Abstract
Toll-like receptors (TLRs) expressed by mucosal epithelium play an essential role in the defense against microbes by recognizing conserved bacterial molecules. For the first time TLR4, TLR5 and TLR9 have been microanatomically localized in patients with noninflamed gastric mucosa and Helicobacter pylori gastritis by immunohistochemistry. Because polarized expression of TLRs in apical and basolateral epithelial compartments is thought to modulate mucosal immunity, subcellular TLR distribution by gastric epithelium was investigated using confocal microscopy. TLR4, TLR5 and TLR9 were expressed by gastric epithelium in antrum and corpus of all patients with H. pylori gastritis (n = 14) and with noninflamed gastric mucosa (n = 5). TLR4 was expressed at the apical and the basolateral pole of the gastric epithelium as well in noninflamed gastric mucosa as in H. pylori gastritis. TLR5 and TLR9 expression in the noninflamed gastric mucosa was identical to that of TLR4 with localization at the apical and the basolateral epithelial pole. However, in H. pylori gastritis TLR5 and TLR9 expression on the gastric epithelium changed to an exclusive basolateral localization without detectable expression at the apical pole. In the human stomach, the gastric epithelium expressed TLR4, TLR5 and TLR9, which gives it the possibility to interact with H. pylori. Furthermore, gastric epithelial TLR4 expression is highly polarized in an apical and a basolateral compartment, whereas TLR5 and TLR9 polarization seems to be a process dynamically influenced by H. pylori infection. This polarized and dynamically regulated gastric epithelial expression of TLRs supports a sentinel role for these receptors in the mucosal immunity to H. pylori.
Keywords: toll-like receptor, TLR, Helicobacter pylori, gastric mucosa, Helicobacter pylori gastritis
INTRODUCTION
Infection of the gastric mucosa by Helicobacter pylori is worldwide one of the most common human bacterial infections. H. pylori causes chronic active gastritis and gastroduodenal ulceration [1–4]. During H. pylori infection the bacterium colonizes the mucus layer overlying the gastric surface epithelium and causes inflammation of the underlying mucosa, which is called chronic active gastritis.
Toll-like receptors (TLRs) are recently found to play an essential role in the first line of host defense by recognition of microbial components. These receptors recognize conserved molecular patterns that are expressed by infectious agents. By this way TLRs mediate the production of proinflammatory cytokines and chemokines resulting in inflammation. Recently, the ligands for most receptors of the TLR family have been identified. TLR2 recognizes and signals bacterial lipoprotein and peptidoglycans from Gram-positive bacteria. TLR3 is considered to recognize double-stranded RNA. Lipopolysaccharides (LPS) from Gram-negative bacteria signal through TLR4. TLR5 is identified to recognize bacterial flagellin and represents therefore a pattern recognition receptor for motile pathogens. Cellular response to the CpG motive of bacterial DNA is mediated by TLR9 [5–9].
First in vitro data suggest that TLRs expressed on gastric epithelial cells interact with H. pylori. It has been demonstrated that TLR5 expressed on human gastric epithelial cell lines is required for H. pylori induced NF-kappaB activation and chemokine expression [10]. TLR4 expression by gastric epithelium is discussed controversely and the precise function of TLR4 is unclear [11,12]. TLR9 has not been investigated in the stomach so far.
In the gut epithelium polarized expression of TLRs in an apical and a basolateral epithelial compartment is thought to be a central principle of mucosal immunity, which discriminates between pathogenic microbes invading the epithelium and nonpathogenic microbes facing the luminal surface [13–15].
TLR expression has not previously been analysed in vivo in the human stomach. Therefore, in this study TLR4, TLR5 and TLR9 were microanatomically localized in patients with noninflammed gastric mucosa and in patients with H. pylori infection. Furthermore, to elucidate a possible role of epithelial polarization in the human stomach during H. pylori infection the subcellular TLR distribution in gastric epithelial cells was investigated in detail by confocal microscopy.
MATERIALS AND METHODS
Patients and selection of gastric tissue
Surgical tissue specimens from gastric antrum and corpus mucosa of 5 patients (4 with tumour of the pancreas, 1 with oesophageal carcinoma) without H. pylori gastritis and of 14 patients with chronic active H. pylori gastritis were investigated in this study by immunohistochemistry and confocal microscopy. In the patients without H. pylori gastritis no inflammatory cells were detectable in the antrum and in the corpus mucosa and H. pylori colonization was excluded in both compartments by a modified Giemsa stain. Chronic active H. pylori gastritis showed a moderate to severe chronic inflammatory infiltrate of T- and B-lymphocytes, plasma cells and monocytes and a mild to severe activity with neutrophils in the lamina propria, within the gastric epithelium and in the foveolar lumen. H. pylori colonization was mild to severe as determined by a modified Giemsa-stain.
