Abstract
It is becoming apparent that γδ T cells form an important part of the adaptive immune response. However, the ligands recognized by γδ T cell receptors (TCRs) and the exact biological function of the cells that express this receptor remain unclear. Numerous studies have shown that the dominant human peripheral blood subset of γδ T cells, which express a Vγ9Vδ2 TCR, can activate in response to low molecular weight nonpeptidic molecules. Some of these components have been purified from bacteria or parasites. We examined the activation of polyclonal γδ T cell lines, clones with Vγ9Vδ2 and Vγ9Vδ1 TCRs, and γδ T cells directly ex vivo in response to multiple phosphate, alkylamine and aminobisphosphonate (nBP) antigens and purified protein derivative from Mycobacterium tuberculosis (PPD). Vγ9Vδ2 T cells were able to respond to multiple small organic molecules of highly variable structure whereas cells expressing a similar Vγ9 chain paired with a Vδ1 chain failed to recognize these antigens. Thus, the TCR δ chain appears to make an important contribution to the recognition of these antigens. The kinetics of responses to alkylphosphate and alkylamine antigens differ from those of responses to the nBP pamidronate. These different classes of antigen are believed to have differed mechanisms of action. Such differences explain why nBPs can be pulsed onto antigen presenting cells (APCs) and still retain their ability to activate γδ T cells while alkylphosphate and alkylamine antigens cannot. We also demonstrate that a substantial proportion of the cells that produce IFNγ directly ex vivo in response to PPD are γδ T cells and that γδ T cell activation requires contact with cells of human origin.
Keywords: T lymphocytes, γδ T cell receptors, alkylphosphate, alkylamine, aminobisphosphonate
INTRODUCTION
The key to adaptive immunity lies in the somatic gene rearrangement of antigen receptors that produces an almost infinite array of specific configurations from a finite number of genes. There are three different types of antigen receptor: the B cell receptor (antibody), the αβ T cell receptor (TCR), which generally recognizes small peptide antigens in the context of self major histocompatibility complex (MHC) molecules presented on the cell surface, and the γδ TCR. These three receptors distinguish the main lymphocyte lineages: B cells, αβ T cells and γδ T cells. The last 40 years have seen much progress in our understanding of how B cells and αβ T cells contribute to immunity. Surprisingly, the ligands recognized by the γδ TCR and the exact biological role of cells that express this receptor remain unclear [1,2].
The potential importance of γδ T cells is evident from the large number of possible γδ T cell receptors (TCRs) [3]. Despite a relatively restricted number of V genes compared with αβ T cells, huge diversity can be generated through the D and J gene segments and it has been estimated that approximately 1020 different γδ TCRs can be created by these gene rearrangements [3]. This is substantially more than the number of possible antibodies or αβ TCRs that the human body can generate although it is not clear to what extent γδ T cells exploit this potential. Hints at an important role for γδ T cells in immunity come from the observed large expansions of these cells that occur in response to a wide range of pathogens. In humans, bacterial infections including Mycobacterium tuberculosis[4], Mycobacterium leprae[5], Salmonella typi[6], Brucella melitensis[7], Francisella tularensis[8], Listeria monocytogenes[9,10] and Ehrlichia chaffeensis[11], viral infections including HIV [12–14], Epstein-Barr Virus [15] and cytomegalovirus [16,17], yeast infection with Candida albicans[18] and parasitic infections including Plasmodium falciparum[19], Plasmodium vivax[20] and Leishmania donovani[21] can all stimulate a dramatic expansion of γδ T cells in vivo. While murine studies have shown that γδ T cells are redundant and nonessential for immunity to many infectious agents (reviewed in [1]) they are indispensable and nonredundant for immunity to other pathogens. Depletion of the γδ T cell subset in mice with TCR-specific antibodies can result in an increase in bacterial titres by 2–3 orders of magnitude after infection with L. monocytogenes[22,23]. A recent study showed that while intranasal inoculation of γδ–/– mice with Nocardia asteroides led to 100% fatality within two weeks there were no deaths in γδ+/+ control mice [24]. Other studies in γδ–/– mice reveal a role for γδ T cells in protection against Mycobacterium bovis bacillus Calmette-Guerin (BCG) [25], M. tuberculosis[26] and L. monocytogenes[27]. In addition, recent experiments in primates highlight the adaptive nature of γδ T cell responses. Macaques infected with BCG showed up to 25 fold increases in circulating Vγ9Vδ2 T cells and exhibited larger expansions during re-infection [28], demonstrating that γδ T cells, in common with their αβ TCR-expressing cousins, possess the capacity for immunological memory. The clonotypic expansions of Vγ9Vδ2 T cells during BCG infection and re-infection infer antigen-specificity in primary and memory γδ T cell-mediated immune responses [28]. Vaccination of humans with BCG induces a marked increase in the capacity of γδ T cells to expand in vitro following stimulation with live BCG or M. tuberculosis lysate [29]. These data suggest that human γδ T cells can mount a memory response [29]. The immune potency of human peripheral blood γδ T cells in vivo has also been demonstrated in the SCID mouse model. Inoculation of SCID mice with human PBMC rescues mice from lethal infection with E. coli[30]. Depletion of Vδ2-expressing T cells from PBMC resulted in a higher bacterial load and lower survival rate [30].
Despite the large number of organisms known to activate γδ T cells, relatively few antigens have been identified. Recent advances have described low molecular weight, nonpeptidic compounds that appear to be able to activate the dominant subset of γδ T cells in human peripheral blood, those with a Vγ9Vδ2 TCR. These compounds include alkylphosphates, alkylamines and aminobisphosphonates (nBPs) [31–33]. Recognition of all these groups of molecules appears to be subject to strict chemical constraints as minor modifications in antigen structure abolish activity [32,34,35]. Many of the γδ T cell phosphoantigens and the antigenic alkylamines are produced by bacteria, suggesting that the recognition of such compounds might be immunologically relevant.
In this study, we examine the recognition requirements of human γδ T cells in detail. We show that Vγ9 T cells expressing a Vδ2 chain recognize multiple small nonpeptidic ligands. In contrast, cells isolated from human peripheral blood that express a Vγ9 chain paired with a Vδ1 chain fail to recognize any of these ligands. A single Vγ9Vδ2 T cell clone activated in response to alkylphosphate, alkylamine, and nBP antigens despite the obvious differences in their chemistry. Recognition of all these compounds was dependent on cell-cell contact. nBP ligands could be pulsed onto antigen presenting cells (APCs), while alkylphosphate and alkylamine antigens needed to be continuously present in the media to activate γδ T cells. Furthermore, all these molecules were only able to activate γδ T cells when the presenting cell was of human origin.
