Abstract
In acute rejection of transplanted organs intragraft fibroblasts increase their production of hyaluronan. Hyaluronan has strong water binding capacity and an increased tissue content of hyaluronan thus contributes to the development of interstitial oedema. The present study examined the effects of commonly used immunosuppressants (prednisolone, cyclosporin, tacrolimus, mycophenolic acid and sirolimus) on fibroblast proliferation, hyaluronan production and cell surface receptor expression. Fibroblasts isolated from rejecting tissue and from normal, non-transplanted tissue were studied in parallel. All substances investigated, except tacrolimus, were found to affect fibroblasts in one way or another. The most striking effect was the almost total inhibition of fibroblast proliferation in the presence of mycophenolic acid. Cyclosporin reduced the proliferation by about 50% and prednisolone had an inhibiting effect on hyaluronan production (50% reduction). These effects were observed on fibroblasts isolated from rat cardiac allografts undergoing rejection as well as on fibroblasts obtained from normal heart tissue. In contrast, sirolimus was found to stimulate the proliferation of fibroblasts from rejecting tissue (100% increase), but not that of normal fibroblasts. The majority of the fibroblasts expressed the hyaluronan receptor CD44, with a more intense expression in cultures of fibroblasts derived at rejection. None of the immunosuppressants affected the staining pattern (number of positive cells or intensity). The inhibitory effects of prednisolone, cyclosporin and mycophenolic acid on fibroblasts may contribute to the overall beneficial effects of these drugs when used for prevention or treatment of rejection.
Keywords: fibroblasts, hyaluronan, immunosuppression, proliferation
INTRODUCTION
In acute rejection of transplanted organs intragraft fibroblasts increase their production of the glycosaminoglycan hyaluronan [1,2]. Hyaluronan has strong water binding capacity [3], and an increased tissue content of hyaluronan thus contributes to the development of interstitial oedema of the grafted organ [1,4]. A severe oedema may directly affect organ function, e.g. in heart transplantation, or lead to increased interstitial pressure with subsequently disturbed microcirculation and local ischaemia as a consequence. Apart from these effects, which can be attributed to the physicochemical properties of the substance, hyaluronan can also affect immunological processes. In general, these effects are mediated through hyaluronan binding to the cell surface receptor CD44 which is expressed, for example, by macrophages and activated T lymphocytes. Hence, there is a co-localization of hyaluronan and inflammatory cells in rejecting allografts [1]. In addition, hyaluronan with a lower molecular weight than the normal approximately 106 kDa has immunostimulatory effects on macrophages [5] and may up-regulate the expression of certain adhesion molecules [6]. Such hyaluronan of a lower molecular weight is found under inflammatory conditions [7].
Fibroblast stimulating substances, e.g. platelet derived growth factor (PDGF), are released during acute rejection. Moreover, several cytokines directly involved in the immune activation within the rejection process, i.e. tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-2 (IL-2), have effects on fibroblast proliferation and/or hyaluronan production [2].
Because immunosuppressive drugs are used both for the prevention and treatment of acute rejection it was of interest to study the effects of these drugs on fibroblasts. In the present study five commonly used immunosuppressive substances (prednisolone, cyclosporin, tacrolimus, mycophenolic acid and sirolimus) were evaluated in terms of fibroblast proliferation, hyaluronan production and cell surface receptor expression. Fibroblasts isolated from rejecting tissue were studied in parallel with fibroblasts isolated from normal, non-rejecting tissue.
MATERIALS AND METHODS
Heart transplantation
Heterotopic heart transplantations were performed using male DA (RT1a) rats as donors and male Lewis (RT1l) rats as recipients (allogeneic transplantation) or male Wistar/Kyoto (RT1lv) rats as donors and recipients (syngeneic transplantation). The animals were obtained from M&B (Skensved, Denmark) and were allowed to settle for at least 1 week before operation, given free access to food and water. Anaesthesia was induced by intraperitoneal injection with a mixture of chloral hydrate (180 mg/kg body weight), pentobarbital (40 mg/kg body weight) and magnesium sulphate (90 mg/kg body weight). The regional ethical committee approved the experiments and all animals received humane care in compliance with The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85–23, revised 1985).
The donor heart was transplanted to the neck vessels of the recipient using a cuff technique. In brief, the right carotid artery and the right jugular vein were dissected free, cross-clamped caudally and cut cranially. Short plastic tubes were placed around the vessels and the vessels were then turned inside out over the tubes and fixed with ligatures. The donor heart was anastomosed by pulling the vessels of the graft over the tubes and fastening them with ligatures. When the transplantation was finished a single dose of cefuroxim (Zinacef®, Glaxo, Greenford, UK), 20 mg/rat, was administered intramuscularly.
Isolation of fibroblasts
Fibroblasts were isolated from normal and transplanted tissues by explant culture.
The grafts were harvested 5 days after transplantation; at that time-point the rejection process is well developed with cellular infiltration, interstitial oedema and hyaluronan accumulation [1]. The animals were anaesthetized and the transplants perfused in situ with Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical, St Louis, MO, USA) containing 50 µg/ml gentamicin (GM; Sigma Chemical) through the aorta. Subsequently, the hearts were removed aseptically and placed in DMEM + gentamycin (GM).
