Abstract
Our previous study indicated that the interleukin (IL)-6/STAT-3 signal was up-regulated in inflammatory bowel disease (IBD) in both humans and animal models. We also discovered phosphorylated STAT-3 in the nucleus of the colonic epithelial cells in IBD mice. Intestinal epithelial cells (IEC) have been shown to secrete IL-6. Therefore, the secretion of IL-6 from IEC may be one of the mechanisms of STAT-3 phosphorylation in IEC during the pathogenesis of IBD, and inhibition of IL-6 production by IEC may be beneficial in preventing IBD. We examined the preventative effect of various types of fucoidans on IL-6 production in a lipopolysaccahride (LPS)-stimulated murine colonic epithelial cells line, CMT-93, in vitro. We also determined in vivo the effect of fucoidans on murine chronic colitis induced with dextran sodium sulphate. Among fucoidans, those from Cladosiphon okamuranus Tokida and Kjellmaniella crassifolia inhibited IL-6 production in CMT-93 cells with the down-regulation of NF-κB nuclear translocation. Analysis of the effect of fucoidan on murine colitis in vivo showed that the disease activity index and myeloperoxidase activity decreased in mice fed Cladosiphon fucoidan, but not Fucus fucoidan. Cytokine profiles in colonic lamina propria indicated that the synthesis of interferon (IFN)-γand IL-6 decreased and that of IL-10 and transforming growth factor (TGF)-βincreased in mice fed Cladosiphon fucoidan, compared with mice fed a standard diet or Fucus fucoidan. The levels of IL-6 mRNA in colonic epithelial cells was lower in colitis-induced Balb/c mice fed Cladosiphon fucoidan than those fed a standard diet. Fucoidan improves murine chronic colitis by down-regulating the synthesis of IL-6 in the colonic epithelial cells. Fucoidan derived from C. o. Tokida may be useful as a dietary substance for the patients with inflammatory bowel disease.
Keywords: experimental colitis, fucoidan, IL-6, inflammatory bowel disease, intestinal epithelial cell, ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) is a severe intestinal inflammation, the pathogenesis of which is not well understood. It is suspected that the disease is due to complex mucosal immune responses to resident enteric bacteria, because intestinal inflammation was absent from various IBD models reared under germ-free conditions [1–7]. Interleukin (IL)-6 is one of the major cytokines secreted by lamina propria cells in the patients with IBD [8–10]. Strong expression of IL-6 has also been reported in murine acute bowel inflammation [11]. Recent studies using antisoluble-IL-6 receptor antibodies demonstrated that IL-6 plays a critical role in the development chronic colitis [12,13]. IL-6 has also been shown to play a central role in arthritis [14]. We and other investigators have confirmed that IL-6 gene-disrupted mice are resistant to arthritis and dextran sodium sulphate (DSS)-induced colitis [15,16]. IL-6 is a cytokine which activates the transcription factor signal transducer and activator of transcription (STAT)-3 via intracellular signalling pathways [17,18]. Recently, we investigated the expression of phosphorylated STAT-3 in the intestinal mucosa of patients with IBD and in murine models [16]. Notably, we observed phosphorylated STAT-3 molecules in the nucleus of colonic epithelial cells. Intestinal epithelial cells (IEC) have been shown to produce several cytokines including IL-6 [19]. Therefore, the autocrine secretion of IL-6 by IEC may be one of the mechanisms of spontaneous STAT-3 phosphorylation in IEC during the pathogenesis of colitis. It is important to identify the stimuli inducing IL-6 production in the pathogenesis of IBD. Bacterial cell components such as lipopolysaccharide (LPS) are known to induce the expression of IL-6 [20–22]. It is recognized that the total number of bacteroides among intestinal flora increases in IBD patients [23]. Rath et al. suggested that overgrowth of bacteroides species induced severe intestinal inflammation in HLA-B27 transgenic rats [24]. Furthermore, Lange et al. described that colitis was less severe in mice with a disrupted toll-like receptor 4 (TLR-4) gene than in control mice [25]. Therefore, LPS signals are essential for the development of inflammatory bowel disease. Intestinal epithelial cells (IEC) are the first line of defence against the intestinal luminal environment. One possibility is that bacterial LPS directly interacts at the apical surface and induces responses in IEC, which in turn produce cytokines and other mediators of inflammation. Evidence is mounting that IEC serve a vital function for the mucosal immune system. Cario et al. described that intestinal cell lines and intestinal epithelial cells in vivo express the TLR family [26,27]. These experimental results led to the hypothesis that LPS-signals delivered through intestinal epithelial cells trigger the production of IL-6 by IEC and initiate colitogenesis. Furthermore, removal of these signals from IEC may contribute to improvement of intestinal inflammation.