MATERIALS AND METHODS
γδ T cell culture
γδ T cells were isolated from human peripheral blood using a magnetic bead-based separation kit (TCR γ/δ Microbead Kit and MS columns, Miltenyi Biotech Ltd., Bisley, UK). Once isolated, cells were resuspended in T cell medium (RPMI supplemented with penicillin, streptomycin and glutamine, 10% FCS, 10% T-STIM (BD Biosciences, Cowley, UK), 200 U/ml Proleukin (Chiron)) containing 2 × 106γ-irradiated human peripheral blood mononuclear cells (PBMC)/ml from three unrelated donors and 2 µg/ml phytohaemagglutinin (PHA). Cells were maintained with T cell medium, and restimulated with mixed irradiated PBMC and PHA every three weeks. For cloning, the same mix was used to grow cells by limiting dilution in 96-well U-bottomed plates. The IMGT system of TCR nomenclature is used throughout this work [36]. Cells that grew were confirmed to be γδ T cells with Vγ9 antibody. 59/60 of Vγ9-expressing clones also stained with Vδ2 antibody. The remaining clone expressed a Vδ1 receptor. Two of the best growing Vγ9Vδ2 clones and the Vγ9Vδ1 clone were taken forward for further examination. Sequencing showed that both Vγ9Vδ2 T cells expressed an identical TCR.
Sequencing γδ TCRs
TCR usage in γδ T cell clones was characterized with a molecular analysis of gene expression. RNA was isolated from 106 cells in TriReagent (Sigma-Aldrich Company Ltd., Gillingham, UK). cDNA was prepared using a poly T oligonucleotide and reverse transcriptase enzyme. Polymerase chain reaction (PCR) was carried out for the gamma chain and for the delta chain using a full panel of variable region primers and a reverse primer to anneal to the constant region. The results of the PCR were consistent with the flow cytometric analysis and demonstrated both a Vγ9Vδ1 and a Vγ9Vδ2 clone.
Antibodies
The following antibodies were used for flow cytometric analysis: FITC-conjugated mouse anti-human Vδ2 mAb clone B6·1 (BD Biosciences Pharmingen, Cowley, UK), FITC-conjugated mouse anti-human Vγ9 mAb clone 7A5 (Endogen), FITC-conjugated mouse anti-human Vδ1 mAb clone TS8·2 (Endogen), PE-conjugated mouse anti-human Vγ9 mAb clone B3·1 (BD Biosciences Pharmingen, Cowley, UK), FITC-conjugated mouse anti-human pan-γδ mAb clone 5A6.E9 (Endogen), PerCP-conjugated mouse anti-human CD3 mAb clone SK7 (BD Biosciences), FITC-conjugated mouse anti-human CD69 mAb clone L78 (BD Biosciences), allophycocyanin (APC)-conjugated mouse anti-human IL2 clone MQ1–17H12 (Caltag-Medsystems Ltd., Siverstone, UK), APC-conjugated mouse anti-human TNFα clone Mab11 (BD Biosciences Pharmingen, Cowley, UK) and APC-conjugated mouse anti-human IFNγ clone B27 (BD Biosciences Pharmingen, Cowley, UK).
γδ T cell antigens
Alkylamines (Sigma) were diluted to a 1 m stock in RPMI media and brought to pH 7·0 with concentrated HCl. Alkylamine stocks were maintained in small aliquots at −80°C. 1 mm isopentenyl pyrophosphate (Sigma), 1 mm pamidronate (Calbiochem) and 100 µm risedronate (Procter & Gamble) stocks in RPMI were stored in the same way. Tuberculin purified protein derivative (PPD) was purchased from the Statens Seruminstitut (Denmark). A cross-linking anti-CD3 monoclonal antibody (clone UCHT-1; Amersham) was used as a positive control.
IFN γ ELISpot
γδ T cells were washed in RPMI and incubated overnight in R10 at 37°C. 96-well nitrocellulose plates (Millipore (UK) Ltd., Watford, UK) were incubated overnight at 4°C with 15 µg/ml anti-human-IFNγ primary antibody (clone 1-D1K; Mabtech, AB, Nacka Strand, Sweden). The plates were then washed twice with RPMI and blocked with R10 for 3 h at 37°C. R10 was decanted by inversion and assays applied to each well before incubation at 37°C as detailed below. Assays were terminated by washing once in water, followed by 5 washes in PBS. Secondary antibody (anti-human-IFNγ-Biotin antibody clone 7-B6-1; Mabtech) was applied at 1 µg/ml and the plate incubated for 100 min at room temperature (RT). The plate was washed 6 times with PBS before application of streptavidin-ALP (1 : 1000 in PBS; Mabtech) for 40 min at RT. After 5 further washes in PBS, spots were revealed by incubation for 15 min at RT with developing buffer (Bio-Rad AP conjugate substrate kit) and counted mechanically using an ELISpot Reader System ELR02 (Autoimmun Diagnostika; Strassberg) after drying. The data displayed has not undergone any manual manipulation. A variety of cell lines were used as antigen presenting cells in IFNγ ELISpot assays. These included the MHC class I-deficient cell lines 721.221 and C1R, the TAP-deficient cell line 721.174, Jurkat T cells, HeLa cells, the β2m-deficient cell line Daudi and several EBV-transformed B cell lines and primary tumour lines.
Intracellular cytokine staining
106 fresh PBMC were incubated in FACS tubes with brefeldin A (10 µg/ml in R10) for 5 h after incubating with relevant antigens for 1 h. The cells were then washed, permeabilized in 20% FACSLyse (BD Biosciences), washed twice in ice cold PBS/0·1% BSA and stained on ice with pretitred APC-conjugated monoclonal antibodies specific for IFNγ, TNFα and IL-2, together with PE-conjugated anti-Vγ9, for 20 min. Cells were then washed, resuspended in PBS and analysed immediately on a FACSCalibur flow cytometer with CellQuest software (BD Biosciences, Cowley, UK).
