Abstract
Human T lymphotrophic virus type-I (HTLV-I), a human retrovirus, infects CD4+ lymphocytes and is thought to modify their function and a possible association with pulmonary diseases has also been suggested. However, little is known about the influence of HTLV-I on diffuse pan-bronchiolitis (DPB), a chronic inflammatory lung disease with infiltration of lymphocytes and hyperplasia of the bronchus-associated lymphoid tissue. In this study, 35 DPB patients with and without HTLV-I infection were examined. HTLV-I positive DPB patients were likely to have a larger affected area with lower FEV1. The CD3+/CD25+ lymphocyte percentage was significantly higher in the BALF of HTLV-I positive patients than in negative patients. MIP-1α, IP-10 and levels in BALF were also significantly higher in HTLV-I positive patients than in negative patients. The levels of MCP-1 and IL-8 were not significantly different. In HTLV-I positive patients, the MIP-1α and IP-10 levels showed a significant positive correlation with the percentage of CD3+/CD25 lymphocytes. BALF cells of all HTLV-I positive DPB patients showed expression of p40tax mRNA. We suggest that HTLV-I infection may modify DPB pathogenesis via activation of T cells. We also found that the frequency of ATL development in HTLV-I positive DPB patients was significantly higher than in all HTLV-I positive patients (OR = 8·22, 95% CI = 2·61–25·9, P < 0·01). The levels of TGF-β in patients who developed ATL were significantly lower than in patients who did not develop ATL. Sensitivity and specificity were 80% and 85·7%, respectively (cut-off = 20 pg/ml). We also propose that these features should be taken into consideration in the treatment of DPB in HTLV-I infected individuals.
Keywords: ATL, IP-10, HTLV-I associated bronchopneumonopathy, MIP-1α, TGF-β
INTRODUCTION
Diffuse pan-bronchiolitis (DPB) is characterized by chronic inflammation of the respiratory bronchioles and infiltration of inflammatory cells [1]. The histological pattern in DPB is characterized by thickening of the walls of respiratory bronchioles, with infiltration of lymphocytes, plasma cells and histiocytes [1]. In addition, hyperplasia of the bronchus-associated lymphoid tissue is usually observed more frequently in DPB than in other respiratory diseases [2]. Furthermore, previous reports [3,4] demonstrated a marked increase of T cells, including in CD4 positive T cells, in BALF of DPB patients compared with healthy subjects. These observations suggest that T cells are also important cellular components of bronchial inflammation in patients with DPB.
On the other hand, human T lymphotrophic virus type-I (HTLV-I), a human retrovirus, is known to cause adult T cell leukaemia (ATL) [5] and preferentially infects CD4+ cells in vivo, modifying the immune system as well as T cell functions [6]. HTLV-I is also associated with several non-malignant disorders such as HTLV-I associated myelopathy (HAM) [7], HTLV-I associated uveitis (HAU) [8] and arthropathy [9]. In addition, several studies from HTLV-I pandemic areas have reported that both ATL patients [10,11] and non-ATL HTLV-I positive people develop frequent pulmonary complications [12–16]. Unlike patients with ATL, the pathogens associated with opportunistic infection are not found in the lungs of non-ATL patients [10,11,17,18]. Bronchoalveolar lavage fluid (BALF) findings in these patients are characterized by increased interleukin-2 receptor (IL-2R/CD25) positive T cells and marked elevation of soluble IL-2R [15]. Other studies showed up-regulated HTLV-I tax gene expression in lung tissue and a close correlation between HTLV-I mRNA expression and lymphocytosis in the lung of HTLV-I positive individuals [19,20]. The association of intrapulmonary chemokine production with the HTLV-I product protein has also been reported in transgenic mice [21]. Furthermore, we have demonstrated an association of HTLV-I in chronic pulmonary disorder, namely HTLV-I associated cryptogenic fobrosing alveolitis [22]. These results led us to hypothesize that HTLV-I might affect DPB.
In the present study, to evaluate the influence of HTLV-I on DPB, we have investigated 35 DPB patients (12 patients were positive for HTLV-I and 23 patients were negative) and have compared the clinical features and cytokine levels in the BALF. We also have found high frequency of ATL development in HTLV-I positive DPB patients during their clinical course.