This study, in which the human tissue was collected from gastrectomy specimens used for routine histopathological diagnosis, has been permitted by a local ethical commission.
Immunhistochemistry
For immunohistological analyses tissue specimens were fixed in 10% formalin buffered at pH 7·0 for 24 h and paraffin-embedded. The following antibodies were used at the dilutions indicated: polyclonal rabbit anti-TLR4 (1 : 500, Zymed, San Francisco, USA), polyclonal rabbit anti-TLR5 (1 : 1000, Zymed), monoclonal mouse anti-TLR9 (1 : 200, Biocarta, Hamburg, Germany). For immunohistochemical staining tissue sections were treated with microwave. Non-specific binding sites were blocked with buffered casein solution (Power Block Universal Blocking Reagent, Bio Genex, San Ramon, USA) for 10min at RT. Sections were incubated with the first-step antibody at 4°C overnight. As second stage reagent a Biotin/Streptavidin-peroxidase detection system (Super Sensitive Multilink HRP Detection System, Bio Genex) was used according to the instruction of the manufacture with 3,3′-diaminobenzidine tetra-hydrochloride (DAB) solution as substrate. To exclude a nonspecific staining in each case a negative control was performed. For this purpose polyclonal TLR4 and TLR5 antibodies were blocked with the peptide used for antibody production (Zymed) at 37°C for 30 min. For TLR9 the first monoclonal antibody was replaced by an isotype control antibody. In both procedures no staining of the gastric tissue was detectable.
Immunofluorescence and confocal microscopy
For confocal microscopy the formalin fixed and paraffin-embedded sections were stained with the TLR4, TLR5 and TLR9 antibodies as described above, followed by incubation with Cy3 conjugated donkey-antimouse IgG antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Nuclei and H. pylori were stained with DAPI (Sigma, Hannover, Germany) and pseudocoloured in blue and green, respectively. Stained tissue sections were observed in a confocal microscope (TCS SP2, Leica, Heidelberg, Germany). To separate DAPI signal from nuclei and bacteria region of interest (ROI) scanning mode was used.
As negative control polyclonal TLR4 and TLR5 antibodies were blocked with the peptide used for antibody production (Zymed, San Francisco, USA) at 37°C for 30 min. For TLR9 the first monoclonal antibody was replaced by an isotype control antibody. In both procedures no staining of the gastric tissue was detectable.
RESULTS
Toll-like receptor expression in noninflamed gastric mucosa and chronic active H. pylori gastritis
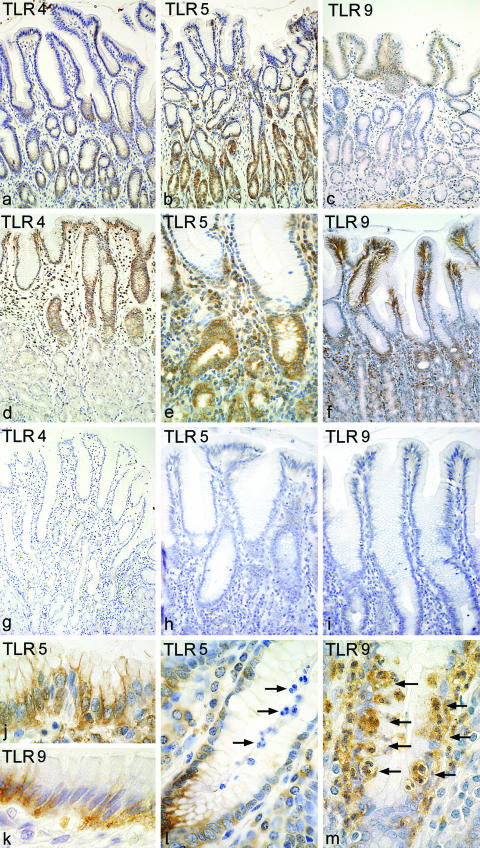
TLR4, TLR5 and TLR9 were expressed by gastric epithelium in antrum and corpus of all patients as well in noninflamed gastric mucosa (n = 5; Fig. 1a–c) as in chronic active H. pylori gastritis (n = 14; Figs 1d–f,j,k). TLR4 and TLR5 expression was accentuated in the glandular neck region and extended to the luminal surface (Figs 1a–b,d,e). In contrast to TLR4 and TLR5, TLR9 expression was predominantly localized at the gastric surface epithelium with nearly no detectable expression in the glandular neck region (Figs 1c,f).
Fig. 1.