The transplants were cut into approximately 50 sections which were placed on Petri dishes, covered with DMEM + GM supplemented with 20% cosmic calf serum (CCS; HyClone, Logan, UT, USA) and incubated at 37°C in 5% CO2. Medium was changed three times weekly. After 10 days the cells were detached by trypsinization (0·05% trypsin + 0·01% ethylenediaminetetra-acetic acid; both from Sigma Chemical) and the outgrowing fibroblasts transferred into 75 cm2 culture flasks and maintained in DMEM + GM + 15% CCS. One week later the cells were trypsinized, centrifuged and resuspended in DMEM + GM + 10% CCS. Dimethyl sulphoxide (J. T. Baker, Deventer, Holland) was added to a final concentration of 10% and the cells were than frozen (1 × 106 cells/vial) and kept at −150° until use.
In parallel, hearts from previously non-operated DA-rats were removed and the biopsies further treated as above.
Experimental design
All experiments were performed on cells in the third passage. The cells were thawed, pooled, placed in 75 cm2 culture flasks and grown in DMEM + GM + 15% CCS for 10 days. After trypsinization, the cells were counted and plated in 12-well plates at two different densities; 15 000 cells/well (‘subconfluent’) and 150 000 cells/well (‘confluent’). The next day, the cells were rinsed with phosphate-buffered saline (PBS; SVA, Uppsala, Sweden) and subjected to serum-starvation by the addition of DMEM + GM containing only 0·5% CCS. Three days later the low-serum containing medium was replaced with 1 ml DMEM + GM + 5%CCS. Fifty µl of the various immunosuppressive drugs at various concentrations (or 50 µl medium) were then added to each well.
Experiments for the analyses of proliferation and hyaluronan production were set up separately.
Immunosuppressive drugs
Prednisolone (Sigma Chemical) was dissolved in ethanol to a concentration of 10 mg/ml, aliquoted and stored at −20°C until use. The stock solution was further diluted in DMEM + GM and the drug was used at concentrations of 0·1, 1, 5, 10, 50, 100 and 1000 ng/ml.
Cyclosporin (Sandimmun®; Novartis, Basel, Switzerland) was diluted in DMEM + GM and used at concentrations of 5, 10, 100, 500, 1000 and 10 000 ng/ml.
Tacrolimus (FK-506; Fujisawa Pharmaceutical Co., Osaka, Japan) in powder form was dissolved in Tween 80 (Merck-Schuchardt, Hohenbrunn bei München, Germany) and methanol (one part Tween 80 and two parts methanol) to a final concentration of 13·3 mg/ml. Further dilutions were performed in DMEM + GM and the drug was studied at concentrations of 0·05, 0·1, 1, 5, 10, 50 and 100 ng/ml.
Mycophenolic acid (Sigma Chemical) was dissolved in methanol to a concentration of 10 mg/ml, diluted further in DMEM + GM and used at concentrations of 1, 10, 50, 100, 500, 1000 and 5000 ng/ml.
Sirolimus (rapamycin; Sigma Chemical) was dissolved in dimethyl sulphoxide (J. T. Baker) to a concentration of 1 mg/ml, aliquoted and stored at −20°C until use. The stock solution was further diluted in DMEM + GM and the drug was used at concentrations of 0·05, 0·1, 0·5, 1, 10, 100 and 500 ng/ml.
Proliferation assay
Fibroblast proliferation was determined by [3H]-thymidine incorporation. One day after the addition of immunosuppressants 100 µl methyl-[3H]-thymidine (Amersham Pharmacia Biotech, Uppsala Sweden), diluted in DMEM + GM, were added to each well at a concentration of 10 µCi/ml (i.e. 1 µCi/well). Controls consisted of wells with cells but without methyl-[3H]-thymidine, and wells with methyl-[3H]-thymidine but without cells. After overnight incubation the cells were trypsinized and transferred to 96-well filter plates (MultiScreen®; Millipore, Molsheim, France) and washed first with PBS and subsequently with 95% ethanol. After drying at 37°C, 25 µl scintillation cocktail (OptiPhase SuperMix; Fisher Chemicals, Loughborough Leics, UK) were added to the wells and the radioactivity measured in a beta counter.
Hyaluronan production
Two days after the addition of immunosuppressants the supernatants were harvested and frozen for subsequent analysis of hyaluronan content.
Hyaluronan was analysed using a radiometric assay (Pharmacia & Upjohn Diagnostics, Uppsala, Sweden). The technique is based on the binding of hyaluronan to specific hyaluronic acid binding proteins (HABP). Briefly, the sample was incubated for 60 min at 4–7°C with [125I]-labelled HABP. Sepharose-coupled hyaluronan was added, and the incubation continued for 45 min at the same temperature. Washing solution was added and the tubes centrifuged at 2000 g for 10 min. After decantation, the radioactivity in the pellet was measured in a gamma counter. A standard curve was constructed from samples with known amounts of hyaluronan. The supernatants from cells cultured at high density (150 000 cells/well) were diluted 11 times in the standard 0-solution, supplied by the manufacturer, before analysis.
Counting of cells
When the supernatants had been frozen for subsequent hyaluronan analysis, the fibroblasts were, in at least one experiment for each drug, trypsinized and counted in a Coulter counter. This was performed to ensure that there was no direct cytotoxic effect of the immunosuppressive substances.