MATERIALS AND METHODS
Subjects
This study was reviewed and approved by the Kagoshima University Faculty of Medicine Committee on Human Research. All cases of patients admitted to the Third Department of Internal Medicine (Kagoshima University Faculty of Medicine) and the Department of Respiratory Medicine (National Minami-Kyushu Hospital) between 1996 and 2003 were carefully reviewed retrospectively by specialists of respiratory medicine. In order to exclude the influence of genetic and environmental factors, we investigated patients from two separate hospitals (Kagoshima City and Aira-gun), wherein a previous study showed a significant difference in HTLV-I prevalence between these two areas [23]. A total of 5342 patients were admitted between 1996 and 2003. The mean age of the patients was 63·2 ± 21·9 years old. Of these, 856 patients (16·02% of all) were infected with HTLV-I.
Study protocol
The following are the steps undertaken during the review process; (1) three specialists of respiratory medicine carefully reviewed the records of all patients who were admitted to our departments; (2) clinical symptoms were carefully investigated and all previous chest radiographs were reviewed; and (3) a diagnosis of DPB was made to the patients who fulfilled all clinical criteria for DPB published by the Japanese Ministry of Health. The clinical criteria of DPB were as follows: (1) symptoms (chronic cough, sputum, and dyspnoea on exertion); (2) physical signs (coarse crackles, rhonchi or wheezes on chest auscultation); (3) chest radiographs (bilateral fine nodular shadows, mainly in the lower lung fields often with hyperinflation of the lungs): chest CTs are more helpful in the diagnosis showing small round areas of high attenuation with a centrilobular distribution, branching linear areas of high attenuation, and hypoattenuation in the peripheral lung; (4) pulmonary function tests and blood gas analysis (FEV1% predicted of < 70% and PaO2 < 80 mmHg); (5) elevated titres of cold haemagglutinin (× 64 or higher); and (7) history or coexistence of chronic parasinusitis. Ten patients (three HTLV-I positive and seven negative patients) were performed histological evaluation. In order to evaluate the influence of HTLV-I infection in DPB, we excluded the patients with systemic diseases such as collagen disease, HIV infection, vasculitis and malignant neoplasms as well as patients with immunological abnormalities that predispose them to opportunistic infection, such as diabetes mellitus and acute or chronic liver disease. For HTLV-I positive patients, we examined the counts of abnormal lymphocytes in the samples at the diagnosis point and excluded the ATL patients.
Determination of serum HTLV-I antibody
As a policy of our departments, serum samples were collected from all subjects and tested for anti-HTLV-I antibody as follows: first, anti-HTLV-I antibody was measured with an Eitest-ATL kit (Eisai Inc., Tokyo), and then the sera were re-examined by Western blot method using MT 2 cell lysate antigens [24] to confirm positivity.
Radiographic analysis
We also examined the affected pulmonary segments on high-resolution computed tomography (HRCT) to evaluate the distribution of the lesion in patients with DPB. The thickness of each slice was 1 cm and 30 slices were examined in total. The ratio of the affected area to the total lung field was judged subjectively by the visual scoring method [25]. Each slice was evaluated individually, and the right and left lungs were graded separately. A score of 0 was given if there were no abnormal shadows on the chest HRCT. If < 25% of the pulmonary parenchyma in a slice was considered to be abnormal, the score was 1; between 25% and 50%, the score was 2; between 50% and 75%, the score was 3; and > 75%, the score was 4. Therefore, the right and left lungs each received a maximum score of 4, and a maximum score of 8 was given per slice. All slices above the level of the diaphragm were assessed in each patient. For each subject, a visual score (VS) in percentage (total of scores for each slice over the total possible maximum score) was calculated. The radiographs of each subject were evaluated independently by two investigators (a pulmonologist and a radiologist) who have been blinded to the clinical data.
