Abstract
CTLA-4 (CD152), the CD28 homologue, is a costimulatory molecule with negative effects on T cell activation. In addition to its role in the termination of activation, CTLA-4 has been implicated in anergy induction and the function of regulatory cells. As an intracellular molecule, it must first relocate to the cell surface and be ligated, in order to inhibit activation. Although some studies have investigated CTLA-4 expression on CD4+ T cells, evidence is lacking regarding the kinetics of expression, and expression on T cell subpopulations. We have investigated CTLA-4 kinetics on human purified peripheral CD4+, naïve, memory, CD4+CD25–, CD4+CD25+ regulatory T cells, and T cell clones. Intracellular stores of CTLA-4 were shown to be very low in naïve T cells, whilst significant amounts were present in memory T cells and T cell clones. Cell surface CTLA-4 expression was then investigated on CD4+CD45RA+ (naïve), CD4+CD45RO+ (memory), CD4+CD25–, and CD4+CD25+ T cells. CD25 and CD45RO are both expressed by regulatory T cells. On naïve and CD4+CD25– T cells, CTLA-4 expression declined after four hours. In contrast, on memory and CD4+CD25+ T cells, high levels of expression were maintained until at least 48 hours. In addition, significant CTLA-4 expression was observed on T cell clones following anergy induction, indicating the potential involvement of CTLA-4 also in this form of tolerance.
Keywords: CD4+, human, memory, naïve, subsets
INTRODUCTION
CTLA-4, the homologue of the T cell costimulatory molecule CD28, is an important negative regulator of T cells, preventing or terminating activation [1,2]. Views on the mechanism of this vary, but three possibilities have been described [3]. Firstly, that by competing for their shared ligands, CTLA-4 may act by depriving the cell of CD28-mediated costimulation [4]. Secondly, CTLA-4 ligation by CD80 and CD86 may trigger a distinct, negative, signalling pathway, or interfere with an activating pathway, thus inhibiting activation downstream of the T cell receptor [5]. For example, recently CTLA-4 has been shown to attenuate TCR signalling by inhibiting the up-regulation of signalling raft domains in human T cells [6]. Thirdly, ligation of CTLA-4 present on a subpopulation of T cells, possibly CD4+CD25+ regulatory T cells, may induce production of a factor(s) that inhibits the activation or proliferation of neighbouring cells [7–9]. It is likely that some or all of these mechanisms apply. Whatever its mode of action, CTLA-4 has been implicated in the maintenance of peripheral tolerance, anergy induction, and in the action of CD4+CD25+ regulatory cells [9,10]. This has proved very hard to investigate in the human, due to the lack of specific ligating or blocking antibodies [7,11–17]. Despite this, CTLA-4 is a potential target molecule for the induction of tolerance in clinical settings, such as the induction of tolerance to allografts.
The murine form of CTLA-4 was identified by Brunet in 1987 [11]. It is a transmembrane protein of the immunoglobulin gene superfamily, with a single variable domain. The complete primary structure of the gene loci has been established, and the human and murine forms show 71% overall homology [12]. CTLA-4 also shares 76% sequence homology with CD28 [13], both of which are predominantly expressed on T cells. However, unlike CD28, surface expression of CTLA-4 is inducible [14], by cell activation [15,16]. Whilst its affinity for their common ligands, CD80 and CD86 is much greater [15–17], its levels of surface expression reach only ∼1/30–50 that of CD28 [18]. CTLA-4 in resting cells is intracellularly localized [18] to clathrin-associated complexes, by sequences on its cytoplasmic tail [19,20]. Cell activation induces relocation to the cell surface, which is transient and rapidly followed by internalization. Therefore, in the words of Linsley et al.[21],
‘CTLA-4 expressed at the cell surface is dynamically regulated by its transit between intracellular stores and the cell surface.’ Expression of CTLA-4 mRNA, rather than cell surface molecules, has been shown to peak between 24 and 48 hours postactivation [18,22], and has been demonstrated at timepoints as early as one hour [22], with functional effects by 12 hours [23], in the mouse. Human CTLA-4 expression has been shown to be up-regulated by activation; directly by IL-2; and indirectly by IFNγ acting via antigen presenting cells [24]. However there is little other data concerning the kinetics of up-regulation and surface expression of human CTLA-4 molecules, especially in T cell subsets.