Toll-like receptor expression in noninflamed gastric mucosa and chronic active H. pylori gastritis determined by immunohistochemistry Gastric epithelial cells as well in noninflamed gastric mucosa (a–c) as in chronic active H. pylori gastritis (d–f,j,k) express TLR4, TLR5 and TLR9. Epithelial TLR4 and TLR5 expression is accentuated in the glandular neck region (a,b,d,e). TLR9 expression was predominantly localized at the gastric surface epithelium (c,f). TLR4 and TLR9 expression by gastric epithelium tends to be stronger in H. pylori gastritis (d,f) than in the noninflamed gastric mucosa (a,c). TLR5 shows a strong expression by gastric epithelium as well in the noninflamed mucosa (b) as in chronic active H. pylori gastritis (e). Fig j and k show TLR5 and TLR9 expression by gastric epithelium in H. pylori gastritis in more detail. Negative controls for TLR4, TLR5 and TLR9 show no staining (g–i) TLR4 and TLR5 stain mononuclear cells in the lamina propria of H. pylori gastritis (d,e). Many neutrophils in chronic active H. pylori gastritis express TLR4 and some neutrophils express TLR9 (arrows, m). TLR5, however, is not found on neutrophils (arrows; l). Magnification: a–d, f × 20; e × 40; g–k × 100.
TLR4 and TLR9 expression by gastric epithelium tended to be stronger in H. pylori gastritis (Figs 1d,f) than in the noninflamed gastric mucosa (Figs 1a,c). TLR5, however, showed a strong expression by gastric epithelium as well in the noninflamed mucosa as in chronic active H. pylori gastritis (Figs 1b,e).
In chronic active H. pylori gastritis some neutrophils localized in the lamina propria, within the gastric epithelium and in the foveolar lumen expressed TLR4 and TLR9 (Fig. 1m). TLR5, however, was not detected on neutrophils (Fig. 1l). TLR4 and TLR5 were additionally expressed by mononuclear cells representing plasmacells and macrophages (Figs 1d,e). TLR9 expression by mononuclear cells was not detectable.
Subcellular distribution of Toll-like receptors by gastric epithelium in noninflamed gastric mucosa and chronic active H. pylori gastritis.
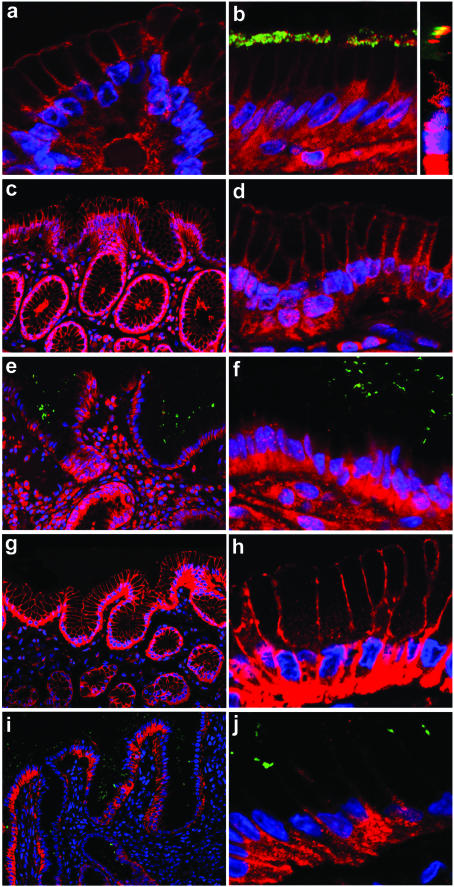
Confocal microscopy confirmed TLR4, TLR5 and TLR9 expression by gastric epithelium as well in noninflamed gastric mucosa (Figs 2a,c,d,g,h) as in chronic active H. pylori gastritis (Figs 2b,e,f,i,j) in all patients.
Fig. 2.
Subcellular distribution of Toll-like receptors in gastric epithelium in noninflamed gastric mucosa and chronic active H. pylori gastritis determined by confocal microscopy Fluorescence microscopy shows TLR4 expression by gastric epithelium at the apical and at the basolateral epithelial pole. This expression pattern was identical in the (a) noninflamed gastric mucosa and (b) in H. pylori gastritis. In H. pylori infection the bacterium was directly attached to TLR4 expressed at the apical epithelial pole (b), which was confirmed by vertical confocal scanning sections (b inset). TLR5 (c, overview; d, detail) and TLR9 (g overview; h, detail) expression in the noninflamed gastric mucosa is identical to TLR4 with expression at the apical and at the basolateral pole of the gastric epithelium. However, in chronic active H. pylori gastritis gastric epithelium with directly overlying H. pylori expressed TLR5 (e, overview; f, detail) and TLR9 (i, overview; j, detail) exclusively at the basolateral pole of the gastric epithelial cells. No TLR5 and TLR9 expression at the apical pole was detectable, so that the apical cell borders are no more visible. TLR expression shows a red fluorescence. Nuclei and H. pylori are stained with DAPI and are pseudocoulored in blue and green, respectively.