Bronchoalveolar lavage fluid (BALF) analysis
The lavage fluid was spun in a cytometer (KN-70, Kubota Ltd, Tokyo, Japan) at 44 g for 5 min and stained with May–Giemsa stain to identify cell populations. Five hundred cells, excluding epithelial cells, were identified per slide to establish differential cell counts, and the counts were expressed in percentages. The subtypes of lymphocytes were analysed by flow cytometry using CD4, CD8 and CD25 monoclonal antibodies (Becton Dickinson Co., Mountain View, CA, USA) and FITC-conjugated anti-CD3 monoclonal antibody. Also, lymphocytes were purified further using iso-osmotic Percoll gradient. At this stage, the purity of lymphocytes was higher than 95% by FACS analysis [26]. BALF fluids were stored at −20°C for further analysis.
Cytokines in BALF
To evaluate the immunological effect of HTLV-I on DPB, we measured the MIP-1α, IP-10, MCP-1, IL-8 and TGF-β concentrations in BALF using an enzyme-linked immunosorbent assay (ELISA) kit purchased from R&D Systems, Minneapolis, MN, USA.
Extraction of RNA and reverse transcription-polymerase chain reaction (RT-PCR)
A total of 5 × 106 cells of BALF cells in HTLV-I positive DPB patients were collected and total RNA was extracted by using Trizol Reagent® according to the manufacturer's protocol. Next, cDNA was synthesized using the sequence-specific primer oligonucleotide pX-9 and Superscript II Reverse Transcriptase (Gibco BRL) according to the manufacturer's protocol. For detection of p40tax mRNA, the cDNA was subjected to two-step amplification using nested primer pairs of the second splice junction site of tax/rex mRNA [19,27]. The reaction mixture including 1·0 µm outer primers (RPX-11: 5′-TAA TAG CCG CCA GTG GAA AG; and pX-9: TGA TCT GAT GCT CTG GAC AG) was then subjected to 20 cycles of amplification. Subsequently, a 1-µl aliquot of the first-step PCR reaction mixture was added to 49 µl of a PCR cocktail containing inner primers (RPX 3: 5′-ATC CCG TGG AGA CTC CTC AA; and RPX 4: AAC ACG TAG ACT GGG TAT CC) and subjected to another 20 cycles. In each cycle of the first and second PCRs, the mixture was subjected to denaturation at 94°C for 2 min, annealing at 60°C for 2 min and extension at 72°C for 2 min. The primers used for the detection of GAPDH were as follows: sense primer: 5′-ACC ACC ATG GAG AAG GCT GG, antisense primer: 5′-CTC AGT GTA GCC CAG GAT GC.
Western blot analysis
Continuous activation of JNK, one of the mitogen-activated protein (MAP) kinase pathway proteins, was reported to be associated with HTLV-I mediated tumorigenesis [28]. Therefore, we evaluated whether MAP kinase proteins were activated in BALF lymphocytes. One million lymphocytes were lysed on ice for 20 min in 1 ml of lysis buffer containing 50 mm HEPES, 150 mm NaCl, 1% Triton X-100, 10% glycerol and a cocktail of protease inhibitors (Roche, Indianapolis, IN, USA). Twenty µl of cell lysates were mixed directly with 20 µl of sample buffer and then boiled for 10 min. Eluted proteins were analysed on 10% polyacrylamide gels by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose membranes at 150 mA for 1 h using a semi-dry system. The membranes were incubated with rabbit IgGs that recognize phosphorylated or non-phosphorylated p44/42 (Erk), p38α and SPARK/JNK (Cell Signalling Technology, MA, USA), followed by sheep antirabbit IgG coupled with horseradish peroxidase (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA). Peroxidase activity was visualized by the Enhanced Chemiluminescence Detection System (Amersham).
Statistical analysis
The χ2 test was used to evaluate the prevalence of HTLV-I infection in DPB patients. The Mann–Whitney U-test was used to see the difference in laboratory findings, lymphocyte subpopulations, VS and the BALF results. A P-value below 0·05 was considered significant. Most values were expressed as mean ± standard deviation (s.d.).
RESULTS
Clinical features and course
Thirty-five patients were diagnosed as having DPB. The prevalence of HTLV-I infection in DPB patients (12/35, 34·3%) was significantly higher than compared to the prevalence in all patients (DPB patients were excluded) admitted to the departments between 1996 and 2003 [odds ratio (OR) = 2·76, 95% confidence intervals (95% CI) = 1·37–5·57, P < 0·01]. Five patients were suffering from HAM; two of them also had HAU.