As the function of CTLA-4 is intimately related to its expression, we have sought to determine the kinetics of CTLA-4 up-regulation in various T cell subsets, including naïve, memory, and CD25+ regulatory cells. Following the link between CTLA-4 and anergy, the up-regulation of CTLA-4 by an activating stimuli was compared to that induced by T:T presentation of specific peptide, which has been shown to induce anergy [25].
MATERIALS AND METHODS
Purification of T cells from PBMCs
For purification of CD4+ T cells, peripheral blood mononuclear cells isolated from healthy individuals were incubated on plastic (4 × 106 cells/ml in RPMI/2%FCS) at 37°C for two hours. The nonadherent population was then incubated with the following antibodies: anti-CD8 (ATCC, USA); anti-CD14 (Sigma, St Louis, USA); anti-CD19 and anti-CD33 (Becton Dickinson, California, USA); anti-CD16 and anti-CD56 (Caltag, California, USA). Anti-CD45RA and anti-CD45RO (Caltag, California, USA) were added for the purification of memory and naïve cells, respectively. Goat antimouse IgG Fc magnetic beads were used to deplete the antibody-bound cells. Where relevant, CD25+ cells were purified using CD25Dynabeads according to the manufacturer's protocol (see also results section).
T cell clones
All human T cell clones used were restricted by HLA-DR, and specific for peptide HA307-319 (7P clones), or HA100-115 (HC3). T cell clones were cultured in 10% human serum and IL-2 (added every two days). They were maintained on a seven to 10 days cycle of stimulation with specific peptide-pulsed autologous PBMC at a 1 : 1 ratio, and were used at least a week after stimulation, and two days after addition of IL-2.
Activation of T cells for analysis of CTLA-4 expression
T cells were stimulated in the following ways: With 50 ng/ml PMA (phorbol ester) and 1 µg/ml ionomycin (calcium ionophore); specific peptide (HA 307–319, or 100–115) was used to stimulate T cell clones (T:T presentation), as were anti-CD3 and anti-CD28 antibodies, and a peptide-pulsed B cell line. T cells were cultured at 37°C until harvested for analysis of CTLA-4 expression.
Immunofluorescence staining and flow cytometric analysis
Cells were analysed for surface CTLA-4 expression using the anti-CD152 PE (Pharmingen, USA) monoclonal antibody. 1 × 105 cells were incubated for 30 minutes at 37°C with the antibody. They were then washed twice with PBS, and fixed with 1% paraformaldehyde. Flow cytometric analysis was performed using a FACSCalibur flow cytometer and CellQuest Software (Becton Dickinson, Oxford, UK). Isotype matched controls were always included. For determination of intracellular CTLA-4 expression, cells were saponin permeabilized, before being incubated with the antibodies for 45 minutes at room temperature.
Anergy induction
Anergy was induced by overnight incubation of 1 × 105 cells of the T cell clones 7P8 and 7P24 with 1 µg/ml of peptide HA 307–319 in a 96 well plate in a total volume of 200 µl. Cell survival was compared with that of 1 × 105 cells of each clone incubated overnight in medium alone (final volume 200 µl). To determine the number of cells remaining viable after anergy induction relative to cells incubated overnight in medium alone, tru-count beads (‘Perfect count microspheres’, Cytognos, Salamanca, Spain) were used in conjunction with Annexin V/7-actinoaminomycin (7AAD) staining. 100 µl of re-suspended cells were transferred to FACS tubes, centrifuged at 615 gfor 5 min and re-suspended in 100 µl of Annexin V staining buffer. The cells were stained with Annexin V-PE and 7AAD for 15 min at room temperature in the dark, washed and re-suspended in 200 µl Annexin V staining buffer. Tru-count beads were vigorously re-suspended and 20 µl added per FACS tube using a reverse pipetting technique. Controls used were un-stained cells, Annexin V-PE alone and 7AAD alone. 2 gates were established, for tru-count beads and for viable T cells, using forward scatter and side scatter. Acquisition was limited to 5000 events in the bead gate. Limiting acquisition using the tru-count beads ensures the number of viable cells acquired reflects the relative concentration of viable cells from the overnight cultures. The relative number of viable cells in each condition was determined using the number of events acquired in the T cell gate which were Annexin V-PE/7AAD double negative. Percent survival was calculated using the formula: (Number of Annexin V/7AAD double negative cells acquired after T:T presentation) divided by (Number of Annexin V/7AAD double negative cells acquired after incubation in medium alone) multiplied by 100.