Fucoidan is a complex sulphated polysaccharide, derived from marine brown seaweed. There have been many reports on the biological effects of fucoidan on mammalian cells [28,29]. Shibata and colleagues indicated that fucoidan derived from Cladosiphon okamuranus Tokida blocked the adhesion of Helicobacter pylori to a human gastric cell line [30]. Therefore, fucoidan may be useful as a dietary substance for preventing human disease because its polysaccharide causes no toxicity or irritation. In this study, we examined the effect of fucoidans derived from various brown seaweeds on the production of IL-6 in a LPS-stimulated murine colonic epithelial cell line. Moreover, we determined the improved effect of fucoidan on murine chronic colitis in vivo.
MATERIALS AND METHODS
Animals
Female Balb/c mice (8 weeks old) were purchased from Japan Clea Laboratory (Tokyo, Japan). They were maintained under specific pathogen-free (SPF) conditions during the experiments.
Preparation of fucoidans
The brown seaweed C. o. Tokida was cultivated in Okinawa, Japan. This seaweed was purchased from the Tropical Technology Centre Co., Ltd (Okinawa, Japan) as salted food. Other brown seaweeds (H. elongata, S. horneri and L. digitata) were kindly provided by SCETI Co., Ltd (Tokyo, Japan) as dried materials. Fucoidan from Fucus vesiculosus was purchased from Sigma (St Louis, MO, USA). Other fucoidans were prepared from brown seaweeds as described in a previous paper [31]. Standard mouse chow (type MF) and fucoidan (C. o. Tokida or F. vesiculosus)-containing MF chows (0·05% w/w) were tableted and provided by Oriental Yeast Co., Ltd (Tokyo, Japan).
Cell culture
The murine colon carcinoma cell line CMT-93 was purchased from the American Type Culture Collection (ATCC). Cells were cultured in 10% fetal calf serum (FCS)/10 mm Hepes/penicillin–streptomycin/non-essential amino acid/DMEM medium under 5% CO2 at 37°C. LPS derived from Escherichia coli was purchased from Sigma. Before the experiment, various concentrations of LPS were examined for their influence on IL-6 synthesis in the CMT-93 cell line. To assess the effect of the fucoidans on the production of IL-6 synthesis in LPS-stimulated CMT-93 cells, 10 µg/ml of LPS was added to cultured CMT-93 with or without 1 µg/ml of the various fucoidans and the cells were cultured for 72 h. After the culture, the supernatants were collected and stored at −84°C until IL-6 ELISA assay. Toxic effects of fucoidans were detrmined by MTT assay and 51Cr-releasing assay and we could find no ant toxic effect of fucoidans against CMT-93 (data not shown).
ELISA
Murine anti-IL-6 MoAbs (clone: MP5–20F3, MP5–32C11) were purchased from BD PharMingen (LA, CA). ELISA was performed according to the standard recommended by the manufactures.
Nuclear protein extraction and the determination of NF-κB amounts
Nuclear proteins were extracted from LPS-stimulated CMT-93 cells that were treated with or without fucoidan derived from C. o. Tokida. The amounts of nuclear-localized NF-κB were determined by using the NF-κB transfactor ELISA kit (CLONTECH Laboratories, Inc.).
Induction of chronic colitis
Chronic colitis was induced in Balb/c mice fed C. o. Tokida or F. vesiculosus-containing MF chows, or conrol MF chow (n = 10, each group) as described by Okayasu et al. [32]. Briefly, mice at 10 weeks of age were treated with 4% DSS (molecular mass, 40 kDa; ICN Biomedicals, Aurora, OH, USA) dissolved in drinking water. The colitis was induced by four administration cycles; each cycle was an alternating regimen of 4% DSS for 7 days followed by drinking water without DSS for the next 7 days.
Assessment of disease severity
A disease activity index confirmed to reflect changes in the clinical status of mice with DSS-induced colitis was calculated by scoring from 0 to 4 abnormalities regarding change in body weight, stool consistency and intestinal bleeding and summing the results [33]. In addition, the length of the colon from the caeco–colonic junction to the anal verge was measured as an established inflammatory parameter in DSS-induced colitis.
Lymphocyte preparation and flow cytometry
Lamina propria lymphocytes (LPLs) were prepared from the large intestine of mice, as described previously [34]. Briefly, a longitudinally opened large intestine was cut into 1-cm pieces. The intestinal segments were incubated with Hanks's balanced salt solution (HBSS) containing DTT (0·45 mm) and EDTA (2 mm) twice for 15 min each at 37°C with agitation. Then, after removal of the epithelial layer by decantation, the resultant intestinal segments were incubated with RPMI-1640 containing 2·5% FCS, collagenase (300 µg/ml, Collagenase-Yakult S, Yakult Honsha Co., Ltd) and DNase I (50 µg/ml, Sigma) three times for 45 min each at 37°C with gentle agitation in a CO2 incubator. Cell pellets were suspended in ice-cold 2·5% FCS/10 mm Hepes/RPMI-1640 and then passed through a nylon column; the lymphocyte population was then isolated from the 44%/100% interface of a Percoll density gradient (Pharmacia, Uppsala, Sweden). Cells were stained with MoAbs against TCR-β, CD4, CD45RB, CD69 or B220. All the MoAbs were purchased from BD PharMingen. The stained cells were analysed with an EPICS EL cell analyser (Beckman Coulter, Inc., Fullerton, CA, USA).