Table 1 shows the clinical data. The affected areas were significantly wider; %FEV1 was significantly lower in HTLV-I positive than in negative patients. The white blood cell count, C-reactive protein levels, mean age, smoking index, male/female ratio and laboratory findings were not significantly different between two groups. There was no significant difference of infectious pathogens in the BALF between HTLV-I positive and negative patients.
Table 1.
Comparison of DPB between HTLV-I positive patients and negative patients
| HTLV-I positive patients (n = 12) | HTLV-I negative patients (n = 23) | P-value | |
|---|---|---|---|
| White blood cells (/µl) | 8923 ± 2344 | 9211 ± 2564 | n.s. |
| C-reactive protein (mg/ml) | 4·33 ± 2·98 | 3·99 ± 3·01 | n.s. |
| HCV ab. (+) | 1/12 | 2/23 | n.s. |
| HBs ag. (+) | 1/12 | 2/23 | n.s. |
| CMV ab (+) | 0/12 | 0/23 | n.s. |
| Adenovirus ab. (+) | 0/12 | 0/23 | n.s. |
| Mycoplasma ab. (+) | 0/12 | 0/23 | n.s. |
| Pulmonary function tests | |||
| %VC (%) | 73·4 ± 19·8 | 75·3 ± 20·2 | n.s. |
| %FEV1 (%) | 60·8 ± 14·2 | 70·1 ± 13·3 | P < 0·05 |
| Radiographic appearance | |||
| Visual score | 30·4 ± 11·8 | 22·3 ± 13·8 | P < 0·05 |
| Ratio of upper lobe involvement | 10/12 | 8/23 | P < 0·05 |
| BALF analysis | |||
| Total cell count (×105/µl) | 32·2 ± 18·8 | 30·1 ± 17·9 | n.s. |
| Macrophage (%) | 20·3 ± 4·66 | 19·6 ± 4·41 | n.s. |
| Lymphocyte (%) | 16·9 ± 4·44 | 16·1 ± 4·38 | n.s. |
| Neutrophile (%) | 68·1 ± 15·1 | 67·6 ± 14·8 | n.s. |
| CD4+ lymphocytes(%) | 37·8 ± 12·1 | 32·8 ± 14·9 | n.s. |
| CD8+ lymphocytes (%) | 33·1 ± 18·1 | 40·5 ± 14·7 | n.s. |
| CD4+/CD8+ | 1·12 ± 1·11 | 0·83 ± 1·22 | n.s. |
| CD3+ lymphocytes (%) | 89·3 ± 13·3 | 86·9 ± 14·5 | n.s. |
| CD3+/CD25+ (%) | 37·6 ± 14·4 | 20·9 ± 15·6 | P < 0·05 |
| CD4+/CD25+ (%) | 10·2 ± 13·4 | 9·8 ± 13·2 | n.s. |
| CD8+/CD25+ (%) | 27·1 ± 13·2 | 10·8 ± 12·6 | P < 0·05 |
| Infectious pathogens in BALF | |||
| P. aeruginosa | 10/12 | 19/23 | n.s. |
| H. influenzae | 2/12 | 4/23 | n.s. |
| C. albicans | 2/12 | 3/23 | n.s. |
| Mycobacteriums | 0/12 | 0/23 | n.s. |
| Others | 1/12 | 0/23 | n.s. |
CMV: cytomegarovirus, ab. antibody, P. aeruginosa: Pseudomonus aeruginosa. H. influenzae: Haemophillus influenzae, C. albicans: Candida albicans. Others in HTLV-I positive patient: Cryptococcus neoformans.