MIP1 β ELISA
5 × 104 clonal Vγ9Vδ1 or Vγ9Vδ2 T cells were incubated with 5 × 104 Spinner HeLa cells for 4 h at 37°C in a 96-well U-bottom plate, in the presence or absence of 1·5 µg/ml anti-CD3 antibody (clone UCHT-1; Amersham plc., Amersham, UK), 10 mm secbutylamine, 10 µm IPP, 1 µm risedronate (Procter & Gamble). MIP-1β concentrations in the cell culture supernatant were determined with a Quantikine MIP1β ELISA kit (R & D Systems, Europe Ltd., Abingdon, UK) as previously described [37].
γδ T cell depletion of PBMC
108 human PBMC were incubated with 10 µg/ml anti-human pan-γδ antibody for 30 min on ice. Cells were washed once in 20X labelling volume with PBS/0·1%BSA and resuspended at 4 × 107 cells/ml in PBS/0·1%BSA. 2 × 107 anti-mouse IgG1 Dynabeads (Dynal Biotech Ltd., Bromborough, UK) washed in PBS/0·1%BSA were added and the cells incubated for 1 h at 4°C with gentle rotation. The tube was then placed in the magnetic particle concentrator (Dynal) and left to separate for 30 min. The supernatant was transferred to a fresh tube. The success of depletion was determined by FACS analysis with the anti-CD3 and pan-γδ antibodies detailed above.
Stimulation of γδ T lymphocytes for subsequent immunoblotting
Cells were washed twice in RPMI and incubated overnight in R10. The following day, FCS was washed off with two changes of RPMI and 106γδ T cells were resuspended in 10 µl of RPMI. After 10 min at 37°C in 5% CO2, T cells were stimulated by incubation with 10 µg/ml anti-CD3 antibody (clone UCHT-1; Amersham) for 3 min. The reaction was stopped by washing once with 1 ml ice-cold PBS, and resuspending the pellet in cold lysis buffer (140 mm NaCl, 20 mm Tris pH 8·0, 10 mm NaF, 2 mm EDTA, 20% glycerol, 1% IGEPAL, 1 mm Na3VO4, 10 µg/ml aprotonin, 10 µg/ml leupeptin) at 5 × 107 cells/ml. Blots were performed as described previously [38].
RESULTS
Activation of human peripheral blood γδ T cells directly ex vivo
Recent studies have described low molecular weight, nonpeptidic compounds that appear to be able to activate the Vγ9Vδ2 dominant subset of γδ T cells in human peripheral blood. These compounds include alkylphosphates [31,39], alkylamines [32] and nBPs [33]. We examined IFNγ production in response to activating alkylphosphate and alkylamine ligands and compared this to PPD by ELISpot (Fig. 1). Significant spot formation was observed with the previously reported [32]γδ antigens secbutylamine and isobutylamine. Between 15 and 220 spots were observed per 5 × 104 peripheral blood mononuclear cells (PBMC) from nine separate healthy donors (Fig. 2a). Curiously, ethylamine, isopropylamine, ethanolamine, and isoamylamine did not appear to activate (Fig. 2b) despite having been previously described as antigenic at the concentration used [32]. However, alkylamines only activate γδ T cells at concentrations close to those that are cytotoxic [32]. It is possible that the assays or cells we used were less sensitive than in previous studies so that cells died at alkylamine concentrations below the activation threshold. Magnetic depletion of γδ T cells from PBMC (judged to be >90% successful by FACS analysis) removed the vast majority of alkylamine-induced spots (Fig. 1a,b). Magnetic depletion similarly removed the majority of spots induced by the phosphate antigen isopentenyl pyrophosphate (IPP) (Fig. 1a,b). Titration showed that IPP was a significantly more potent antigen than the alkylamines (Fig. 2b). High concentrations of isobutylamine were toxic [32] (Figs 1a and 2b) and also prevented peptide-induced spot formation by αβ TCR-expressing cytotoxic T lymphocytes (data not shown). Magnetic-depletion of γδ T cells was observed to remove approximately a third of IFNγ spot forming cells in response to PPD during overnight ELISpot assay (Fig. 1). Similar results were obtained using PBMC from two other individuals (data not shown). Vγ9 T cells were shown to respond to IPP, risedronate and PPD in direct ex vivo intracellular cytokine staining (ICS) assays (Fig. 2c). Examination of T cell activation by CD69 up-regulation (Fig. 2d) confirmed the presence of PPD-reactive γδ T cells in the PBMC of more than half of the individuals studied. The responses measured by 6 h ICS were significantly lower than those measured by overnight ELISpot or CD69 up-regulation. Far fewer peripheral blood γδ T cells up-regulated surface expression of CD69 (<5%) after only 6 h incubation with PPD (data not shown); thus, the difference between ICS and other assays likely reflects the duration of antigen exposure. Overnight ICS assay was toxic to cells.
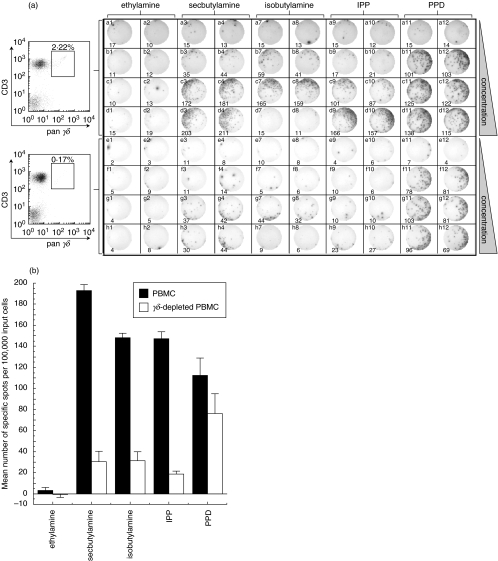
Fig. 1.
γδ T cells respond to alkylphosphate, alkylamine and PPD antigens directly ex vivo. (a) Demonstration of γδ T cell activation by direct ex vivo IFNγ ELISpot assay. The top half of the plate was set up with 100 000 PBMC/well and the bottom half with 100 000 γδ-depleted PBMC per well. FACS analysis with pan-γδ and anti-CD3 antibody (left) showed that γδ-depletion was >92% successful. Cells were activated with ethylamine in columns 1 & 2, secbutylamine in columns 3 & 4, isobutylamine in columns 7 & 8, IPP in columns 9 & 10 and PPD in columns 11 & 12. Two replicates were performed for each experimental condition. Rows a and e are control rows without added antigen. Alkylamine antigens were added at 1 mm in rows b & f, 10 mm in rows c & g and 50 mm in rows d & h. IPP was added at 10 µm in rows b & f, 100 µm in rows c & g and 1 mm in rows d & h. PPD was added at 5 µg/ml in rows b & f, 10 µg/ml in rows c & g and 20 µg/ml in rows d & h. The ELISpot photograph and number of spots (bottom of each panel) were generated mechanically using an ELISpot Reader System ELR02 (Autoimmun Diagnostika; Strassberg). (b) Number of specific spots (sample minus background) per 100 000 PBMC (▪) and γδ-depleted PBMC (□) induced by specified antigens. Bars represent spots induced by 50 mm ethylamine, 50 mm secbutylamine, 10 mm isobutylamine (50 mm isobutylamine was toxic to cells), 1 mm IPP and 20 µg/ml PPD. Error bars show the standard deviation from the mean of two replicate wells.