Cell culture and cytokine assay
LPLs (1·0 × 106 cells) were cultured in 24-well tissue culture plates in 10% FCS/10 mm Hepes/2-ME/RPMI-1640 under stimulation with or without immobilized anti-TCR-β MoAb (H57–597, 10 µg/ml); anti-CD28 MoAb (37·51, 1 µg/ml) was added subsequently. After 48 h of culture, supernatants were collected and stored at −84°C until the assays. Cytokine-specific ELISA was performed using the following antibody combinations: anti-interferon (IFN)-γ (clone: XMG1·2, R4–6A2), anti-IL-4 (clone: 11B11, BVD6–24G2); all from BD PharMingen. For the assay of IL-10 and TGF-β1, assay kits were purchased from BioSource International Inc. (Camarillo, CA, USA) and Genzyme Corp. (Cambridge, MA, USA), respectively.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis for IL-6 mRNA on colonic epithelial cells
Colonic epithelial cells were isolated from colitis-uninduced Balb/c mice or from colitis-induced Balb/c mice fed a normal diet, or fucoidan derived from C. o. Tokida, and total RNA was prepared from the three groups of mice as described previously [35]. The total RNA (1·0 µg) was reverse-transcribed and then PCR was performed using specific primers for G3PDH, IL-6, tumour necrosis factor (TNF)-α or TLR-4 [36]. After gel electrophoresis, PCR products were visualized with ethidium bromide.
Myeloperoxidase assay
Tissue MPO activity was measured as previously described [37,38]. In brief, the colonic tissue was homogenized with a Polytoron homogenizer in hexadecyltrimethylammonium bromide (Sigma) buffer. The suspension was sonicated on ice and then centrifuged at 15·000 r.p.m. for 30 min. The supernatant was mixed with an enzyme substrate buffer containing 0·167 mg of O-dianisidine hydrochloride (Sigma) per ml and 0·0005% hydrogen peroxide. The changes in the absorbance at 405 nm were measured.
Measurement of immunoglobulin contents in colonic segments
A colonic homogenate sample was prepared as described above. Total IgG-, IgG1- or IgG2a-specific sandwich ELISA assays were performed as described elsewhere [38].
Statistics
All data were expressed as the mean ± s.e. and evaluated by Tukey or Tukey–Kramer test for multiple comparisons. P-values of less than 0·05 were considered to be statistically significant.
RESULTS
IL-6 production by CMT-93 cells after stimulation with LPS
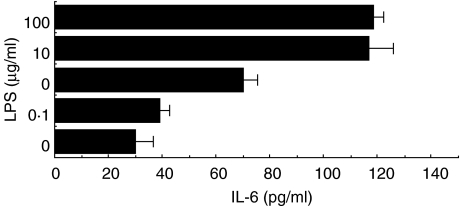
CMT-93 cells were cultured in the presence or absence of various concentrations of LPS. Maximum IL-6 production by CMT-93 was observed at 5–10 µg/ml LPS (Fig. 1).
Fig. 1.
Effects of various doses of LPS on IL-6 synthesis in the murine colonic epithelial cell line CMT-93. Cells were cultured in the absence or presence of various doses of LPS. After 72 h, the amount of IL-6 in the culture supernatant was determined by sandwich ELISA. Similar results were obtained from five independent experiments. Data are mean ±s.d.
Effect of fucoidans in IL-6 production on CMT-93 stimulated with LPS
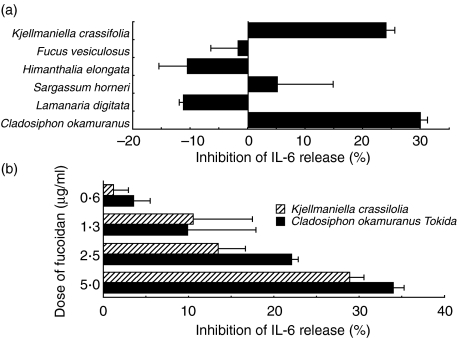
To clarify whether fucoidans modulate the production of IL-6 in LPS-stimulated CMT-93 cells, various fucoidans isolated from brown seaweed were tested. Fucoidans derived from C. o. Tokida and K. crassifolia repressed IL-6 synthesis in LPS-stimulated CMT-93 (Fig. 2a). However, the fucoidans from F. vesiculosus, H. elongata, S. horneri and Lamanaria digitata did not. The inhibitory effect on IL-6 release in LPS-stimulated CMT 93 cells by the fucoidan derived from C. o. Tokida or from K. crassifolia was dose-dependent (Fig. 2b). We did not detect any up-regulation in the release of IL-6 from CMT-93 cells cultured in the presence of single fucoidans (data not shown).