Immunohistochemical stainings
To be able to perform immunohistochemical stainings, fibroblasts from normal and rejecting tissue were grown on cover slips (20 mm × 20 mm). A total of 3000 fibroblasts in 100 µl DMEM + GM + 15% CCS were placed on each coverslip. After overnight incubation the medium was replaced with starvation medium (DMEM + GM + 0·5% CCS) and the incubation continued for 3 more days. At that time-point the cells were exposed to the various immunosuppressive drugs, either prednisolone (100 ng/ml), cyclosporin (5000 ng/ml), tacrolimus (50 ng/ml), mycophenolic acid (1000 ng/ml) or sirolimus (100 ng/ml). Two days later the fibroblasts were washed in PBS and thereafter fixed in methanol (15 min), air-dried and frozen (−70°C).
Immunohistochemical stainings were performed using a peroxidase–antiperoxidase (PAP) technique. In brief, the sections were incubated in 0·3% H2O2 in PBS to inhibit endogenous peroxidase and thereafter with normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) to prevent non-specific background staining before incubation with the primary antibody. A secondary antibody, goat antimouse IgG (Jackson ImmunoResearch Laboratories), was used and added in excess. Then, the sections were incubated with horseradish peroxidase–mouse antiperoxidase (Dakopatts, Glostrup, Denmark). Finally, H2O2 as substrate and 3-amino-9-ethyl-carbazole (AEC) as electron donor were added to react with the horseradish peroxidase. Counterstaining was performed with Mayer's haematoxylin. Negative controls were obtained by omitting the primary antibody. Positive controls consisted of 6 µm thick acetone-fixed cryostat sections of frozen biopsies of rejecting heart tissue.
The following monoclonal antibodies were used as primary antibodies: OX50 (reacts with CD44 positive cells), OX6 (reacts with MHC class II positive cells), OX8 (reacts with CD8 positive cells, W3/25 (reacts with CD4 positive cells), OX39 (reacts with IL-2 receptor positive cells) and VIM 13·2 (reacts with vimentin − an intermediate filament protein of, e.g. fibroblasts). All antibodies were acquired from Serotec (Oxford, UK), except for VIM 13·2, which was purchased from Sigma Chemical. The slides were evaluated blindly according to a four-step scale depending on the percentage of cells staining positive for the various antibodies: + + + (100% positive), + + (75–99% positive), + (1–74% positive), – (0% positive). In parallel, the stain of the individual cell was judged as strong (+), weak (+/–) or negative (–).
Statistics
The results are shown as means and standard errors of the mean (s.e.m.) of the different experiments (n = 3–5). Every experiment consisted of triplicates. The data are related to the proliferation and hyaluronan concentration, respectively, of cells not exposed to any immunosuppressive drugs, which are set to 100%. Multiple comparisons between data were performed by analysis of variance (anova; StatView, SAS Institute Inc., Cary, NC, USA) followed by Fisher's protected least significant difference (PLSD) post hoc test when appropriate. A P level of less than 0·05 was regarded as statistically significant.
RESULTS
Basal hyaluronan production and proliferation
The hyaluronan production and proliferation of fibroblasts isolated from normal and rejecting rat heart tissue have been reported previously [2]. In brief, both parameters are elevated markedly in fibroblasts obtained from rejecting tissue. The hyaluronan production is about 65% higher in fibroblasts from rejecting tissue than in fibroblasts from normal tissue, both for subconfluent and confluent cells (P < 0·001), whereas the proliferation is increased by about 720% for subconfluent cells (P < 0·01) and 235% for confluent cells (P < 0·001).
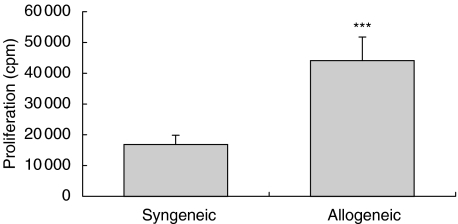
To ensure that the increased fibroblast activation is not only a result of the transplantation procedure as such, also fibroblasts from syngeneically transplanted hearts were isolated and studied. The proliferation of these cells were considerably lower (P < 0·001) than that of fibroblasts from rejecting tissue (Fig. 1).
Fig. 1.
Proliferation, measured as [3H]-thymidine incorporation, of confluent fibroblast cultures (n = 10). The fibroblasts were isolated either from syngeneically or allogeneically transplanted heart tissue. ***P < 0·001.
Effects of immunosuppressive drugs on fibroblast hyaluronan production and proliferation
None of the immunosuppressive substances investigated appeared to have any direct toxic effect on fibroblasts, i.e. there was no difference in cell number at the termination of the experiments when comparing fibroblasts exposed to the highest drug concentrations and fibroblasts incubated in the absence of any drug.
Prednisolone
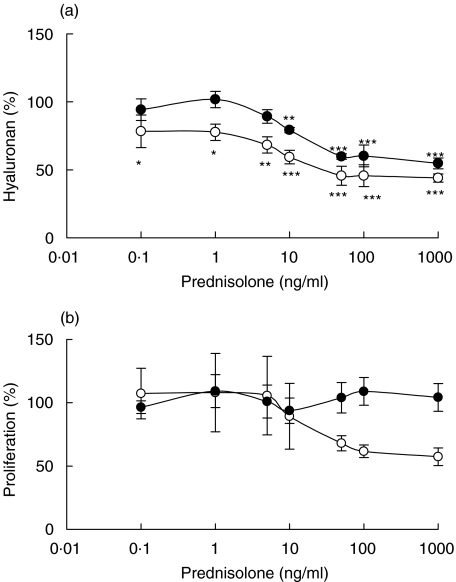
The hyaluronan production was inhibited in the presence of prednisolone (Fig. 2a). This effect was seen in all cultures at prednisolone concentrations above 10 ng/ml; in normal, confluent fibroblasts the inhibitory effect was seen even at the lowest concentration investigated, i.e. 0·1 ng/ml. Prednisolone had no effects on the proliferation of fibroblasts (Fig. 2b), regardless of source (normal or rejecting tissue) or density (confluent or subconfluent).