BALF analysis
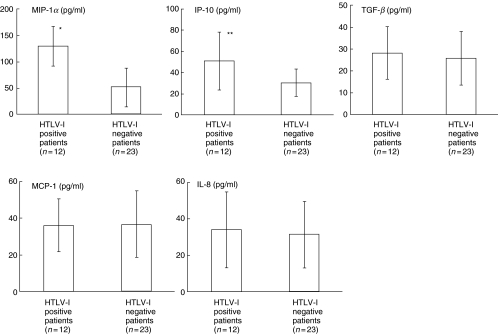
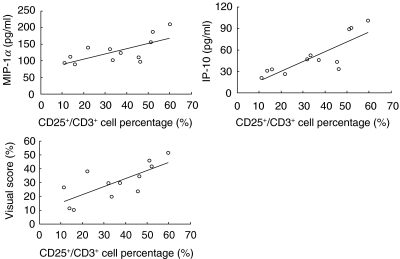
There was no significant difference in lymphocyte numbers and CD3+ lymphocyte percentage; however, CD3+/CD25+ percentage was significantly higher in HTLV-I positive patients than in negative patients (Table 1). MIP-1α and IP-10 levels were significantly higher in HTLV-I positive patients than in negative patients. The levels of TGF-β, MCP-1 and IL-8 were not significantly different (Fig. 1). In HTLV-I positive patients, the BALF MIP-1α and IP-10 levels showed significant positive correlation with the CD3+/CD25+ percentage in BALF. VS in HTLV-I positive patients were significantly higher than in negative patients (HTLV-I positive patients = 31·2 ± 12·7, negative patients = 18·9 ± 15·3, P < 0·05). There was no significant correlation between BALF cytokine levels and CD3+/CD25+ lymphocyte percentages in HTLV-I negative DPB patients. In HTLV-I positive DPB patients, VS showed significant positive correlation with the percentage of CD3+/CD25+ lymphocytes in BALF (Fig. 2), while no significant correlation in HTLV-I negative DPB patients were observed. In addition, CD8+/CD25+ lymphocyte percentages in HTLV-I positive patients than in negative patients while CD4+/CD25+ lymphocyte percentages showed no significant difference between two groups. CD8+/CD25+ lymphocyte percentages also showed a positive correlation with MIP-1α (r = 0·601, P < 0·05), IP-10 (r = 0·701, P < 0·01) and VS (r = 0·665, P < 0·01).
Fig. 1.
Comparison of cytokine levels in BALF between HTLV-I positive and negative DPB patients. MIP-1α and IP-10 levels were significantly higher in HTLV-I positive patients than in negative patients. The levels of TGF-β, MCP-1 and IL-8 were not significantly different. Bars represent standard deviations. *P < 0·01, **P < 0·05.
Fig. 2.
Correlation between percentage of CD3+/CD25+ lymphocytes and cytokine levels in BALF of HTLV-I positive DPB patients. The levels of MIP-1α (r = 0·549, P < 0·05), IP-10 (r = 0·698, P < 0·01) and VS (r = 0·654, P < 0·01) showed significant positive correlations with the percentage of CD3+/CD25+ lymphocytes.
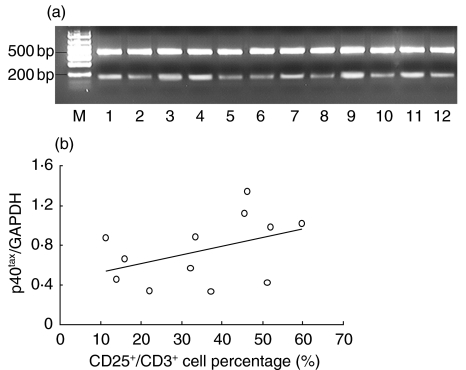
Detection of p40tax mRNA in BALF cells of HTLV-1 positive patients
To evaluate the existence of HTLV-I in lung, we amplified HTLV-I p40tax mRNA by nested RT-PCR using BALF cells. In all positive DPB patients, positive bands of p40tax mRNA were detected at the second PCR (Fig. 3). The obtained bands of amplified DNA were quantified using the NIH image analysis software and expressed relative to that of GAPDH. As shown in Fig. 3, the percentage of CD3+/CD25+ lymphocytes increased with higher expression of p40tax mRNA in BALF of HTLV-I positive patients. However, the correlation between the two parameters was not significant.
Fig. 3.