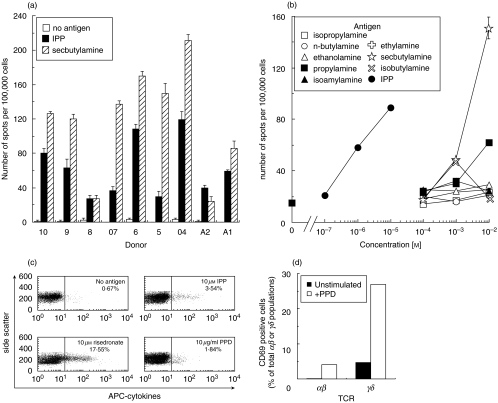
Fig. 2.
Further characterization of direct ex vivo responses to γδ T cell antigens. (a) IFNγ ELISpot reponses to 100 µm IPP and 10 mm secbutylamine in PBMC from 9 separate individuals. All individuals also responded to n-butylamine, propylamine and isobutylamine (data not shown) although the response to these antigens was generally lower than that with secbutylamine. Bars show the standard deviation from the mean of two replicate assays. (b) The effect of antigen concentration on the activation of γδ T cells directly ex vivo. The number of spots per 100 000 input PBMC is shown. PBMC from other individuals gave similar results (data not shown). Isobutylamine is toxic at high concentration [32]. Ethylamine, isopropylamine, ethanolamine, and isoamylamine, previously reported to be antigenic [32], did not induce spots. Error bars show standard deviation from the mean of two replicate assays. The errors were generally smaller than the plot symbols. (c) 106 fresh PBMC from a BCG vaccinated healthy donor were exposed to indicated antigens for 6 h and then stained with anti-Vγ9 antibody and for intracellular cytokines (IFNγ, TNFα and IL2) as described in the Materials and Methods. Plots show side scatter vs. staining with APC-cytokine antibodies for the Vγ9 positive population and indicate the fraction of cytokine positive cells. (d) PPD induced CD69 up-regulation in almost 30% of human peripheral blood γδ T cells. PBMCs were washed in RPMI and resuspended in RPMI + 10% FCS at 106 cells/ml and incubated ± 10 µg/ml PPD at 37°C for 12 h. Cells were stained with FITC-conjugated anti-CD69 antibody, PE-conjugated anti-Vγ9 antibody and PerCP-conjugated anti-CD3 antibody prior to FACS analysis.
Characterization of γδ T cell clones
In order to characterize peripheral blood γδ T cells further, we grew γδ T cell clones from human PBMC and screened for those bearing the dominant Vγ9 receptor by flow cytometry with monoclonal antibody clone 7A5 (Endogen). Curiously, 1/60 of the clones that grew expressed a Vγ9 receptor paired with a Vδ1 chain. Two robust Vγ9Vδ2-expressing and the Vγ9Vδ1-expressing clone were taken forward for further analysis. The TCR sequences of these clones (Fig. 3) confirmed these designations but showed that both Vγ9Vδ2-expressing cells expressed an identical TCR. The CDR3 regions of the Vγ9 chain differed in the Vδ1 and Vδ2 clone (Fig. 3). Both Vγ9 chains used a joining segment encoded by the TCRGJP (JγP) gene (IMGT nomeclature [36]). The Vγ9Vδ1 and Vγ9Vδ2 clones expressed high levels of cell surface TCR (Fig. 4a) and could be activated through this TCR by CD3-crosslinking (Fig. 4b). The Vγ9Vδ2 clone made substantial amounts of MIP1β in response to anti-CD3 cross-linking, IPP, secbutylamine and risedronate (Fig. 4c). In contrast, the Vγ9Vδ1 clone only responded to anti-CD3 cross-linking and was unable to recognize alkylphosphate, alkylamine or nBP antigens (Fig. 4c). IFNγ ELISpot analysis confirmed that the Vγ9Vδ2 clone activated in response to IPP, secbutylamine and isobutylamine (Fig. 4d). Activation of the Vγ9Vδ2 clone by low molecular weight nonpeptidic compounds required the presence of both antigen-presenting cells and antigen (data not shown). Curiously, PPD was unable to activate the Vγ9Vδ2 T cell clone. This result contrasts with those of another study which indicated that all Vγ9Vδ2 T cells respond to M. tuberculosis extract including those that are CD4-CD8-, CD8+ or CD4+ [40]. However, these differences might be due to the fact that M. tuberculosis extracts are known to contain pyrophosphate antigens that are lost in the production of PPD. Neither alkylphosphate or alkylamine antigens were able to activate the Vγ9Vδ1-expressing clone in IFNγ ELISpot assay (data not shown).
Fig. 3.
The γ and δ chain sequences of γδ T cell clones. The TCR sequences for the Vγ9Vδ1 (top box) and Vγ9Vδ2 (bottom box) T cell clones (IMGT nomenclature [36] is used throughout this work). The γ and δ chain sequences for the Vγ9Vδ1 clone have the accession numbers AJ583014 and AJ583015, respectively, at the EMBL Nucleotide Sequence Database. The accession numbers AJ583012 and AJ583013 have been assigned to the γ and δ chains for the Vγ9Vδ2 clone. The CDR1, 2 and 3 sequences are indicated by shaded boxes. The differences in the Vγ9 CDR3 region between the Vδ1 and Vδ2 clones are underlined. Both Vγ9 chains use the JγP joining region which contains two germline encoded lysine residues (shown in bold text within the CDR3 region) that have been shown to be essential for recognition of alkylphosphate and alkylamine ligands [46].
Fig. 4.