Fig. 2.
Effect of various fucoidans on IL-6 synthesis in LPS-stimulated CMT-93 cells (a). Various fucoidans were isolated from marine seaweed as described in Materials and methods. Cells were cultured in the presence of both purified fucoidans (2·5 µg/ml) and LPS (10 µg/ml) for 72 h. Dose-dependent inhibition of IL-6 release by fucoidans in an LPS-stimulated colonic epithelial cell line CMT-93 (b). CMT-93 was cultured with various doses of the fucoidan derived from C. o. Tokida or from K. crassifolia in the presence of LPS for 72 h. The amount of IL-6 in the culture supernatant was measured by an IL-6-specific sandwich ELISA. Three independent experiments gave similar results. Data are mean ±s.d.
The effect of fucoidan on NF-κB recruitments in LPS-stimulated CMT-93 cells
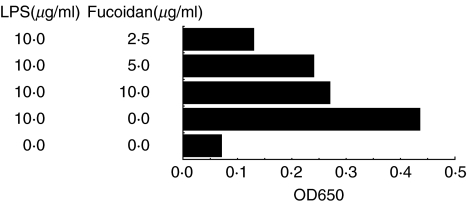
The amounts of nuclear-localized p65 NF-κB in CMT-93 cells that were treated with or without fucoidan derived from C. o. Tokida were determined. The amounts of nuclear-localized p65 NF-κB in LPS-stimulated CMT-93 cells were down-regulated by the treatment of the Cladpsiphon fucoidan (Fig. 3).
Fig. 3.
The effects of fucoidan in NF-κB activities in LPS-stimulated CMT-93 cells. The amounts of nuclear-localized p65 NF-κB in LPS-stimulated CMT-93 were determined by transfactor ELISA system. The amounts of nuclear-localized NF-κB in LPS-stimulated CMT-93 cells were down-regulated by treatment of Cladosiphon fucoidan. Two independent experiments gave similar results. Data are mean of duplicate assays.
In vivo effect of fucoidans on murine chronic colitis
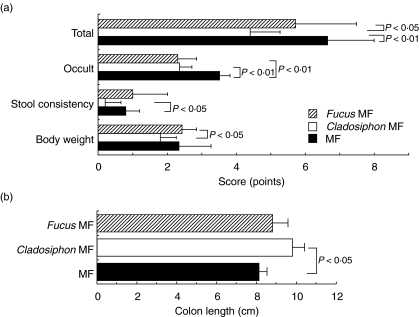
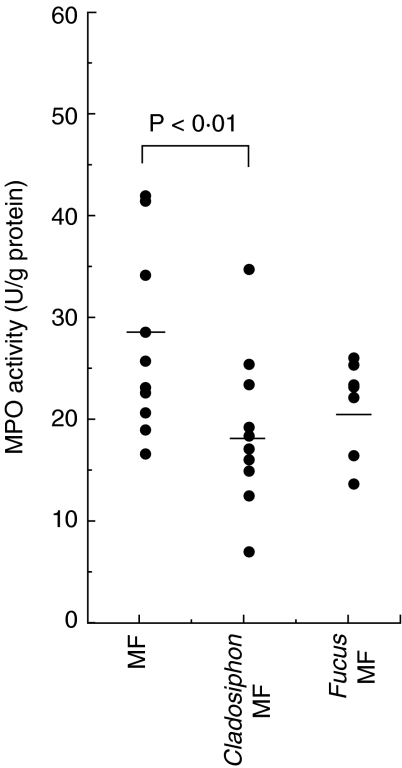
To examine the effect of fucoidans on murine chronic colitis in vivo, colitis was induced in Balb/c mice fed standard mouse chow (MF), MF chow containing fucoidan from C. o. Tokida (0·05% w/w) or MF containing fucoidan from F. vesiculosus (0·05% w/w) by administering the animals DSS in drinking water and the effect was analysed using various disease parameters. The scores of all the parameters in the disease activity index such as loss of body weight, diarrhoea and occult blood were reduced in Balb/c mice fed Cladosiphon fucoidan compared with those fed the standard diet or Fucus fucoidan (Fig. 4a). The scores are comparable between mice fed Fucus fucoidan and those fed standard diet. Moreover, the length of the colon was longer in colitic mice fed Cladosiphon fucoidan than those fed standard diet (Fig. 4b). However, the length of the colon shortened due to severe inflammation in the mice fed the standard diet or Fucus fucoidan. To confirm these results, the MPO activity in colonic tissue was compared among the three groups. The activity was lower in colitic Balb/c mice fed Cladosiphon fucoidan than those fed standard diet or Fucus fucoidan (Fig. 5).