T cell proliferation assays
To assess the regulatory capacity of CD4+CD25+ T cells, their ability to suppress the proliferation of CD4+CD25– T cells at a 1 : 1 ratio was measured. 1 × 104 CD4+CD25– T cells were stimulated alone, or in the presence of 1 × 104 CD4+CD25+ T cells, with 0·05 µl of Dynal anti-CD3/anti-CD28 coated beads. On day five of culture the wells were pulsed with 1 µCi/well 3H-thymidine (Amersham International, Amersham, UK). Thymidine incorporation was measured after 20 hours by a liquid scintillation counter (Wallac, Turku, Finland).
Assessment of proliferation of T cells to peptide-pulsed B cells (to assess anergy induction in T cell clones), was carried out in flat-bottomed 96-well plates. B cells were pulsed the night before with peptide, and then washed and irradiated (160Gy) prior to coculture with T cells at a 1 : 3 ratio. On day three of culture wells were pulsed with 1 µCi/well 3H thymidine. Thymidine incorporation was measured after 20hours by a liquid scintillation counter.
RESULTS
Memory CD4+ T cells and T cell clones contain significant intracellular reservoirs of CTLA-4
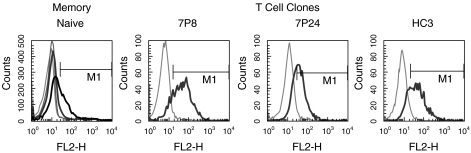
As cell surface CTLA-4 can be recruited from intracellular stores, the presence of a pre-existing reservoir would play a large role in determining the capacity for cell surface expression. Hence we investigated intracellular CTLA-4 reservoirs in resting memory and naïve CD4+ T cells, and in the T cell clones 7P8, 7P24 and HC3 (Fig. 1).
Fig. 1.
Memory but not naïve T cells have intracellular stores of CTLA-4. Saponin permeabilized, fixed, unstimulated T cells were assessed for total CTLA-4 expression by incubation with an anti-CTLA-4PE mAb for 45 minutes at room temperature. Expression levels were analysed using a Becton Dickinson FACSCalibur, and CellQuest software. Isotype controls are shown as thin lines, and anti-CTLA-4PE as thick lines. CTLA-4 expression in naïve cells is shown in dark grey, and in memory cells in light grey.
Naïve T cells possessed a small intracellular reservoir of CTLA-4, whereas memory cells contained a larger reservoir. The panel of T cell clones used comprised CD4+, Th0 clones, specific for different peptide/MHC complexes. T cell clones are of a phenotype consistent with repeated stimulation, and as such bear a greater resemblance to memory cells. Unsurprisingly, the percentage of each T cell clone possessing an intracellular reservoir was higher again than memory cells.
PMA and ionomycin induce rapid and sustained up-regulation of CTLA-4 expression
We next went on to investigate the kinetics and stability of CTLA-4 expression (Fig. 2). PMA/ionomycin activation was used, which induces similar profiles of expression to those seen in response to stimulation with anti-CD3 and anti-CD28 antibodies (data not shown). CTLA-4 expression was analysed on both freshly prepared peripheral blood CD4+ T cells, and on the human T cell clone HC3. Staining was carried out at 37°C, as described in the material and methods section, and as published [21].
Fig. 2.
Sustained expression of CTLA-4 in CD4+ T cells. Cell surface CTLA-4 expression was analysed on purified CD4+ T cells, and the T cell clone HC3, after PMA/ionomycin stimulation for the duration shown. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines.
CD4+ T cells showed up-regulation by two hours which peaked around four hours (Fig. 2a), and was then sustained for 48 hours (Fig. 2b). Differences seen between the two four-hour timepoints are indicative of interassay variation, whilst the kinetics shown are representative of several independent experiments.