Vγ9Vδ1 and Vγ9Vδ2 T cells respond to different antigens. (a) Both the Vγ9Vδ1 (upper panels) and Vγ9Vδ2 (lower panels) T cell clones were shown to express high levels of TCR by FACS. Clones were stained with PE-conjugated anti-human Vγ9 mAb clone B3·1 (all panels), FITC-conjugated anti-human Vδ1 mAb clone TS8·2 (left panels) and FITC-conjugated anti-human Vδ2 mAb clone B6·1 (right panels). (b) Both clones can be activated through their TCR by anti-CD3 antibody. Clones were stimulated with anti-CD3 antibody (10 µg/ml) for 3 min, washed once with PBS, and lysed, before separation by SDS-PAGE alongside unstimulated controls. Proteins bearing phosphorylated tyrosine residues were revealed by immunoblotting with anti-phosphotyrosine antibody clone 4G10 as described [38]. The lysates of 106 cells per lane were loaded as follows: Vγ9Vδ1 T lymphocytes before (lane 1) and after (lane 2) stimulation; Vγ9Vδ2 T lymphocytes before (lane 3) and after (lane 4) stimulation. (c) Vγ9Vδ2, but not Vγ9Vδ1 T cells, are able to recognize alkylphosphate, alkylamine and nBP antigens. MIP1β release from 5 × 104 clonal T cells as described in the Materials and Methods section is shown in response to 1·5 µg/ml anti-CD3 antibody clone UCHT-1, 10 µm IPP, 10 mm secbutylamine and 1 µm risedronate. (d) IFNγ ELISpot assay with a titration of potential antigens. 1000 Vγ9Vδ2 T cells were used with 25 000 EBV transformed B cells (Sparky line) per well as antigen presenting cells. The Vγ9Vδ1 clone failed to activate in response to any of these antigens (data not shown).
Vγ9Vδ2 T cells respond to alkylphosphate/alkylamine antigens and nBP antigens with different kinetics
Recent studies have shown that nBP antigens can be pulsed onto tumour cells [41,42]. We used IFNγ ELISpot to examine whether alkylphosphate, alkylamine and aminobisphosphonate antigens could be pulsed onto antigen presenting cells (Fig. 5). Alkylphosphate and alkylamine antigens were unable to induce lasting changes to antigen presenting cells and activation was only observed when these antigens were present throughout the assay. In contrast, the nBP antigen, pamidronate, was able to effect changes in antigen presenting cells so that they remained antigenic even after washing (Fig. 5).
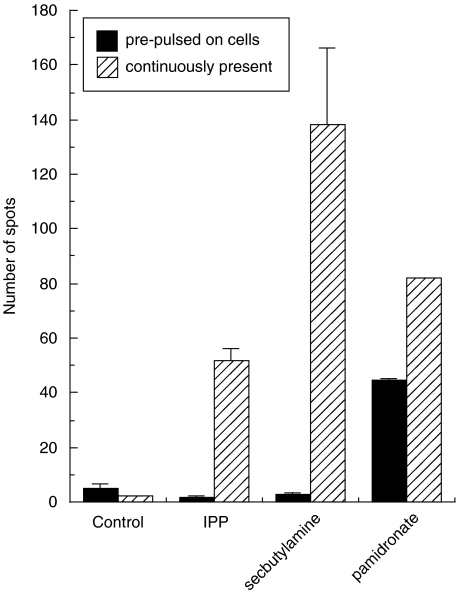
Fig. 5.
Alkylamine and alkylphosphate antigens exhibit different pharmacokinetics than nBP antigens. Mean number of spots induced by IPP and secbutylamine when 1000 cells from the Vγ9Vδ2 T cell clone Bob were incubated with 25 000 BCL line Sparky in the continuous presence (striped bars) of 100 µm IPP, 10 mm secbutylamine and 1 mm pamidronate during a 12 h IFNγ ELISpot. The filled bars are from a parallel assay where the BCL were incubated for 12 h in R10 containing the above concentrations of antigens and then washed once and resuspended in fresh media without antigen prior to the experiment. The ‘control’ targets were not pulsed with antigen. Bars show the standard deviation from the mean of two replicate assays. Only pamidronate could be ‘pulsed’ onto cells. Similar results were obtained with γδ T cell lines from three separate individuals (data not shown).
γδ T cell activation by phosphate and alkylamine antigens requires contact with cells of human origin
Analysis of T cell activation by IFNγ ELISpot has the benefit of utilizing very few cells. Such a low number of cells spread out in a 96-well flat bottomed plate are unlikely to contact each other. Indeed no spots were seen if antigen was added in the absence of presenting cells (Fig. 6). Antigen recognition could be restored by the addition of cell lines of human origin. The appearance of spots when Vγ9Vδ2 cells were plated at a higher density indicated that γδ T cells were able to present antigen to themselves. The mouse myeloma P3/NS1/1-Ag4-1 (ECACC 85011427) and the rat myeloma Y3.AG.1.2.3 (ECACC 85110502) cell lines were unable to ‘present’ phosphate or alkylamine antigens to Vγ9Vδ2 T cells. We tested nine different human cell lines, including those deficient in β2-micoglobulin and MHC class I, and were unable to find a nucleated human cell line that did not present these antigens, or nBP antigens, to Vγ9Vδ2 T cells (data not shown). Activation always required both antigen presenting cells (APC) and antigen. Human erythrocytes were unable to function as APC in our assays (data not shown).
Fig. 6.
Recognition of alkylamine and alkylphosphate antigens by Vγ9Vδ2 T cells requires contact with cells of human origin. Mean number of spots in IFNγ ELISpot assay with and without antigen presenting cells. 1000 γδ T cells/well were plated with 25 000 EBV-transformed human B cells, Spinner Hela cells, rat myeloma Y3.AG.1.2.3 or mouse myeloma P3/NS1/1-Ag4·1. Only human cells were able to act as antigen presenting cells in this assay. The use of 25 000 γδ cells allowed cell-cell contact and confirms that these cells have some ability to present alkylphosphate and alkylamine antigens to each other. Secbutylamine and isobutylamine were added to assays at 10 mm; IPP was added at 100 µm. Assays were performed over 12 h as described in the Materials and Methods. Standard deviation from the mean of four replicates is shown.