TLR4 was expressed at the apical and the basolateral pole of the gastric surface epithelium. This expression pattern was identical in the noninflamed gastric mucosa and in H. pylori gastritis (Figs 2a,b). In H. pylori gastritis the bacterium was directly attached to the TLR4 receptor expressed at the apical epithelial pole (Fig. 2b), which was confirmed by vertical confocal scanning sections (Fig. 2b inset). This suggests that TLR4 may not only in vitro, but also in vivo function as a cellular binding receptor for H. pylori[12].
On subcellular level TLR5 (Figs 2c,d) and TLR9 (Figs 2g,h) were expressed in the noninflamed gastric mucosa of all patients as well at the apical pole as at the basolateral pole of the gastric epithelium.
In contrast, in chronic active H. pylori gastritis TLR5 (Figs 2e,f) and TLR9 (Figs 2i,j) expression of the gastric epithelium was exclusively localized at the basolateral pole of the epithelial cells without detectable expression at the apical pole.
Our data demonstrate that TLR expression by gastric epithelium is highly polarized. Moreover, TLR5 and TLR9 polarization was found to differ between noninflamed gastric mucosa and H. pylori gastritis and seems therefore to be a dynamical process during H. pylori infection.
DISCUSSION
In H. pylori infection the bacterium colonizes the mucus layer overlying the gastric surface epithelium and causes inflammation and immune response of the underlying mucosa, which is called chronic active gastritis. There is evidence that gastric epithelial cells themselves play an active role in the innate immunity against H. pylori particulary by secreting chemokines. But so far the receptors, which trigger this epithelial driven inflammatory response, are not clearly defined and localized in the human stomach. Mucosal epithelium has been reported to express several TLRs, suggesting that these receptors play a role in gastrointestinal epithelial innate immune response.
In our study for the first time TLR4, TLR5 and TLR9 were localized in vivo in the human stomach. TLR4, TLR5 and TLR9 were expressed by gastric epithelial cells not only in chronic active H. pylori gastritis, but also constitutively in noninflamed gastric mucosa. TLR4 expression by gastric epithelium is discussed controversely, because TLR4 is expressed on gastric epithelial cell lines, but primary gastric epithelial cells were not found to express TLR4 [11,12]. We have identified for the first time TLR4 expression in vivo in the human stomach and can demonstrate a clear TLR4 protein expression of gastric epithelial cells as well in noninflamed gastric mucosa as in chronic active gastritis. The precise function of TLR4 on gastric epithelium, however, remains unclear, as TLR4 seems not to be involved in NF-kappaB activation of the gastric epithelium [11,12]. In a very recent study in the gastric mucosa a nonconventional role of TLR4 beyond innate immunity is discussed [12] in analogy to the intestinal epithelium [16].
TLR5 expressed on gastric epithelial cell lines was found to recognize the H. pylori flagellin, which results in NK-kappaB activation with subsequent chemokine expression by epithelial cells [10]. In respect to these in vitro data, TLR5 expression by gastric epithelium in vivo implies that TLR5 is involved in the epithelial driven inflammatory response to H. pylori. Indeed, in the human stomach H. pylori induces gastric epithelial cells to produce cytokines and chemokines, predominantly IL-8 and Groa, which coordinate neutrophil trafficking from the mucosal vessel into the gastric epithelium to the site of infection [17].
TLR9 expression is not investigated in the human stomach so far. For the first time we could demonstrate TLR9 expression by gastric epithelium. This is in line with very recent in vitro data demonstrating that colonic epithelial cell lines express TLR9 mRNA. Because these cell lines respond to E.coli DNA via TLR9 by increased IL-8 production [18], further studies have to investigate whether H. pylori DNA induces a similar response of the gastric epithelial with IL-8 production and following neutrophil recruitment.
A crucial question in mucosal immunity is how to discriminate between pathogenic and nonpathogenic microbes. Now there is first evidence that compartimentalization of TLRs may play an important role in this process. In the gut the polarized expression of TLRs in an apical and and a basolateral epithelial compartment is thought to be a central principle for the discrimination between pathogenic bacteria invading the epithelium and nonpathogenic microbes facing the luminal surface [13,14,19]. In the stomach epithelium we could verify this principle of a highly polarized TLR expression for the investigated TLRs.