CTLA-4 expression on HC3 cells was noticeable by one hour post activation (Fig. 2a) and continued to increase until 48 hours (Fig. 2b). The beginning of noticeable cell death prevented subsequent timepoints from being investigated.
Despite similar early kinetics, by 24 hours post activation, only naive cells have begun to down-regulate CTLA-4
Purified naïve (CD4+CD45RA+) and memory (CD4+CD45RO+) T cells were also analysed for CTLA-4 expression post PMA/ionomycin activation (Fig. 3). Both subpopulations showed similar kinetics of early up-regulation (Fig. 3a). Levels at four hours were almost identical. However, at later timepoints, CTLA-4 expression differed markedly between the naïve and memory subpopulations (Fig. 3b). Whereas naïve cells down-regulated CTLA-4 from four hours to 48 hours, the expression on memory cells was sustained for at least 48 hours.
Fig. 3.
Memory but not naïve T cells have sustained expression of CTLA-4. Cell surface CTLA-4 expression was analysed on purified naïve and memory CD4+ T cells, after PMA/ionomycin stimulation for the duration shown. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines.
CD4+CD25– and CD4+CD25+ cells display fundamental differences in their pattern of CTLA-4 expression
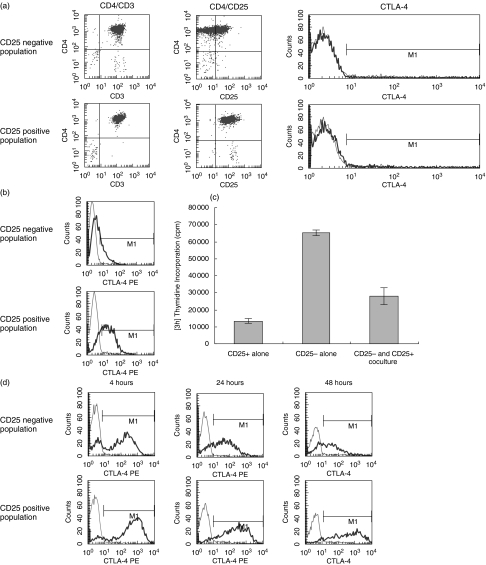
CTLA-4 has been linked not only to anergy and tolerance, but also to the action of regulatory T cells [9]. CD4+CD25+ regulatory cells have been shown to express significant levels of CTLA-4 [9,10]. CD4+CD25+ cells were obtained by positive selection, and CD4+CD25– cells by negative selection, using CD25Dynabeads according to manufacturer's protocol. ‘CD4+CD25+’ defined a population of high CD25 expressers, whilst low and negative expressers were contained within the ‘CD4+CD25–’ subset. The purity of the two populations was assessed by immunofluorescence staining for CD3, CD4, and CD25, as shown in Fig. 4a. Baseline CTLA-4 expression was also analysed, and was shown to be marginally greater on CD4+CD25+ T cells than on CD4+CD25– T cells (MFI of 10·59 as compared to 4·69).
Fig. 4.
Expression of CTLA-4 is sustained in activated CD4+ CD25+ T cells. (a) Purity of unstimulated purified CD4+CD25+ and CD4+CD25– T cells was assessed, and shown as dot blots. Cell surface CTLA-4 expression was analysed on the resting cells and is shown as peaks. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines. (b) Intracellular CTLA-4 expression was analysed on purified resting CD4+CD25+ and CD4+CD25– T cells. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines. (c) 1 × 104 CD4+CD25+ or CD4+CD25– T cells were stimulated alone with 0·05 µl of dynal anti-CD3/anti-CD28 coated beads. The ability of CD4+CD25+ T cells to suppress CD4+CD25– T cell proliferation was then measured by coculturing the two populations at a 1 : 1 ratio. The plate was pulsed after five days with 3H thymidine and harvested after 16 hours. (d) Cell surface CTLA-4 expression was analysed on purified CD4+CD25+ and CD4+CD25– T cells, after PMA/ionomycin stimulation for the duration shown. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines.