Fig. 2.
Hyaluronan content in supernatants of confluent fibroblasts exposed to different concentrations of prednisolone for 48 h (a) and proliferation, measured as [3H]-thymidine incorporation, of confluent fibroblasts exposed to different concentrations of prednisolone for 48 h (b). The fibroblasts were isolated from cardiac allografts undergoing rejection (•) or from normal heart tissue (○). The results are mean values ± s.e.m. of three or four different experiments where every experiment consisted of triplicates. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with hyaluronan content or proliferation of fibroblasts incubated in the absence of prednisolone.
Cyclosporin
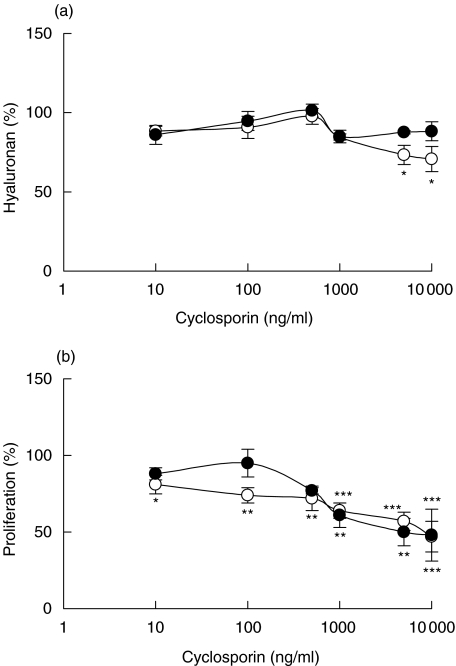
In the presence of cyclosporin slightly decreased amounts of hyaluronan were found in the supernatants of normal, confluent fibroblasts at the highest cyclosporin concentrations investigated, otherwise no effects on hyaluronan production were seen (Fig. 3a). However, the proliferation of normal, confluent fibroblasts was inhibited, as was the proliferation of both confluent and subconfluent fibroblasts obtained from rejecting tissue (Fig. 3b).
Fig. 3.
Hyaluronan content in supernatants of confluent fibroblasts exposed to different concentrations of cyclosporin for 48 h (a) and proliferation, measured as [3H]-thymidine incorporation, of confluent fibroblasts exposed to different concentrations of cyclosporin for 48 h (b). The fibroblasts were isolated from cardiac allografts undergoing rejection (•) or from normal heart tissue (○). The results are mean values ± s.e.m. of three or four different experiments where every experiment consisted of triplicates. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with hyaluronan content or proliferation of fibroblasts incubated in the absence of cyclosporin.
Tacrolimus
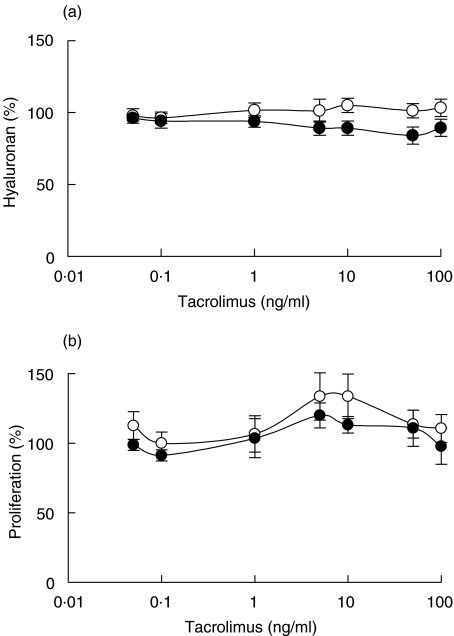
Tacrolimus did not affect either hyaluronan production (Fig. 4a) or proliferation (Fig. 4b) of confluent or subconfluent fibroblasts obtained from normal or rejecting tissue.
Fig. 4.
Hyaluronan content in supernatants of confluent fibroblasts exposed to different concentrations of tacrolimus for 48 h (a) and proliferation, measured as [3H]-thymidine incorporation, of confluent fibroblasts exposed to different concentrations of tacrolimus for 48 h (b). The fibroblasts were isolated from cardiac allografts undergoing rejection (•) or from normal heart tissue (○). The results are mean values ± s.e.m. of three or four different experiments where every experiment consisted of triplicates.
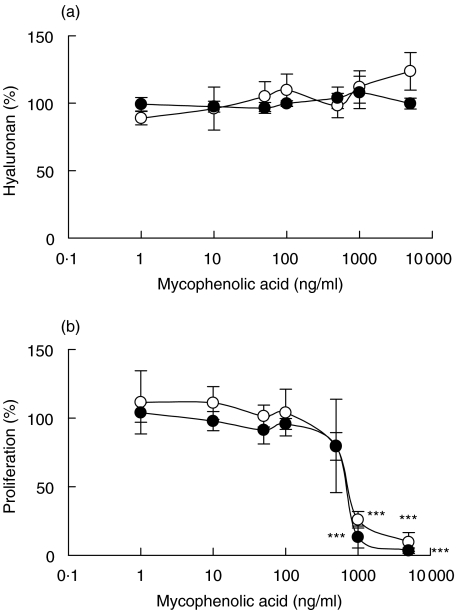
Mycophenolic acid
Mycophenolic acid had no effect on hyaluronan production (Fig. 5a), but strongly affected fibroblast proliferation (Fig. 5b). At mycophenolic acid concentrations of 1000 and 10 000 ng/ml the proliferation of the cells was almost totally abolished. This effect was seen in 17 of 18 tests; the single exception was in one experiment with non-confluent, normal fibroblasts.