Fig. 4.
Disease activity index (a) and colon length (b) in colitis-induced Balb/c mice fed normal diet or fed diets containing fucoidans (n = 10, each group). Disease activity index and colon length were determined in colitis-induced Balb/c mice fed normal diet, or fed Cladosiphon fucoidan or Fucus fucoidan as described in Materials and methods. Similar results were obtained from three independent experiments. Data are mean ± s.d.
Fig. 5.
MPO activity of the colonic tissue in colitis-induced Balb/c mice fed normal diet or fed fucoidan-containing diets (n = 10, each group). Colonic tissues were homogenated in HTMB buffer with a polytron-homogenizer. After centrifugation, MPO activity in the supernatants was measured as described in Materials and methods. Down-regulation of MPO activity in the colonic tissue in colitis-induced Balb/c mice fed Cladosiphon fucoidan observed in separate experiments is plotted as dots. Bars represent mean values.
Immunological characterization
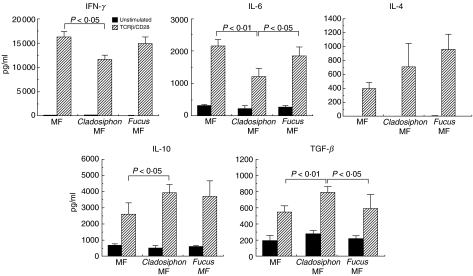
At the beginning of this experiment, we compared LI–LPL phenotypes between control Balb/c mice and mice with colitis induced by DSS. To summarize the results, the total number of B220-positive B cells increased markedly after induction of colitis (data not shown). The increase in the number of TCR-αβ T cells was small. Among CD4+ TCR-αβ T cells, the up-regulation of CD69 molecules was pronounced (data not shown). Moreover, cell with the CD45RBhigh CD4+ TCR-αβ T cell phenotype decreased in number after the induction of colitis. Based on these results, we compared the phenotypes of LI–LPLs among three groups of colitis-induced Balb/c mice fed standard diet, Cladosiphon fucoidan or Fucus fucoidan. Total numbers of B220-positive B cells were lower in the mice fed Cladosiphon fucoidan than those fed standard diets or Fucus fucoidan (Table 1). The numbers of TCR-αβ T cells were comparable among the three groups. The expression of CD69 and CD45RB antigen was also comparable among the groups (data not shown). Cytokine proflies in TCR-β/CD28-stimulated LI–LPLs indicated that the production of proinflammatory cytokines such as IFN-γ and IL-6 was down-regulated in colitis-induced mice fed Cladosiphon fucoidan compared to those fed standard diet or Fucus fucoidan (Fig. 6). In contrast, the production of TGF-β was greater in mice fed Cladosiphon fucoidan than standard diet or Fucus fucoidan. Moreover, more IL-10 was produced in mice fed Cladosiphon fucoidan than standard diet. The analysis of IgG contents in the colonic mucosa in three experimental groups showed that the amounts of total IgG, IgG1 and IgG2a increased in colitis-induced Balb/c mice fed standard diet and Fucus fucoidan, but not those fed Cladosiphon fucoidan (Fig. 7).
Table 1.
The cell number of large intestinal lamina propria cells in colitis induced Balb/c mice fed standard diet or fucoidan-containing diets
| Diets | No. of TCR-β cells | No. of B220 cells |
|---|---|---|
| Control MF | (5·70 ± 0·64) × 106 | (9·77 ± 1·91) × 106 |
| Chadosiphon MF | (5·01 ± 0·93) × 106 | (5·38 ± 1·23) × 106a,b |
| Fucus MF | (4·33 ± 1·52) × 106 | (8·53 ± 1·29) × 106 |
Significantly different between control MF and Cladosiphon MF-treated mice (P < 0·01).
Significantly different between Cladosiphon MF and Fucus MF treated mice (P < 0·05).
Fig. 6.
Cytokine profiles in the culture supernatants of TCR-β/CD28-stimulated LI–LPLs in colitis-induced Balb/c mice fed standard diet or fucoidan-containing diets (n = 10, each group). LI–LPLs were stimulated with or without MoAbs TCR-β/CD28. After 48 h culture, amounts of each cytokine were analysed by sandwich ELISA. Similar results were obtained from two independent experiments. Data are mean ± s.d.
Fig. 7.