Intracellular levels of CTLA-4 were analysed in CD4+CD25– and CD4+CD25+ populations (Fig. 4b). Both possessed intracellular CTLA-4, but this reservoir was much greater in CD4+CD25+ T cells. The regulatory ability of CD4+CD25+ T cells was also demonstrated. CD4+CD25+ T cells alone were shown to have only a limited proliferative capacity in response to anti-CD3 and anti-CD28 antibody stimulation, whereas CD4+CD25– T cells proliferated greatly. This proliferation was suppressed when CD4+CD25+ cells were added to the culture, at a 1 : 1 ratio with the CD4+CD25– T cells (Fig. 4c).
Expression of CTLA-4 on CD4+CD25– and CD4+CD25+ populations was analysed over a 4–48 hour timecourse (Fig. 4d). Like naïve and memory subpopulations, at early timepoints both CD4+CD25– and CD4+CD25+ populations showed similar kinetics of up-regulation. However, again like the naïve and memory population, whilst CD4+CD25– cells down-regulated CTLA-4 from four hours, CD4+CD25+ cells maintained high levels of CTLA-4 expression up to 48 hours. This sustained CTLA-4 expression may be critical to the function of CD4+CD25+ regulatory T cells.
T cells rendered anergic express high levels of CTLA-4
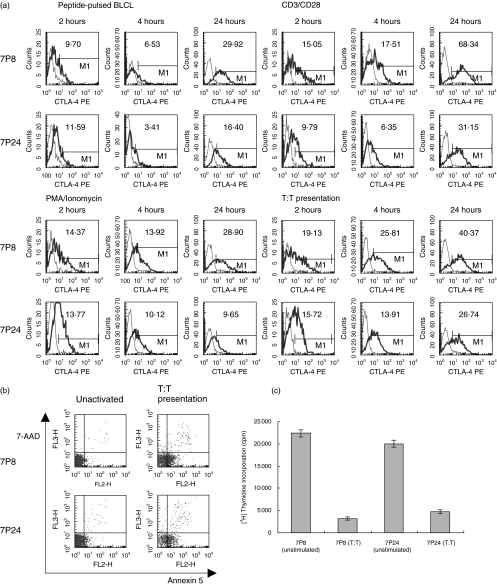
In view of the many common features shared by CD4+CD25+ and anergic T cells, up-regulation of CTLA-4 was investigated post anergy induction in T cell clones (Fig. 5). The two T cell clones 7P8 and 7P24, which were from the same donor, and had the same specificities, were stimulated with anti-CD3 and anti-CD28 antibodies, with PMA and ionomycin, by peptide-pulsed B cells, or by T:T presentation of peptide. Both activating, and anergising (T:T presentation) stimuli induced CTLA-4 up-regulation within four hours (Fig. 5a). However, in the case of T:T presentation, these high levels were seen despite the induction of anergy, rather than activation.
Fig. 5.
T cells rendered anergic express high levels of CTLA-4. 7P8 and 7P24 cells were stimulated with either anti-CD3 and anti-CD28 antibodies, PMA and ionomycin, peptide pulsed BLCLs, or by T:T presentation of peptide. (a) Cell surface CTLA-4 expression in response to the various stimuli was measured after two, four, and 24 hours. Isotype controls are shown as thin lines, and anti-CTLA-4PE mAb as thick lines. (b) Parallel cultures were set up which were stimulated for 24 hours, after which time the cells double stained with the vital dyes 7AAD and annexin 5, and counted using tru-count beads. (c) Proliferation of unstimulated cells or cells stimulated by T:T presentation, in response to subsequent challenge by BLCL presenting specific peptide, was then investigated. Recovered cells were cocultured with peptide-pulsed BLCL, at concentrations of 5 × 103 and 3 × 104, respectively. 3H thymidine incorporation was used as a measure of proliferation.
It was noted that the levels of CTLA-4 expressed on the clone 7P8 in response to T:T presentation were much higher than the levels expressed on 7P24 (Mean fluorescence intensity is shown in each plot). T:T presentation in T cell clones is associated with a variable degree of cell death [26]. Whether the level of cell death was related to the level of CTLA-4 expression, was investigated by double staining the cells after stimulation, with the vital dyes 7AAD and annexin 5, which are incorporated into apoptotic or necrotic cells (Fig. 5b). After T:T presentation, 55·30% of 7P8 cells remained viable (98·40% viability when unstimulated), as compared to only 41·65% of 7P24 cells (98·45% viability when unstimulated).