Fig. 5.
Hyaluronan content in supernatants of confluent fibroblasts exposed to different concentrations of mycophenolic acid for 48 h (a) and proliferation, measured as 3H-thymidine incorporation, of confluent fibroblasts exposed to different concentrations of mycophenolic acid for 48 h (b). The fibroblasts were isolated from cardiac allografts undergoing rejection (•) or from normal heart tissue (○). The results are mean values ± s.e.m. of three or four different experiments where every experiment consisted of triplicates. **P < 0·01 and ***P < 0·001 compared with hyaluronan content or proliferation of fibroblasts incubated in the absence of mycophenolic acid.
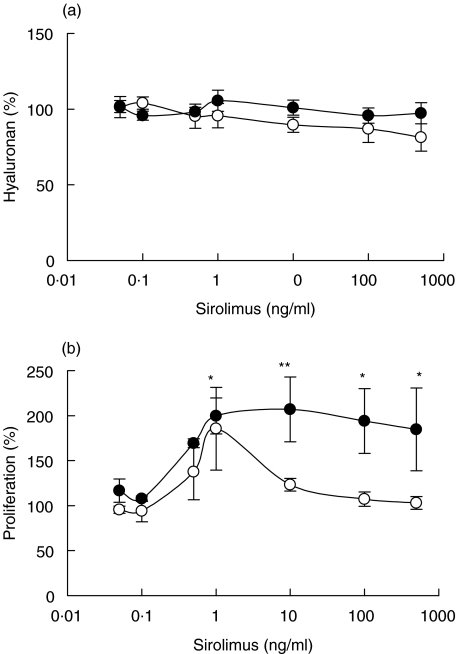
Sirolimus
The fibroblast supernatant concentrations of hyaluronan were not affected in the presence of sirolimus (Fig. 6a). However, in confluent cultures of fibroblasts obtained from rejecting tissue the proliferation was stimulated (Fig. 6b). A tendency towards increased proliferation at low concentrations was also seen in normal fibroblasts; this was, however, not statistically significant. In contrast, in non-confluent cultures there was a tendency (statistically non-significant) towards a decreased proliferation.
Fig. 6.
Hyaluronan content in supernatants of confluent fibroblasts exposed to different concentrations of sirolimus for 48 h (a) and proliferation, measured as [3H]-thymidine incorporation, of confluent fibroblasts exposed to different concentrations of sirolimus for 48 h (b). The fibroblasts were isolated from cardiac allografts undergoing rejection (•) or from normal heart tissue (○). The results are mean values ± s.e.m. of three or four different experiments where every experiment consisted of triplicates. *P < 0·05 and **P < 0·01 compared with proliferation of fibroblasts incubated in the absence of sirolimus.
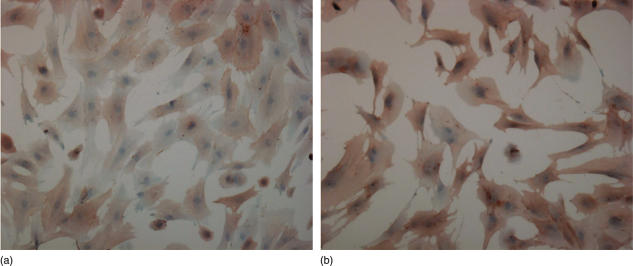
Immunohistochemical stainings
The absolute majority of the fibroblasts expressed CD44 on their surfaces, irrespective of whether the cells were isolated form normal or rejecting hearts (Table 1). Often the staining was more intense in cultures of fibroblasts from rejecting tissue than in cultures of fibroblasts from normal tissue (Fig. 7). There was no obvious difference in the staining pattern (number of positive cells or intensity) in the presence of any of the drugs. IL-2 receptors, CD4 or MHC class II antigens were not detected in any of the cultures. The antibody recognizing CD8, which, when applied on frozen tissue provides a distinct staining, resulted in a diffuse labelling of the fibroblasts, irrespective of source (normal or rejecting tissue) or drug exposure. All cells stained positive for vimentin.
Table 1.
Immunohistochemical stainings of fibroblasts. Fibroblasts isolated from normal heart tissue or from transplanted hearts undergoing rejection were grown on coverslips, fixed and stained. The slides were evaluated blindly according to a four-step scale depending on the percentage of cells staining positive for the various antibodies: + + + (100% positive), + + (75–99% positive), + (1–74% positive), – (0% positive). In parallel, the stain of the individual cell was judged as strong (+), weak (+/–) or negative (–). The table shows median values of three or four experiments
| Number of positive cells | Intensity | |||
|---|---|---|---|---|
| Normal | Rejection | Normal | Rejection | |
| CD44 | ++/+++ | +++ | +/– | + |
| IL-2 receptor | – | – | – | – |
| CD4 | – | – | – | – |
| MHC class II | – | – | – | – |
| CD8 | ++ | ++ | +/– | +/– |
| Vimentin | +++ | +++ | + | + |
Fig. 7.