(a) P40tax mRNA expression in BALF cells of HTLV-I positive DPB patients. The lower band (145 bp) shows p40tax and the upper band (508 bp) represents GAPDH. All patients’ BALF cells expressed p40tax mRNA. M: marker. (b) Correlation of p40tax mRNA expression level and CD3+/CD25+ lymphocytes in BALF. The percentage of CD3+/CD25+ lymphocytes increased with higher expression of p40tax mRNA in BALF of HTLV-I positive patients. However, the correlation between the two parameters was not significant (r = 0·498, P = 0·059).
High incidence of ATL development in HTLV-I positive DPB patients
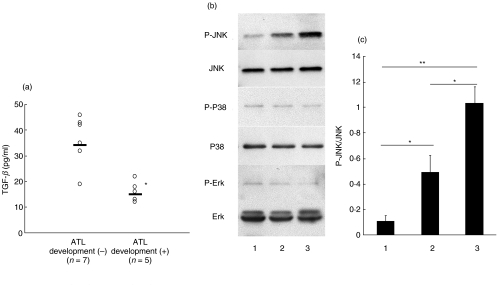
The frequency of ATL development in HTLV-I positive DPB patients (5/12, 41·7%, mean period from DPB diagnosis to ATL development = 35·8 ± 22·5 months) were significantly higher than in all HTLV-I positive patients (60/856, 7·01%, OR = 8·22, 95% CI = 2·61–25·9, P < 0·01). All patients were treated with macrolides. Four HTLV-I positive patients and seven HTLV-I negative patients developed pneumonia during their clinical course as investigated in this study. The levels of TGF-β in patients who developed ATL are significantly lower than in patients who did not develop ATL. Sensitivity and specificity were 80% and 85·7%, respectively (cut-off = 20 pg/ml). HTLV-I oncoprotein Tax represses TGF-β signalling in human T cells via JNK activation [29] and this activation is considered as a potential mechanism of HTLV-I leukaemogenesis [28]. Therefore, we have examined whether JNK is activated in BALF lymphocytes and compared TGF-β levels in BALF. As shown in Fig. 4, JNK was activated in BALF lymphocytes from HTLV-I positive DPB patients. There was no significant difference of chemokine levels measured in this study, VS and CD3+/CD25+ lymphocyte percentages between the patients who developed ATL and the patients who did not develop ATL (data not shown).
Fig. 4.
(a) Comparison TGF-β levels in BALF between HTLV-I positive DPB patients who developed ATL and patients who did not develop ATL. TGF-β levels were significantly higher in HTLV-I positive DPB patients who developed ATL than those who did not develop ATL (*P < 0·01). (b) Comparison of MAP kinase protein phosphorylations. The JNK phosphorylation level of HTLV-I positive DPB patients who developed ATL (lane 3) was higher than those who did not develop ATL (lane 2) and HTLV-I negative DPB patients (lane 1). Data show the representative data of three different patients in each group. (c) Results of densitometry analysis of P-JNK/JNK. The JNK phosphorylation level of HTLV-I positive DPB patients who developed ATL (lane 3) was significantly higher than those who did not develop ATL (lane 2) and HTLV-I negative DPB patients (lane 1) (*P < 0·01, **P < 0·001, Bonferroni/Dunn with one-way factorial anova). Data are shown with mean ± standard deviation, n = 3 in each group).
DISCUSSION
In this study, we evaluated clinical and immunological HTLV-I infection influence on DPB. There have been several reports describing possible association of HTLV-I infection and pulmonary disorders [12,16,17,30]. Accordingly, a new clinical entity, namely HTLV-I associated fibrosing alveolitis (HAFA) [22], has been suggested. However, to our knowledge there has been no report to suggest a high prevalence of HTLV-I infection in patients with DPB. Although fibrosing alveolitis and DPB are different disease, the immunological aspects seen in HAFA and HTLV-I positive DPB patients are very similar. In both conditions, activation of T lymphocytes seemed to affect their clinical features.