DISCUSSION
Recent studies have documented that T cells expressing a Vγ9Vδ2 TCR, usually the dominant subset of γδ T cells in human peripheral blood, can activate in response to alkylphosphate, alkylamine or nBP antigens [31–33,39]. We have extended these observations by showing that all of these antigens can stimulate a population of γδ T cells in direct ex vivo IFNγ ELISpot assay. The numbers of Vγ9Vδ2 T cells measured by FACS analysis of PBMC indicate that only approximately 10% of these cells respond to alkylphosphate, alkylamine and nBP antigens. This may reflect the proportion of cells that recognize these antigens or the proportion that make IFNγ when they respond. However, only approximately 30% of a clonal Vγ9Vδ2 T cell population were able to produce spots in IFNγ ELISpot assay (Fig. 4d). This percentage is roughly the same as that observed for more than 20 different αβ TCR-expressing cytotoxic T lymphocyte (CTL) clones in response to cells presenting their cognate peptide-MHC antigen (unpublished data). We also show that the number of cells that activate in IFNγ ELISpot is dependent on the antigen-presenting cell (Fig. 6). Variation in the ability of different cells to act as presenting cells may reflect differences in the surface expression of ICAM-1 [42] or differences in the surface expression a true γδ T cell ligand presentation molecule. A recent study defined four subsets of human Vδ2 T cells in peripheral blood based on the expression of CD45RA and CD27 [43]. Only the CD45RA–CD27– population of Vδ2-expressing cells were able to make large amounts of IFNγ in response to IPP [43]. CD45RA–CD27– were found to make up 26·5 ± 15·3% of Vδ2 T cells in the peripheral blood of 15 healthy adults [43]. It is possible that we are only able to detect this population of Vδ2 cells in our IFNγ ELISpots. We also find that in more than half the individuals we examined, 25–35% of the cells that produce IFNγ in response to PPD express a γδ TCR (Fig. 1). PPD-induced activation of γδ T cells was confirmed by FACS analysis (Fig. 2c). These data are in accordance with early studies that show an expansion of γδ T cells in the lungs of mice exposed to aerosolized PPD [44] as well as those showing expansion of the human Vγ9Vδ2 T cell population in vivo in M. tuberculosis infection [4] and after BCG vaccination [29]. We have not determined the exact nature of the γδ T cell ligands in PPD and it is possible that the PPD-induced activation of γδ T cells could have been due to low-level contamination of this preparation with mycobacterial alkylphosphate antigens or to the presence of Toll-like receptor ligands.
To examine their activation requirements further, we cloned Vγ9-expressing T cells from peripheral blood. We found that Vγ9Vδ1 T cells are unable to recognize the plethora of nonpeptide antigens recognized by cells expressing Vγ9 in its more common pairing with a Vδ2 chain (Fig. 4c). This result is in agreement with those of a previous study that showed Vγ9Vδ1 T cell clones failed to respond to monoethyl phosphate [31]. A previous study demonstrated that transfection of TCR-negative Jurkat T cells with a Vγ9Vδ2 TCR, but not a Vδ1 TCR (not paired with Vγ9), enabled recognition of alkylphosphate antigen [45]. These elegant experiments further highlight that T cell recognition of such antigens requires the expression of a specific γδ TCR. Minato and colleagues have recently extended these observations by showing that recognition of alkylphosphate, alkylamines and nBP antigens by Vγ9Vδ2 T cells requires that the Vγ9 segment be joined to a JγP joining region [46]. Previous experiments have shown that the proportion of V9JPC1 γ chains is significantly higher in the peripheral blood than in thymocytes [47]. The peripheral expansion of Vγ9 cells using JγP appears to be antigen-driven [48]. The γδ T cells in cord blood mononuclear cells (CBMC) express a diverse repertoire of γδ TCRs [48]. Stimulation of CBMC with alkylphosphate antigen, but not with PHA, induces the preferential expansion of T cells bearing Vγ9Vδ2 TCRs [48]. 70% of TCRs expanded with alkylphosphate antigen use a JγP joining region compared to just 20% of those cells expanded with PHA [48]. The JγP joining region differs from other possible joining regions in that it contains two germline encoded lysine residues (K1 & K2) in addition to a third lysine (K3) that is conserved in all Jγ[36,46]. Mutation of these lysine residues completely abrogated the ability of these TCRs to respond to nonpeptide antigens [46]. Both the Vγ9Vδ1 and Vγ9Vδ2 clones tested here bear Vγ9JP chains (Fig. 3). As only the Vδ2 clone is able to respond to alkylphosphate, alkylamine and nBP antigens (Fig. 4c) it would appear that the Vδ2 chain may also make an essential contribution to the recognition of these ligands. Recent results appear to confirm that specific features of the Vδ2 chain are required for recognition of nonpeptide antigens by Vγ9Vδ2 T cells [48]. It is further noteworthy that the amount of MIP1β manufactured by Vγ9Vδ2 T cells (Fig. 4c) is comparable to that we have measured after antigen-induced activation of CTL [37]. The release of β-chemokines by γδ T cells may act to recruit immune cells to sites of infection [49].
We show that a clonal population of Vγ9Vδ2 T cells can recognize alkylphosphate, alkylamine and nBP ligands (Figs 4 and 5). Bukowski et al. [32] also demonstrated that a single T cell clone could recognize two types of ligand; alkylamines and IPP. The authors suggest that, given the small sizes of these antigens, a single γδ TCR might either possess two different binding sites or a single binding site that can accommodate both types of ligand. Our finding that the recognition of both these antigens and nBP antigens requires cell surface presentation makes it highly unlikely that there are separate binding sites for these classes of ligand. Indeed, it is very difficult to envisage just how single Vγ9Vδ2 TCRs are able to respond to such different chemical species and yet still exhibit tight structural constraints within each class of ligand. The most obvious similarity between the recognized ligands is their alkyl chains. While it is known that phosphate antigens require their phosphate and nBPs and alkylamines require their amino groups for their antigenicity [32,33,39] it is possible that this is necessary for anchoring them to a presenting molecule rather than forming a component of the TCR-docking structure. Indeed, the finding that both antigenic alkylphosphate and alkylamine ligands require similar alkyl chains for recognition lends some credence to such a hypothesis and has lead to attempts at molecular prediction [50]. However, it is noticeable that the most potent nBP antigen to date, risedronate, which can stimulate γδ T cell expansion from human PBMC at concentrations as low as 10 nm[35] was not included in this predictive exercise [50]. We have also shown that risedronate can activate Vγ9Vδ2 T cells in IFNγ ELISpot assay at concentrations of <1 µm (Fig. 4c and data not shown). Risedronate has an aromatic ring and does not fit with the pattern recognition hypothesis championed by Gossman and Oldfield [50].