CD44 expression on fibroblasts isolated from normal (a) and rejecting (b) heart tissue as visualized by immunoperoxidase staining with the monoclonol antibody OX50. Counterstaining was performed with Mayer's haematoxylin.
DISCUSSION
The present study demonstrates that some of the most commonly used immunosuppressive drugs have the capacity to directly affect graft-derived fibroblasts. Effects were seen on fibroblast proliferation and/or hyaluronan production. Prednisolone had a strong inhibiting effect on hyaluronan production whereas fibroblast proliferation was reduced by cyclosporin, and in the presence of mycophenolic acid the proliferation was almost totally abolished. These effects were seen on fibroblasts isolated from rat cardiac allografts undergoing rejection as well as on fibroblasts obtained from normal, non-transplanted heart tissue. In contrast, sirolimus was found to stimulate the proliferation of fibroblasts from rejecting tissue but not that of normal fibroblasts. Tacrolimus was the single substance investigated where no effects were seen on any parameter.
The immunosuppressive drugs in current use in organ transplantation are more or less specific. Many of the drugs used for maintenance therapy are generally regarded as ‘cell-specific’, e.g. the calcineurin-inhibitors as ‘T lymphocyte-specific’ and mycophenolic acid as ‘lymphocyte-specific’. However, even those drugs can affect other cell types, such as endothelial cells and keratinocytes for the calcineurin-inhibitors [8,9] and vascular smooth muscle cells and endothelial cells for mycophenolic acid [10,11]. The most unspecific substances in common use as immunosuppressants are the steroids, which are utilized not only for prophylaxis but also for therapeutic intervention at already established rejection.
The question of whether the immunosuppressive drugs can exert effects on fibroblasts is not just of theoretical interest, but is probably also clinically relevant. Fibroblasts of rejecting tissue are activated in that they have an increased proliferation rate and an increased production of hyaluronan [2]. Local hyaluronan accumulation has negative effects on organ viability by contributing to interstitial oedema and cell infiltration. As proof of this, treatment with the hyaluronan-degrading enzyme hyaluronidase leads to decreased oedema and decreased interstitial pressure as well as reduced cell infiltration and prolonged graft survival [4,12]. The effects on cell infiltration and graft survival are most probably exerted through interference with the interaction between hyaluronan and the cell surface receptor CD44. Hence, such effects can also be obtained by treatment with low molecular weight hyaluronan [13].
An enhanced fibroblast proliferation, resulting in increasing numbers of cells, should also affect the tissue concentration of other substances than hyaluronan being produced by fibroblasts. Today there is increasing evidence of extracellular matrix proteins, such as laminin and fibronectin, as being of importance in allograft rejection [14]. Thus, local treatment with antilaminin antibodies was recently shown to ameliorate the rejection process [15].
In the present study it was shown that fibroblasts isolated from rejecting tissue displayed a more intense staining for CD44 than normal fibroblasts. This finding is in agreement with that of studies performed on human lung allografts where the fibroblast expression of CD44 was increased in biopsies from patients with acute rejection and/or obliterative bronchiolitis [16].
In view of the undesired effects of excessive amounts of extracellular matrix substances, it would be advantageous to treat rejection with a drug that suppresses not only the immunocompetent cells but also the activated fibroblasts. In contrast, an immunosuppressive drug with stimulating effects on fibroblasts might worsen the injuries of the tissue.
The hyaluronan concentration in supernatants of fibroblasts isolated from an immunologically active environment was reduced in the presence of prednisolone. This hyaluronan inhibition might, by improving the viability of the organ, contribute to the often very good effects of steroids in the treatment of acute rejection. Prednisolone was also found to decrease the hyaluronan production of normal fibroblasts − and at lower concentrations than for the already stimulated fibroblasts. This indicates that the low dose of steroids traditionally being included in maintenance therapy protocols has favourable effects on fibroblasts. Steroids have been shown previously to suppress the production of hyaluronan in fibroblasts of other sources, e.g. synovial and skin fibroblasts [17,18].
Hyaluronan can bind to CD44 on fibroblasts and subsequently become internalized and degraded [19]. Thus, a reduced supernatant content of hyaluronan may be the result not only of a decreased synthesis but also of an increased CD44 expression. However, no such tendency was seen in the analysis of the immunohistochemical stainings. In addition, the mRNA expression of one of the hyaluronic acid synthases, HAS2, has in recent experiments been demonstrated to be suppressed in the presence of steroids [20].
The effects of the two calcineurin inhibitors were not similar; tacrolimus did not affect either fibroblast proliferation or hyaluronan production, whereas cyclosporin reduced proliferation. Inhibiting effects of cyclosporin on normal fibroblasts were observed even at the lowest concentration studied, i.e. 10 ng/ml, thus it is unlikely that the lack of effect of tacrolimus is a consequence of the drug concentrations chosen for the study. Consequently, the effect of cyclosporin on cardiac fibroblasts appears to be non-calcineurin-dependent. The inhibitory effect might thus be associated with cyclosporin binding to cyclophilin. Previous experiments on cultured fibroblasts have noticed that the folding of procollagen I is slowed down in the presence of cyclosporin [21].