To demonstrate that T:T presentation induced anergy in the clones, after recovery the clones were rechallenged with peptide pulsed BLCLs. T cell clones exposed to T:T presentation were shown to be hyporesponsive compared to previously unstimulated cells (Fig. 5c).
DISCUSSION
In this study various aspects of CTLA-4 expression on a range of human T cell subsets and clones have been described. CTLA-4 was shown to be up-regulated on the cell surface at timepoints early after stimulation, in response to either activating or anergising stimuli. Expression levels on whole CD4+ T cells, or T cell clones, were maintained up to 48 hours after activation. Differential expression of CTLA-4 was seen in naïve, memory, CD4+CD25– and CD4+CD25+ T cells. Whilst CD4+CD25+ and memory T cells showed sustained CTLA-4 expression post stimulation, expression levels on naïve and CD4+CD25– T cells declined rapidly after initial up-regulation. CD4+CD25+ cells have previously been shown to constitutively express CTLA-4, whereas CD4+CD25– cells do not. The data described here is in accordance with this, but further suggests that maintenance of expression may be a more important defining feature than resting expression levels.
Memory T cells and T cell clones were shown to contain a significant intracellular reservoir of CTLA-4. This reservoir was much smaller in naïve T cells, although these cells did possess some CTLA-4. This expression observed in naïve cells contrasts with a study that showed that naïve resting T cells did not express CTLA-4 mRNA or surface protein [27]. However this study looked at murine cells, whereas in this study the cells used were human. Certainly resting human whole CD4+ T cells have been shown to express intracellular CTLA-4 [24]. The increase in CTLA-4 expression from naïve to memory to T cell clones suggests that previous stimulation, be it the stimuli that drive differentiation from naïve to memory, or the stimulation with IL-2 and PBMC that is required to maintain T cell clones, is a necessary factor for the production of CTLA-4 protein. Thus the size of the CTLA-4 reservoir may be determined by the stimulation history of the T cell. Thus the reservoir was significant in cells that had differentiated to the memory phenotype, and greater in T cell clones, which were maintained on a regular cycle of stimulation.
CD4+ T cells and the T cell clone HC3 were shown to up-regulate cell surface CTLA-4 within two hours of activation, whereas previous studies have only examined mRNA expression at this time. The presence of CTLA-4 at such early timepoints lends weight to the theory [3] that CTLA-4 may not only be involved in the termination of activation, but also in the prevention of inappropriate activation. CTLA-4 expression was maintained for up to 48 hours, allowing also for a more sustained inhibitory action.
Naïve and memory T cells were shown to have similar early CTLA-4 expression, but only memory cells retained expression by 24 and 48 hours. This suggests deficiencies in naïve cells in either their de novo production, or their ability to recruit CTLA-4 from intracellular stores by tyrosine phosphorylation. The latter is consistent with the differences in CTLA-4 intracellular reservoirs seen between naïve and memory cells, in that the reservoir seen in naïve cells is much smaller that than in memory cells, and thus may become exhausted sooner, thus causing surface expression to decline.
The discrepancy in sustained expression may relate to the ‘need’ for CTLA-4. In naïve cells, which neither have to be long-lived, nor function as regulatory cells [28], CTLA-4 may only be needed early after activation to control the response. Memory cells however, have to be long-lived, and CTLA-4 has been linked to protection from apoptosis [29]. This suggests that the sustained expression after activation of CTLA-4 may be important to memory cell survival. Also, the regulatory activity of CD4+CD25+ cells, which may act partly via CTLA-4, has been shown to be confined to the CD45RO+ subset. The small percentage of CD4+ T cells that are both CD25+ and CD45RA+ (4·99%), do not possess regulatory capacity [28]. Sustained memory cell CTLA-4 expression may thus be a prerequisite for regulatory activity.
Both CD4+CD25– and CD4+CD25+ T cells showed high CTLA-4 expression at four hours. However, whilst the CD4+CD25– cells had down-regulated CTLA-4 by 48 hours, CD4+CD25+ cells showed sustained expression up to 48 hours. Following the hypothesis that CTLA-4 may play a role in the regulatory function of CD4+CD25+ T cells [9,10,30] sustained CTLA-4 expression would be of great benefit. These data correlate well with the sustained expression of CTLA-4 also seen on memory cells, in that functional regulatory T cells are CD4+CD25+CD45RO+.