In this report, we showed that HTLV-I positive DPB patients were likely to have wide lesions. Of course, we cannot deny the possibility of coincidence; however, we think that DPB patients with HTLV-I infection are likely to have incurred more damage to the lung parenchyma because of the following reasons: (1) the percentage of activated T cells in BALF was significantly higher in HTLV-I positive patients than in negative patients; (2) BALF chemokine levels were correlated with activated T cell percentages only in HTLV-I positive DPB patients; and (3) the histological pattern in DPB is characterized by infiltration of lymphocytes [1] and hyperplasia of the bronchus-associated lymphoid tissue is usually observed more frequently in DPB than in other respiratory diseases [2].
Similar to the results in HAFA [22], our study showed increased MIP-1α and IP-10 BALF levels that correlated with activated T cell percentages (CD3+/CD25+ lymphocytes) in HTLV-I positive patients. MIP-1α is known to regulate the trafficking and activation state of select subgroups of inflammatory cells, including lymphocytes [31]. It modulates leukocyte adhesion to the endothelium and contributes to leukocyte recruitment into the lungs [32]. In 2001, Kadota et al. suggested its contribution to the pathogenesis of DPB [33]. On the other hand, IP-10 is chemotactic for activated T cells and plays an important role in recruiting activated effector T cells into tissue inflammation sites. T cells can be the cellular source of MIP-1α[34] and IP-10 productions [35]. An in vivo study showed that p40tax protein of HTLV-I could induce chemokine production in the lung including MIP-1α and IP-10 [21]. In addition, CD8+/CD25+ lymphocyte percentage was significantly higher in HTLV-I positive DPB patients than in negative patients. HTLV-I can infect CD8+ lymphocytes [36] and CD8+ lymphocytes play an important role in the pathogenesis of DPB [4]. Therefore, we think it is possible that HTLV-I infected lymphocytes may contribute to the differential levels of chemokines and cytokines in BALF between HTLV-I positive and negative DPB patients.
In our study, there was no significant difference of MCP-1 and IL-8 levels between HTLV-I positive and negative patients despite the significant difference of VS and pulmonary functions. MCP-1 and IL-8 have been reported to be elevated in bronchoalveolar lavage fluids from patients with DPB and to be decreased after appropriate therapy, suggesting roles in airway inflammatory processes [37,38]. MCP-1 is a major chemoattractant for monocytes in inflammation and immune responses [39]. On the other hand, IL-8 is a potent chemoattractant for neutrophils and plays a pivotal role in DPB by recruiting and activating neutrophils [40]. Macrophages or epithelial cells, not lymphocytes that were the targets of HTLV-I infection, were the main cellular source of MCP-1 and IL-8 productions in DPB [38,41]. Therefore we think that only lymphocytes, not monocytes and neutrophils, are responsible for the difference between HTLV-I positive and negative patients.
To our knowledge, this is the first report that showed a high incidence of ATL development in HTLV-I positive patients compared with all HTLV-I positive patients and the clinical value of BALF TGF-β measurement to estimate the ATL development. The prognosis of ATL is poor and there is a long latency period before the onset of ATL, indicating the multistep mechanisms of leukaemogenesis [42]. The cumulative incidence of ATL among HTLV-I carriers in Japan is estimated at 2·5% (3–5% in males, 1–2% in females) [43]. Therefore, a valuable clinical marker that can estimate the likelihood of ATL development in HTLV-I carriers is needed [42]. In our study, the levels of TGF-β in patients who developed ATL are significantly lower than in patients who did not develop ATL and the sensitivity and specificity were 80% and 85·7%, respectively (cut-off = 20 pg/ml). Therefore, in the case of DPB, we propose that measurement of TGF-β in BALF can be one of the estimating factors of ATL development.
We also have examined whether JNK is activated in BALF lymphocytes and compared TGF-β levels in BALF, because HTLV-I oncoprotein Tax represses TGF-β signalling in human T cells via JNK activation [29] and this activation is considered as a potential mechanism of HTLV-I leukaemogenesis [28]. Similar to previous reports, our results showed that JNK, not other MAP kinases such as P38 or p44/42, was activated in BALF lymphocytes from HTLV-I positive DPB patients. In addition, activation of JNK was higher in HTLV-I positive DPB patients who developed ATL than HTLV-I positive patients who did not develop ATL and HTLV-I negative DPB patients. It is possible that HTLV-I leukaemogesis is likely to occur in HTLV-I positive DPB patients with lower BALF TGF-β levels by the decreased TGF-β signalling. This study must be repeated with a second cohort to confirm these findings, because of the potential problems encountered when undertaking multiple comparisons. Further studies addressing these points are necessary to clarify the influence of HTLV-I in DPB.