TLR4 was expressed at the apical and the basolateral membrane compartment of the gastric epithelium. The pattern of TLR4 compartimentalization did not change in vivo in the human stomach during H. pylori infection. Having in mind that TLR4 is a binding receptor for H. pylori in vitro[12] and that H. pylori was directly attached to apical expressed TLR4 in patients with gastritis, it seems probably that TLR4 may also in vivo function as a bacterial binding receptor.
In the noninflamed gastric mucosa TLR5 and TLR9 showed an identical subcellular expression by gastric epithelium as found for TLR4 with TLR expression at the apical and the basolateral epithelial pole. In this study for the first time we could demonstrate that TLR9 was not only expressed in the cytoplasma, but also on the cell surface. To exclude an unspecific cell surface staining, we tested the human B cell line TK6 with the same TLR9 antibody. Here we found an exclusive intracellular localization of TLR9, which is in line with literature (data not shown).
However, in patients with chronic active H. pylori gastritis TLR5 and TLR9 expression by gastric epithelium changed to an exclusive basolateral localization.
Because in the intestinum basolaterally expressed TLR5 will only activate proinflammatory gene expression by microbes that have invaded or translocated their flagellin across the epithelium [13,14], further studies have to investigate whether translocation of H. pylori ligands to the basolateral pole of the gastric epithelium is necessary for mediating inflammation of the underlying gastric mucosa.
For the first time we were able to demonstrate in vivo for TLR5 and TLR9 that subcellular TLR distribution in an apical and a basolateral epithelial compartment is not a static, but a dynamic process, which seems to be regulated by H. pylori infection. A similar process of dynamic regulation of TLR polarization is described in vitro for intestinal epithelium, which shows a TLR4-trafficking from apical membrane to cytoplasmic compartments in response to the TLR4 ligand LPS [20]. In analogy the dynamic regulation of TLR5 and TLR9 receptor polarization may be influenced by H. pylori flagellin or DNA, the specific ligands for TLR5 and TLR9.
In vivo TLR expression by gastric epithelium confirm in vitro data that TLRs are involved in the innate immune response to H. pylori. Moreover, the principle of epithelial TLR compartimentalization seems to be not only valid in the gut, but also in the human stomach. In conclusion the polarized and dynamically regulated gastric epithelial expression of TLRs supports a sentinel role for these receptors in gastric mucosal immunity.
Acknowledgments
This study was supported by the ‘Deutsche Forschungsgemeinschaft’ grant EC 203/1 and EC 203/2. We thank E. Bachmann and E. Schmitt for excellent technical assistance and E. Geissinger for reading the manuscript.
References
- 1.Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;4:1273–5. [PubMed] [Google Scholar]
- 2.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;16:1311–4. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 3.Rauws EAJ, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GNJ. Campylobacter pyloridis-associated chronic active antral gastritis. Gastroenterology. 1988;94:33–40. [PubMed] [Google Scholar]
- 4.Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991;6:125–30. doi: 10.1111/j.1440-1746.1991.tb01451.x. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 6.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–10. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 8.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 9.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;17:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 10.Smith MF, Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–60. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 11.Backhed F, Rokbi B, Torstensson E, et al. Gastric mucosal recognition of Helicobacter pylori is independend of Toll-like receptor 4. J Infect Dis. 2003;187:829–36. doi: 10.1086/367896. [DOI] [PubMed] [Google Scholar]
- 12.Su B, Ceponis PJ, Lebel S, Huynh H, Shermann PM. Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun. 2003;71:3496–502. doi: 10.1128/IAI.71.6.3496-3502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 14.Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoe by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. Immunol. 2002;168:2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz AT. Intestinal epithelial toll-like receptors: to protect. & serve? Curr Pharm Des. 2003;9:1–5. doi: 10.2174/1381612033392422. [DOI] [PubMed] [Google Scholar]
- 17.Eck M, Schmaußer B, Scheller K, Toksoy A, Kraus M, Menzel T, Muller-Hermelink HK, Gillitzer R. CXC chemokines Gro (alpha)/ IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. 2000;122:192–9. doi: 10.1046/j.1365-2249.2000.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependend, NF-kappaB-independend pathway. FASEB J. 2003;17:1319–21. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 19.Xavier RJ, Podolsky DK. Microbiology. How to get along-friendly microbes in a hostile world. Science. 2000;289:1483–4. doi: 10.1126/science.289.5484.1483. [DOI] [PubMed] [Google Scholar]
- 20.Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol. 2002;160:165–73. doi: 10.1016/S0002-9440(10)64360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]