Studies of cyclosporin have been performed on gingival fibroblasts to elucidate the mechanisms behind gingival overgrowth during cyclosporin therapy. Most authors have concluded that cyclosporin stimulates the proliferation of these cells [22,23]. In addition, there are indications of increased production of extracellular matrix substances, including hyaluronan [24], although it has been proposed recently that gingival overgrowth could be attributed to a reduced fibroblast degradation of extracellular matrix substances [25]. In our studies on cardiac fibroblasts obtained from normal or rejecting tissue no effects were seen on hyaluronan production/degradation or CD44 expression.
The most striking result of our experiments was the total inhibition of fibroblast proliferation in the presence of mycophenolic acid. Because the hyaluronan levels were maintained and the actual cell numbers not affected at this early time point, toxic effects can be excluded. The average plasma trough level of mycophenolic acid in kidney-transplanted patients given a daily dose of mycophenolic mofetil of 1·5 g for prevention of rejection is about 3 µg/ml [26]. Hence, the concentrations where we observed the suppressed proliferation (1 and 10 µg/ml) should be biologically relevant.
The antiproliferative effect of mycophenolic acid on vascular smooth muscle cells has been suggested to contribute to the positive effects of the drug in preventing intimal thickening associated with chronic rejection in experimental models [27]. In addition, a multivariate analysis of risk factors for chronic allograft failure in about 8500 kidney-transplanted patients treated with mycophenolic mofetil showed that the drug reduces the risk of developing chronic allograft failure. This effect is attributed not only to a reduced number of acute rejection episodes [28]; a reduced proliferation of intragraft fibroblasts might very well contribute to such an effect by reducing the deposition of extracellular matrix substances.
There are several reports on sirolimus as an inhibitor of growth-factor induced proliferation of fibroblasts from various sources [29–31]. In analogy, it is well known that wound healing may be disturbed in transplanted patients treated with sirolimus. In our study, where the fibroblasts were obtained from an immunologically active environment but not stimulated further in vitro, the proliferation was increased. Thus, our in vitro data may call for increased surveillance of possible negative effects related to an additional activation of fibroblasts when sirolimus is given in episodes of rejection. However, no stimulatory effects were seen on normal fibroblasts, hence the data do not indicate a counterproductive effect of sirolimus when used for prophylaxis of rejection.
Several of the drugs investigated affected fibroblast proliferation but not hyaluronan synthesis. However, it can be speculated that a reduced cell number will, over time, result in lower amounts of hyaluronan being produced, even if the production rate is maintained on a single cell basis. On the other hand, an increasing cell number should result in higher hyaluronan levels. Similarly, the amounts of other substances produced by fibroblasts should also be affected by a stimulated or suppressed proliferation.
To summarize, several of the immunosuppressive drugs used in organ transplantation have effects on fibroblasts. Suppression of fibroblast activity might have favourable effects on graft viability, which would thus contribute to the overall effects of the drugs.
Acknowledgments
The technical assistance of Charlotta Jonsson and My Quach is gratefully acknowledged. Financial support was obtained from the Swedish Research Council (K2002–73X-14249–01 A), the Lennart Jacobssons Fund and Njurfonden (Riksförbundet för Njursjukas Njurfond).
References
- 1.Hällgren R, Gerdin B, Tengblad A, Tufveson G. Accumulation of hyaluronan (hyaluronic acid) in myocardial interstitial tissue parallels development of transplantation edema in heart allografts in rats. J Clin Invest. 1990;85:668–73. doi: 10.1172/JCI114490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellkvist J, Tufveson G, Gerdin B, Johnsson C. Characterization of fibroblasts from rejecting tissue: the hyaluronan production is increased. Transplantation. 2002;74:1672–7. doi: 10.1097/00007890-200212270-00004. [DOI] [PubMed] [Google Scholar]
- 3.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;8:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Johnsson C, Hällgren R, Elvin A, Gerdin B, Tufveson G. Hyaluronidase ameliorates rejection-induced edema. Transplant Int. 1999;12:235–43. doi: 10.1007/s001470050216. [DOI] [PubMed] [Google Scholar]
- 5.McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. J Clin Invest. 1996;98:2403–13. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oertli B, Beck-Schimmer B, Fan X, Wüthrich RP. Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-κB and activating protein-1. J Immunol. 1998;161:3431–7. [PubMed] [Google Scholar]
- 7.Schenck P, Schneider S, Miehlke R, Prehm P. Synthesis and degradation of hyaluronate by synovia from patients with rheumatoid arthritis. J Rheumatol. 1995;22:400–5. [PubMed] [Google Scholar]
- 8.Sharpe RJ, Arndt KA, Bauer SI, Maione TE. Cyclosporin inhibits basic fibroblast growth factor-driven proliferation of human endothelial cells and keratinocytes. Arch Dermatol. 1989;125:1359–62. [PubMed] [Google Scholar]