It is noteworthy that antigenic nBPs, and not their corresponding nonantigenic nonaminobisphosphonates (BPs), are potent inhibitors of the mevalonate pathway (reviewed in [51]). It must be no coincidence that IPP, a potent activator of Vγ9Vδ2 T cells, is part of this metabolic pathway. Indeed, treatment of cells with the nBPs zoledronate and pamidronate has recently been shown to lead to an accumulation of IPP [52]. It thus seems likely that antigenic nBPs exert their effects via IPP and not directly as previously assumed [41,42,50]. The results of De Libero and colleagues [52] elegantly demonstrate this mode of action for nBPs by two different means. First, they show that nBPs, unlike IPP, require internalization in order to exert their effects [52]. Second, they show that blocking the production of IPP inhibits the ability of nBPs to activate γδ T cells [52]. A model in which nBPs function via metabolites in the mevanolate pathway conveniently explains some current anomalies in the activation of γδ T cells by these compounds. Firstly, it indicates how the nBPs risedronate (Fig. 5) and zoledronate [52], the aromatic ring structures of which do not fit with the established pattern recognition motif for γδ antigens [50], might lead to activation. Secondly, it explains why BPs that do not inhibit the mevalonate pathway [51] fail to act as antigens ([52] and our unpublished data). This mechanism of action also explains why the nBP pamidronate has been described as being ‘rather unique’ amongst non peptide antigens in that it could be pulsed onto antigen presenting cells and retain its antigenicity after these cells had subsequently been washed ([41,42] and Fig. 5). However, although unlikely, it still remains possible that nBPs activate Vγ9Vδ2 Tcells directly. Some of the findings of Gober et al.[52] could potentially be due to the toxic effects of statins. Statins and bisphosphonates have inhibitory effects on almost all cells as blocking the mevanolate pathway leads to the loss of Ras and other receptor signals due to the blockade of protein prenylation [53]. nBPs block farnesyl synthase and may act synergistically with inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase to kill cells. These effects may be overcome by further addition of intermediates in the mevalonate pathway. The significance of these issues is apparent in the clinical setting. nBPs have become well established as clinically successful antiresorptive agents for the treatment of osteoporosis (reviewed in [51]) and, more recently, multiple myeloma [54,55]. These drugs can induce a transient fever and biological changes, including specific cytokine production, that are suggestive of an acute phase response (e.g. [56–58]). It is possible that the build up of IPP and other metabolites resulting from the actions of these drugs on the mevalonate pathway and the ensuing activation of γδ T cells in vivo might contribute to these side-effects. Further work aimed at testing this hypothesis is required.
Any attempt to understand the recognition requirements of γδ T cells should also take the absolute requirement for species-specific cell-cell contact into account. The use of IFNγ ELISpot has allowed us to conclusively prove the need for cell-cell contact with a cell of human origin. The results of Morita et al. [40] showing that monoethyl phosphate-induced calcium flux in Vγ9Vδ2 T cells required that the cells be centrifuged and resuspended, and those of Lang et al. [59] showing that Vγ9Vδ2 T cells responded to mycobacterial phosphoantigens poorly when kept in suspension first hinted that cell-cell contact might be important for activation. During the preparation of this manuscript another group have shown that recognition of the nBP pamidronate also requires species-specific cell-cell contact [42]. This study showed that 33 tumour cell lines from 12 different species, including other primates, failed to mediate Vγ9Vδ2 T cell activation in response to pamidronate [42]. Curiously, pamidronate did not activate γδ T cells when ‘presented’ by 5 human tumour cell lines [42]. Recognition of some, but not all, of these cell lines could be restored by transfection with ICAM-1 [42]. However, pamidronate likely functions indirectly via its actions on IPP synthesis rather than as a direct ‘ligand’ as this study assumed and it remains possible that these cell lines have an abnormal mevalonate pathway. It is important that these experiments be repeated with IPP itself. Human cell lines that fail to present Vγ9Vδ2 T cell antigens are likely to be very useful tools in the elucidation of the antigen-presentation mechanism.
That γδT cell activation requires cell-cell contact with a cell of human origin (Fig. 6) opens up a number of possibilities. First, it could be indicative of a human-specific γδ T cell ligand-presentation molecule. The ability of multiple cell lines from different tissues and individuals to act as antigen-presenting cells argues against any kind of large variability in this molecule as seen for the MHC genes. Alkylphosphate and alkylamine antigens are unable to effect stable changes in the antigen presenting cells as their effects are readily removed by dilution and washing [40] (Fig. 5, and our unpublished observation). This argues against major changes in the surface of the presenting cell such as up-regulation of a γδ TCR ligand or heat shock proteins such as MIC-A and MIC-B [60–62]. Second, the requirement for presentation by a human cell may be indicative of a human specific coreceptor element or the requirement for additional costimulatory signals. Distinguishing between these various models awaits the characterization of the true ligand for peripheral blood Vγ9Vδ2 T cells. The recent production of a high purity, soluble Vγ9Vδ2 TCR [63] should aid in this process. Only when we understand exactly what the γδ TCR recognizes will we be able to unlock the forgotten third arm of adaptive immunity that γδ T cells control.
Acknowledgments
We thank Anita Milicic for careful reading of the manuscript and Paul Klenerman for kindly donating blood for these experiments on several occasions. We are indebted to Ian Matthews for help in locating commercial sources of antigen and discussions on antigen patterns and Mike Simpkins for providing cells to test in antigen presentation assays. This work was funded by the Wellcome Trust. AKS is a Wellcome Trust Senior Fellow. AEG is a Wellcome Trust Prize Student. AL and DJ were Nuffield Department of Medicine vacation scholars. BT performed part of this work as her undergraduate research project. DAP is a Medical Research Council Clinician Scientist. REH is funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland.