Immunoglobulin contents of colonic tissue in colitis-induced Balb/c mice fed normal diet, Cladosiphon fucoidan or Fucus fucoidan (n = 10, each group). The IgG, IgG1 and IgG2a contents in the colonic tissue were examined by sandwich ELISA using colonic tissue homogenates isolated from the three groups of mice. The total IgG contents of the mice fed Cladosiphon fucoidan was lower than that of the others and the differences were significant. The IgG2a content was significantly lower in the mice fed Cladosiphon fucoidan than Fucus fucoidan. Each plot shows individual data and bars represent mean values.
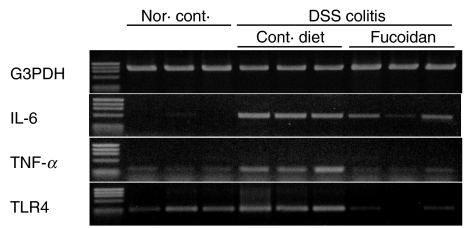
IL-6 mRNA analysis of colonic epithelial cells
The levels of IL-6 mRNA expression in the colonic epithelial cells that were isolated from colitis-uninduced Balb/c mice, or colitis-induced Balb/c mice fed either a normal diet or Cladosiphon fucoidan were determined by RT-PCR. The mRNA of IL-6 increased markedly in colonic epithelial cells of the mice fed standard diet on induction of colitis (Fig. 8). However, the expression of IL-6 mRNA was down-regulated in colonic epithelial cells of Balb/c mice fed fucoidan derived from C. o. Tokida. The mRNA expression of TNF-α and TLR-4 were also down-regulated in colonic epithelial cells of mice fed fucoidan derived from C. o. Tokida (Fig. 8).
Fig. 8.
RT-PCR analysis of cytokines or TLR-4 mRNA in colonic epithelial cells from colitis-uninduced Balb/c mice, or from colitis-induced Balb/c mice fed normal diet or Cladosiphon fucoidan. Total RNA was obtained from the colonic epithelial cell fraction of three experimental groups of mice. RT-PCR was performed with each specific primer for G3PDH, IL-6, TNF-α and TLR-4. After electrophoresis, the gel was stained with ethidium bromide and PCR products were visualized by trans-illuminator.
DISCUSSION
In the present study, we examined the inhibitory effects of fucoidan derived from Cladosiphon okamuranus Tokida, a traditional food in Japan, on the production of IL-6 in an LPS-stimulated murine colonic epithelial cell line and also examined the effect of these fucoidans on murine chronic experimental colitis in vivo. Moreover, we compared the effects with those of other fucoidans both in vitro and in vivo.
IL-6 is a 21–28-kDa glycoprotein that is secreted by monocytes, macrophages, lymphocytes and epithelial cells, including intestinal epithelial cells [19,39]. IL-6 is important for host defence and excessive production of IL-6 is thought to contribute to inflammatory disorders, including rheumatoid arthritis and inflammatory bowel disease [15,16]. The IL-6 receptor is present in a variety of cells such as monocytes, macrophages and epithelial cells, including intestinal epithelial cells [40]. It is known that high levels of IL-6 in serum are detected in patients with inflammatory bowel disease [40]. IL-6 has been shown to play important roles on the pathogenesis of murine Th-1-mediated colitis [12,13]. Intestinal epithelial cells and lamina propria lymphocytes are a major source of IL-6 in inflammatory bowel disease [16,19]. Sitaraman et al. showed that IL-6, released by the cultured intestinal epithelial cell line T84, induced an intracellular (Ca++) flux and degranulation in neutrophils [41]. These results suggested that secretion of IL-6 by intestinal epithelial cells plays a critical role in the pathogenesis of IBD and the prevention of this secretion may be useful in the treatment of inflammatory bowel disease. To test this possibility, we screened for an inhibitory effect of fucoidans isolated from various brown seaweeds on IL-6 secretion in LPS-stimulated CMT-93 and discovered that the fucoidans derived from C. o. Tokida and K. crassifolia had such an effect. However, fucoidans from other brown seaweeds such as S. horneri, F. vesiculosus and L. digitata had no effect. Moreover, we discovered the down-regulation of NF-κB signalling in LPS-stimulated CMT-93 cells by treatment of fucoidans derived from C. o. Tokida. We also discovered that the fucoidan derived from C. o. Tokida prevented the expression of IL-6 mRNA in colonic epithelial cells of colitis-induced Balb/c mice in vivo. Next, we examined in vivo the effect of the fucoidan-derived form C. o. Tokida on murine chronic colitis and compared it with that of the other fucoidans. Administration of Cladosiphon fucoidan to Balb/c mice with chronic colitis induced by DSS improved both the disease parameters such as disease activity index and the MPO activity in the colonic tissue that compared with these parameters in the mice fed standard diet or Fucus fucoidan. Moreover, the colon of the colitis-induced mice fed Cladsiphon fucoidan was the same length as that of normal mice. However, the colon length of mice fed standard diet or Fucus fucoidan was reduced due to severe intestinal inflammation. Analysis of the immunological parameters also showed that the production of proinflammatory cytokines in LI–LPLs decreased and that of T regulatory (Tr) and T helper type 3 (Th-3) cytokines, such as IL-10 and TGF-β increased in colitic mice fed Cladosiphon fucoidan compared with mice fed standard diet or Fucus fucoidan. These results are important. It is well known that proinflammatory cytokines such as IFN-γ and IL-6 are causative factors in the development of DSS-induced colitis [11]. On the other hand, Tr and Th-3 cytokines improved intestinal inflammation in various models of murine experimental colitis [42–44]. The mechanisms underlying the induction of Tr and Th-3 cytokines in colitis-induced mice fed Cladosiphon fucoidan should be investigated further.