CTLA-4 up-regulation induced by anergy induction was also investigated, following the many similarities between anergic T cells and CD4+CD25+ T cells. Presentation of specific peptide by T cell clones did not induce a significant proliferative response, and rendered the cells anergic to subsequent stimulation. Such T:T presentation was shown to induce significant early levels of CTLA-4 expression, highlighting the fact that CTLA-4 is present (and in high amounts) in situations not only of activation, but also of anergy induction. Not only this, but increased levels of CTLA-4 expression have been correlated to reduced levels of T:T-induced apoptosis. These results suggest that CTLA-4, which has already been shown not to increase the rate of apoptosis [31], may furthermore protect against apoptosis. We have shown this with regard to anergy induction. CTLA-4-induced protection from apoptosis [29] may also apply to memory cells and CD4+CD25+ T cells, which, like anergic cells, express high levels of CTLA-4.
Acknowledgments
We thank Dr Federica Marelli-Berg and Dr Anthony Dorling for critical reading of the manuscript. C.B. Jago is the recipient of a research studentship from the Medical Research Council of Great Britain. This work was supported by an MRC programme grant.
References
- 1.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansom DM. CD28, CTLA-4 and their ligands. who does what and to whom? Immunology. 2000;101:169–77. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–27. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance? J Immunol. 1997;158:1989–93. [PubMed] [Google Scholar]
- 6.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–81. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge. lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–31. [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily – CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 12.Ling V, Wu PW, Finnerty HF, Sharpe AH, Gray GS, Collins M. Complete sequence determination of the mouse and human CTLA4 gene loci: cross-species DNA sequence similarity beyond exon borders. Genomics. 1999;60:341–55. doi: 10.1006/geno.1999.5930. [DOI] [PubMed] [Google Scholar]
- 13.Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–44. [PubMed] [Google Scholar]
- 14.Freeman GJ, Lombard DB, Gimmi CD, et al. CTLA-4 and CD28 mRNA are coexpressed in most T cells after activation. Expression of CTLA-4 and CD28 mRNA does not correlate with the pattern of lymphokine production. J Immunol. 1992;149:3795–801. [PubMed] [Google Scholar]
- 15.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 16.Guinan EC, Gribben JG, Boussiotis VA, Freeman GJ, Nadler LM. Pivotal role of the B7: CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84:3261–82. [PubMed] [Google Scholar]
- 17.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradshaw JD, Lu P, Leytze G, Rodgers J, Schieven GL, Bennett KL, Linsley PS, Kurtz SE. Interaction of the cytoplasmic tail of CTLA-4 (CD152) with a clathrin-associated protein is negatively regulated by tyrosine phosphorylation. Biochemistry. 1997;36:15975–82. doi: 10.1021/bi971762i. [DOI] [PubMed] [Google Scholar]
- 20.Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–9. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–43. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 22.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–99. [PubMed] [Google Scholar]
- 23.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XB, Zheng CY, Giscombe R, Lefvert AK. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol. 2001;54:453–8. doi: 10.1046/j.1365-3083.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 25.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldman M, et al. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargreaves RG, Borthwick NJ, Montani MS, Piccolella E, Carmichael P, Lechler RI, Akbar AN, Lombardi G. Dissociation of T cell anergy from apoptosis by blockade of Fas/Apo-1 (CD95) signaling. J Immunol. 1997;158:3099–107. [PubMed] [Google Scholar]
- 27.Metz DP, Farber DL, Taylor T, Bottomly K. Differential role of CTLA-4 in regulation of resting memory versus naive CD4 T cell activation. J Immunol. 1998;161:5855–61. [PubMed] [Google Scholar]
- 28.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Da Rocha Dias S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001;97:1134–7. doi: 10.1182/blood.v97.4.1134. [DOI] [PubMed] [Google Scholar]
- 30.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 31.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [published erratum appears J Exp Medical 1996 July 1;184 (1):301] [DOI] [PMC free article] [PubMed] [Google Scholar]