- 9.Furue M, Gaspari AA, Katz SI. The effect of cyclosporin A on epidermal cells. II. Cyclosporin A inhibits proliferation of normal and transformed keratinocytes. J Invest Dermatol. 1988;90:796–800. doi: 10.1111/1523-1747.ep12462009. [DOI] [PubMed] [Google Scholar]
- 10.Gregory CR, Pratt RE, Huie P, et al. Effects of treatment with cyclosporine, FK 506, rapamycin, mycophenolic acid, or deoxyspergualin on vascular muscle proliferation in vitro and in vivo. Transplant Proc. 1993;25:770–1. [PubMed] [Google Scholar]
- 11.Mohacsi PJ, Tüller D, Hulliger B, Wijngaard PLJ. Different inhibitory effects of immunosuppressive drugs on human and rat aortic smooth muscle and endothelial cell proliferation stimulated by platelet-derived growth factor or endothelial cell growth factor. J Heart Lung Transplant. 1997;16:484–92. [PubMed] [Google Scholar]
- 12.Johnsson C, Tufveson G, Hällgren R. Monitoring of intragraft pressure of rejecting organs. Increased tissue pressure can be reduced by hyaluronidase therapy. Transplantation. 2000;70:1575–80. doi: 10.1097/00007890-200012150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Knoflach A, Azuma H, Magee C, et al. Immunomodulatory functions of low-molecular weight hyaluronate in an acute rat renal allograft rejection model. J Am Soc Nephrol. 1999;10:1059–66. doi: 10.1681/ASN.V1051059. [DOI] [PubMed] [Google Scholar]
- 14.Coito AJ, Kupiec-Weglinski JW. Extracellular matrix proteins in organ transplantation. Transplantation. 2000;69:2465–73. doi: 10.1097/00007890-200006270-00001. [DOI] [PubMed] [Google Scholar]
- 15.Riederer I, Silva-Barbosa SD, Rodrigues ML, Savino W. Local antilaminin antibody treatment alters the rejection pattern of murine cardiac allografts: correlation between cellular infiltration and extracellular matrix. Transplantation. 2002;74:1515–22. doi: 10.1097/00007890-200212150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Zander DS, Baz MA, Massey JK. Patterns and significance of CD44 expression in lung allografts. J Heart Lung Transplant. 1999;18:646–53. doi: 10.1016/s1053-2498(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 17.Saarni H, Tammi M, Vuorio E. Effects of cortisol on glycosaminoglycans synthesized by normal and rheumatoid synovial fibroblasts in vitro. Scand J Rheumatol. 1977;6:222–4. doi: 10.3109/03009747709095454. [DOI] [PubMed] [Google Scholar]
- 18.Smith TJ. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. J Clin Invest. 1984;74:2157–63. doi: 10.1172/JCI111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116:1055–60. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Watson CE, Liu C, Williams KJ, Werth VP. Glucocorticoids induce a near-total suppression of hyaluronan synthase mRNA in dermal fibroblasts and in osteoblasts: a molecular mechanism contributing to organ atrophy. Biochem J. 2000;349:91–7. doi: 10.1042/0264-6021:3490091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinmann B, Bruckner P, Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans isomerase. J Biol Chem. 1991;266:1299–303. [PubMed] [Google Scholar]
- 22.Bartold PM. Regulation of human gingival fibroblast growth and synthetic activity by cyclosporine-A in vitro. J Periodontal Res. 1989;24:314–21. doi: 10.1111/j.1600-0765.1989.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 23.Willershausen-Zonnchen B, Lemmen C, Schumacher U. Influence of cyclosporin A on growth and extracellular matrix synthesis of human fibroblasts. J Cell Physiol. 1992;152:397–402. doi: 10.1002/jcp.1041520222. [DOI] [PubMed] [Google Scholar]
- 24.Newell J, Irwin CR. Comparative effects of cyclosporin on glycosaminoglycan synthesis by gingival fibroblasts. J Periodontol. 1997;68:443–7. doi: 10.1902/jop.1997.68.5.443. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H, Nishimura F, Naruishi K, et al. Phenytoin and cyclosporin A suppress the expression of MMP-1, TIMP-1, and cathepsin L, but not cathepsin B in cultured gingival fibroblasts. J Periodontol. 2000;71:955–60. doi: 10.1902/jop.2000.71.6.955. [DOI] [PubMed] [Google Scholar]
- 26.Smak-Gregoor PJ, van Gelder T, van Besouw NM. Mycophenolic acid trough levels after kidney transplantation in a cyclosporin-free protocol. Transplant Int. 2000;13:S333–5. doi: 10.1007/s001470050355. [DOI] [PubMed] [Google Scholar]
- 27.Räisänen-Sokolowski A, Vuoristo P, Myllarniemi M, Yilmaz S, Kallio E, Häyry P. Mycophenolate mofetil (MMF, RS-61443) inhibits inflammation and smooth muscle cell proliferation in rat aortic allografts. Transplant Immunol. 1995;3:342–51. doi: 10.1016/0966-3274(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 28.Meier-Kriesche H-U, Ojo AO, Arndorfer JA, et al. Mycophenolate mofetil decreases the risk for chronic renal allograft failure. Transplant Proc. 2001;33:1005–6. doi: 10.1016/s0041-1345(00)02305-8. [DOI] [PubMed] [Google Scholar]
- 29.Akselband Y, Harding MW, Nelson PA. Rapamycin inhibits spontenous and fibroblast growth factor β-stimulated proliferation of endothelial cells and fibroblasts. Transplant Proc. 1991;23:2833–6. [PubMed] [Google Scholar]
- 30.Migita K, Eguchi K, Aoyagi T, et al. The effects of the immunosuppressant rapamycin on the growth of rheumatoid arthritis (RA) synovial fibroblast. Clin Exp Immunol. 1996;104:86–91. doi: 10.1046/j.1365-2249.1996.d01-651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salas-Prato M, Assalian A, Mehdi AZ, Duperre J, Thompson P, Brazeau P. Inhibition by rapamycin of PDGF- and bFGF-induced human tenon fibroblast proliferation in vitro. J Glaucoma. 1996;5:54–9. [PubMed] [Google Scholar]