The mechanisms of the inhibitory effect of Cladosiphon fucoidan on the secretion of IL-6 by LPS-stimulated CMT-93 cells are unknown. It is clear that LPS signals are important for the development of murine colitis [25]. Cario et al. reported the over-expression of TLR-4 in the colonic epithelial cells in a patient with inflammatory bowel disease [27]. In the present study, we observed the up-regulation of TLR-4 mRNA in colonic epithelial cells isolated from colitis-induced Balb/c mice and also the down-regulation in those fed Cladosiphon fucoidan. The mechanisms underlying the down-regulation of TLR-4 mRNA in the colonic epithelial cells of mice fed Cladosiphon fucoidan were unclear. It was reported that low-dose exposure to LPS induced LPS tolerance in cultured macrophages through a reduction in both the cell surface expression of TLR-4/MD2 complex and TLR-4 mRNA [45]. It is possible that fucoidan interacts with TLR-4 and then induces LPS tolerance. If so, there may be some molecular mimicry between LPS and fucoidan. Further analysis may clarify this point.
Another report suggested that intravenous injection of fucoidan derived from F. vesiculosus into mice with DSS-induced colitis prevented colito-genesis through the inhibition of selectin function and lymphocyte rolling [46]. However, we did not detect a clear positive effect of F. vesiculosus fucoidan on chronic colitis. Shibata et al. also reported that anti-ulcer effects of Cladosiphon fucoidan were higher than those of F. vesiculosus fucoidan [30]. The difference in effect on colito-genesis between Cladosiphon fucoidan and F. vesiculosus fucoidan may be due to structural differences. Nagaoka et al. has reported that the basic structure of the backbone chains of these fucoidans is the same [31]. However, there were differences in sulphate content and branched sugars. Cladosiphon fucoidan has a sulphate group for every 2 mol of fucose, and F. vesiculosus fucoidan 2–3 mol of fucose. Moreover, Cladosiphon fucoidan has one glucuronic acid residue for every 6 mol of fucose as branched chains, and F. vesiculosus fucoidan has fucose branches [31]. These structural differences may influence their anticolitogeneic activity. This possibility is currently being investigated.
Other possibilities for the improved effect of Cladosiphon fucoidan should be discussed. C. o. Tokida blocked the adhesion of H. pylori to a human gastric cell line [30]. It is well known that the numbers of mucosal adherent bacteria was increased in the intestinal mucosa IBD [47]. Fucoidan may inhibit the adhesion of commensal bacteria to the colonic epithelial cells. Moreover, the adhesion of fucoidan to epithelial cells may modulate epithelial barrier functions. Further analysis may clarify this point.
In conclusion, we discovered that fucoidan derived from C. o. Tokida inhibited the synthesis of IL-6 in both an LPS-stimulated colonic epithelial cell line in vitro and colonic epithelial cells in mice with DSS-induced colitis in vivo. Moreover, we observed a positive effect of Cladosiphon fucoidan on murine chronic colitis. Fucoidan derived from C. o. Takida may be useful as a dietary substance for preventing inflammatory bowel disease in humans.
Acknowledgments
The authors are grateful to Dr Y. Umesaki for the critical reading of the manuscript and to Miss N. Watanabe for her expert technical help. We are indebted to the staff people in the animal facility of Yakult Central Institute for their expertise in animal care.
