758-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirito, M., Q. Lin, T. Maity, and M. Sawadogo. 1994. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res. 22:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi, Y., K. Nakayama, S. Itoh, Y. Fujii-Kuriyama, and T. Kamataki. 1997. Inhibition of the transcription of CYP1A1 gene by the upstream stimulatory factor 1 in rabbits. Competitive binding of USF1 with AhR/Arnt complex. J. Biol. Chem. 272:30025-30031. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, M., M. Gertsenstein, J. Rossant, and A. Nagy. 1997. Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev. Biol. 190:55-65. [DOI] [PubMed] [Google Scholar]
- 43.Tian, H., R. E. Hammer, A. M. Matsumoto, D. W. Russell, and S. L. McKnight. 1998. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12:3320-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiesener, M. S., H. Turley, W. E. Allen, C. Willam, K. U. Eckardt, K. L. Talks, S. M. Wood, K. C. Gatter, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and P. H. Maxwell. 1998. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 92:2260-2268. [PubMed] [Google Scholar]
- 45.Yang, X. P., L. A. Freeman, C. L. Knapper, M. J. Amar, A. Remaley, H. B. Brewer, Jr., and S. Santamarina-Fojo. 2002. The E-box motif in the proximal ABCA1 promoter mediates transcriptional repression of the ABCA1 gene. J. Lipid Res. 43:297-306. [PubMed] [Google Scholar]