References
- 1.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Sciammas R, Tatsumi Y, Sperling AI, Arunan K, Bluestone JA. TCR gamma delta cells: mysterious cells of the immune system. Immunol Res. 1994;13:268–79. doi: 10.1007/BF02935618. [DOI] [PubMed] [Google Scholar]
- 3.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 4.Balbi B, Valle MT, Oddera S, Giunti D, Manca F, Rossi GA, Allegra L. T-lymphocytes with gamma delta+ V delta 2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–90. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 5.Uyemura K, Deans RJ, Band H, Ohmen J, Panchamoorthy G, Morita CT, Rea TH, Modlin RL. Evidence for clonal selection of gamma/delta T cells in response to a human pathogen. J Exp Med. 1991;174:683–92. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara T, Mizuno Y, Takaki K, et al. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–10. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertotto A, Gerli R, Spinozzi F, et al. Lymphocytes bearing the gamma delta T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–80. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 8.Sumida T, Maeda T, Takahashi H, Yoshida S, Yonaha F, Sakamoto A, Tomioka H, Koike T. Predominant expansion of V gamma 9/V delta 2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–8. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertotto A, Spinozzi F, Gerli R, Bassotti G, Forenza N, Vagliasindi C, Vaccaro R. Peripheral blood gamma delta T cells in human listeriosis. Acta Paediatr. 1995;84:1434–5. doi: 10.1111/j.1651-2227.1995.tb13584.x. [DOI] [PubMed] [Google Scholar]
- 10.Jouen-Beades F, Paris E, et al. In vivo and in vitro activation and expansion of gammadelta T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–72. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell CW, Everett ED, McDonald G, Yesus YW, Roland WE, Huang HM. Apoptosis of gamma/delta T cells in human ehrlichiosis. Am J Clin Pathol. 1996;105:640–6. doi: 10.1093/ajcp/105.5.640. [DOI] [PubMed] [Google Scholar]
- 12.De Paoli PD, Gennari D, Martelli P, Basaglia G, Crovatto M, Battistin S, Santini G. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clin Exp Immunol. 1991;83:187–91. doi: 10.1111/j.1365-2249.1991.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L. Selective increase of a subset of T cell receptor gamma delta T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:917–9. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 14.Boullier S, Cochet M, Poccia F, Gougeon ML. CDR3-independent gamma delta V delta 1+ T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418–31. [PubMed] [Google Scholar]
- 15.De Paoli P, Gennari D, Martelli P, Cavarzerani V, Comoretto R, Santini G. Gamma delta T cell receptor-bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis. 1990;161:1013–6. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- 16.Dechanet J, Merville P, Lim A, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merville P, Dechanet J, Berge F, et al. Cytomegalovirus infection in kidney allograft recipients is followed by a prolonged expansion of gammadelta T lymphocytes. Transplant Proc. 2000;32:357–9. doi: 10.1016/s0041-1345(99)00978-1. [DOI] [PubMed] [Google Scholar]
- 18.Jones-Carson J, Vazquez-Torres A, van der Heyde HC, Warner T, Wagner RD, Balish E. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–7. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 19.Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–41. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 20.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–5. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raziuddin S, Telmasani AW, el-Hag el-Awad M, al-Amari O, al-Janadi M. Gamma delta T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992;22:1143–8. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 22.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 23.Hiromatsu KY, Yoshikai G, Matsuzaki S, Ohga K, Muramori K, Matsumoto JA, Bluestone K. Nomoto. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King DP, Hyde DM, Jackson KA, et al. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–6. [PubMed] [Google Scholar]
- 25.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guerin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995;25:838–46. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 26.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877–81. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 27.Ladel CH, Blum C, Kaufmann SH. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect Immun. 1996;64:1744–9. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–54. [PubMed] [Google Scholar]
- 30.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2001;108:1349–57. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 33.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 34.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human gamma delta T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 35.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–8. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 36.Lefranc M-P, Lefranc G. The T Cell Receptor Factsbook. London: Academic Press; 2001. [Google Scholar]
- 37.Price DA, Sewell AK, Dong T, Tan R, Goulder PJ, Rowland-Jones SL, Phillips RE. Antigen-specific release of beta-chemokines by anti-HIV-1 cytotoxic T lymphocytes. Curr Biol. 1998;8:355–8. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson SL, Wooldridge L, Tafuro S, et al. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–93. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 40.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167:5092–8. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species–specific interactions for the activation of human gamma delta T cells by pamidronate. J Immunol. 2003;170:3608–13. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 43.Dieli F, Poccia F, Lipp M, Caccamo N, Sano Di, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Augustin A, Kubo RT, Sim GK. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989;340:239–41. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 45.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 46.Miyagawa F, Tanaka Y, Yamashita S, Mikami B, Danno K, Uehara M, Minato N. Essential contribution of germline-encoded lysine residues in Jgamma1.2 segment to the recognition of nonpeptide antigens by human gammadelta T cells. J Immunol. 2001;167:6773–9. doi: 10.4049/jimmunol.167.12.6773. [DOI] [PubMed] [Google Scholar]
- 47.Davodeau F, Peyrat MA, Hallet MM, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J Immunol. 1993;151:1214–23. [PubMed] [Google Scholar]
- 48.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human gammadelta T cells. Int Immunol. 2003;15:1301–7. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 49.Price DA, Klenerman P, Booth BL, Phillips RE, Sewell AK. Cytotoxic T lymphocytes, chemokines and antiviral immunity. Immunol Today. 1999;20:212–6. doi: 10.1016/s0167-5699(99)01447-4. [DOI] [PubMed] [Google Scholar]
- 50.Gossman W, Oldfield E. Quantitative structure – activity relations for gammadelta T cell activation by phosphoantigens. J Med Chem. 2002;45:4868–74. doi: 10.1021/jm020224n. [DOI] [PubMed] [Google Scholar]
- 51.Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9(Suppl. 2):S66–80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- 52.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 54.Kanis JA, McCloskey EV. Bisphosphonates in multiple myeloma. Cancer. 2000;88:3022–32. doi: 10.1002/1097-0142(20000615)88:12+<3022::aid-cncr19>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 55.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–7. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 56.Sauty A, Pecherstorfer M, Zimmer-Roth I, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–9. doi: 10.1016/8756-3282(95)00448-3. [DOI] [PubMed] [Google Scholar]
- 57.Pietschmann P, Stohlawetz P, Brosch S, Steiner G, Smolen JS, Peterlik M. The effect of alendronate on cytokine production, adhesion molecule expression, and transendothelial migration of human peripheral blood mononuclear cells. Calcif Tissue Int. 1998;63:325–30. doi: 10.1007/s002239900535. [DOI] [PubMed] [Google Scholar]
- 58.Thiebaud DA, Sauty P, Burckhardt P, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–92. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- 59.Lang F, Peyrat MA, Constant P, et al. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–94. [PubMed] [Google Scholar]
- 60.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 61.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–40. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 63.Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820–4. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]