References
- 1.Duchmann R, Kaiser I, Herhann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duchmann R, May E, Heike M, Neurath M, et al. T cell specifity and cross reactivity towards enterobacteria, Bacteroides, Bifidobacterium, and antigen from resident intestinal flora in humans. Gut. 1999;44:812–8. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchmann R, Schmitt E, Knolle P, et al. Tolerance towards resident intestinal flora in mice is aborogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–8. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 4.Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 5.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwaguchi-Miyashita M, Shimada S, Kurosu H, et al. An accessory role of TCR-γδ+ cells in the exacerbation of inflammatory bowel disease in TCRα mutant mice. Eur J Immunol. 2001;31:980–8. doi: 10.1002/1521-4141(200104)31:4<980::aid-immu980>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 9.Gross V, Andus T, Caesar I, et al. Evidence for continuous stimulation of IL-6 production in Crohn's disease. Gastroenterology. 1992;102:514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 10.Fuss IJ, Neurath M, Boirivant M, et al. Desperate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 11.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th-1 cell mediated murine colitis. J Immunol. 2000;164:4878–82. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- 13.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn's disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 14.Ohshima S, Saeki Y, Mima T, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–64. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 19.Hungness ES, Pritts TA, Luo GJ, et al. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1beta-stimulated human enterocytes. Shock. 2000;14:386–91. doi: 10.1097/00024382-200014030-00025. [DOI] [PubMed] [Google Scholar]
- 20.Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 21.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 22.Skidmore BJ, Morrison DC, Chiller JM, Weigle WO. Immunologic properties of bacteroides lipopolysaccharide (LPS). The unresponsiveness of C3H/HeJ mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975;142:1488–504. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keighley MR, Arabi Y, Dimock F, et al. Influence of inflammatory bowel disease on intestinal microflora. Gut. 1978;19:1099–104. doi: 10.1136/gut.19.12.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rath HC, Ikeda JS, Linde HJ, et al. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology. 1999;116:310–9. doi: 10.1016/s0016-5085(99)70127-7. [DOI] [PubMed] [Google Scholar]
- 25.Lange S, Delbro DS, Jennische E, Mattsby-Baltzer I. The role of the Lps gene in experimental ulcerative colitis in mice. APMIS. 1996;104:823–33. doi: 10.1111/j.1699-0463.1996.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 26.Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing toll-like receptors. J Immunol. 2000;164:966–72. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 27.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR-3) and TLR-4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahony MC, Oehninger S, Clark GF, Acosta AA. Fucoidan inhibits the zona pellucida-induced acrosome reaction in human spermatozoa. Contraception. 1991;44:657–65. doi: 10.1016/0010-7824(91)90085-t. [DOI] [PubMed] [Google Scholar]
- 29.Baba M, Snoeck R, Pauwels R, DeClerq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1994;32:1742–5. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata H, Kimura-Takagi I, Nagaoka M, et al. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobactor pylori to human gastric cells. J Nutr Sci Vitaminol. 1999;45:325–36. doi: 10.3177/jnsv.45.325. [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka M, Shibata H, Kimura-Takagi I, et al. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconj J. 1999;16:19–26. doi: 10.1023/a:1006945618657. [DOI] [PubMed] [Google Scholar]
- 32.Okuyasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 33.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 34.Matsumoto S, Watanabe N, Umesaki Y. Roles of chloroform-resistant variants in mouse models of experimental colitis. Microb Ecol Health Dis. 2000;12:102–8. [Google Scholar]
- 35.Matsumoto S, Nonno M, Watanabe N, Miyashita M, et al. Physiological roles of γδ T-cell receptor intraepithelial lymphocytes in cytoproliferation and differentiation of mouse intestinal epithelial cells. Immunology. 1999;97:18–25. doi: 10.1046/j.1365-2567.1999.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi T, Matsuguchi T, Tsuboi N, et al. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoclasts via toll-like receptors. J Immunol. 2001;166:3574–9. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- 37.Krawisz JE, Sharon P, Stenson F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 38.Matsumoto S, Watanabe N, Imaoka A, Okabe Y. Preventive effects of Bifidobacterium- and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse (SAM) P1/Yit strain mice. Digestion. 2001;64:92–9. doi: 10.1159/000048846. [DOI] [PubMed] [Google Scholar]
- 39.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;12:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 40.Hosokawa T, Kushigami K, Ando T, et al. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J Gastroenterol Hepatol. 1999;14:987–96. doi: 10.1046/j.1440-1746.1999.01989.x. [DOI] [PubMed] [Google Scholar]
- 41.Sitaraman SV, Merlin D, Wang L, et al. Neutrophil–epithelial crosstalk at the intestinal luminal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–9. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powrie F, Leach MW, Mauze S, et al. Inhibition of Th-1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhigh CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 43.Neurath MF, Kelsall BL, Presky DH, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β-mediated oral tolerance. J Exp Med. 1996;183:2605–16. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagenbaugh A, Sharma S, Dubinett SM, et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–10. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura F, Araki S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–9. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XZ, Liu Q, Thorlacius H. Inhibition of selectin function and leukocyte rolling protects against dextran sodium sulfate-induced colitis. Scand J Gastroenterol. 2001;36:270–5. doi: 10.1080/003655201750074555. [DOI] [PubMed] [Google Scholar]
- 47.Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]