Acknowledgments
We thank Mrs Rumi Matsuyama for her excellent technical help with this study.
References
- 1.Homma H, Yamanaka A, Tanimoto S, et al. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983;83:63–9. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- 2.Sato A, Chida K, Iwata M, Hayakawa H. Study of bronchus-associated lymphoid tissue in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1992;146:473–8. doi: 10.1164/ajrccm/146.2.473. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami K, Kadota J, Iida K, et al. Phenotypic characterization of T cells in bronchoalveolar lavage fluid (BALF) and peripheral blood of patients with diffuse panbronchiolitis; the importance of cytotoxic T cells. Clin Exp Immunol. 1997;107:410–6. doi: 10.1111/j.1365-2249.1997.259-ce1139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukae H, Kadota J, Kohno S, et al. Increase in activated CD8+ cells in bronchoalveolar lavage fluid in patients with diffuse panbronchiolitis. Am J Respir Crit Care Med. 1995;152:613–8. doi: 10.1164/ajrccm.152.2.7633715. [DOI] [PubMed] [Google Scholar]
- 5.Blayney DW, Blattner WA, Jaffe ES, Gallo RC. Retroviruses in human leukemia. Hematol Oncol. 1983;1:193–204. doi: 10.1002/hon.2900010302. [DOI] [PubMed] [Google Scholar]
- 6.Mortreux F, Gabet AS, Wattel E. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 7.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 8.Nakao K, Ohba N, Matsumoto M. Noninfectious anterior uveitis in patients infected with human T lymphotropic virus type I. Jpn J Ophthalmol. 1989;33:472–81. [PubMed] [Google Scholar]
- 9.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;1:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Yokota T, Mashita R, Tamura S. Pulmonary manifestations in adult T cell leukemia at the time of diagnosis. Respiration. 1993;60:115–9. doi: 10.1159/000196184. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka R, Yamaguchi K, Yoshinaga T, Takatsuki K. Pulmonary complications in patients with adult T cell leukemia. Cancer. 1985;55:2491–4. doi: 10.1002/1097-0142(19850515)55:10<2491::aid-cncr2820551030>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama I, Thihara J, Sakashita R, et al. HTLV-I associated bronchopneumonopathy − a new clinical entity? Am Rev Respir Dis. 1988;137:46. [Google Scholar]
- 13.Sugimoto M, Nakashima H, Watanabe S, et al. T lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet. 1987;2:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto M, Nakashima H, Kawano O, Ando M, Araki S. Bronchoalveolar T lymphocytosis in HTLV-1-associated myelopathy. Chest. 1989;95:708. doi: 10.1378/chest.95.3.708a. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto M, Nakashima H, Matsumoto M, Uyama E, Ando M, Araki S. Pulmonary involvement in patients with HTLV-I-associated myelopathy: increased soluble IL-2 receptors in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1989;139:1329–35. doi: 10.1164/ajrccm/139.6.1329. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto M, Mita S, Tokunaga M, et al. Pulmonary involvement in human T cell lymphotropic virus type-I uveitis: T lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur Respir J. 1993;6:938–43. [PubMed] [Google Scholar]
- 17.Seki M, Higashiyama Y, Kadota J, et al. Elevated levels of soluble adhesion molecules in sera and BAL fluid of individuals infected with human T cell lymphotropic virus type 1. Chest. 2000;118:1754–61. doi: 10.1378/chest.118.6.1754. [DOI] [PubMed] [Google Scholar]
- 18.Tateishi U, Nishihara H, Miyasaka K. HTLV-1-associated bronchopneumonopathy (HAB): CT pathological correlation. Clin Radiol. 2001;56:664–6. doi: 10.1053/crad.2001.0677. [DOI] [PubMed] [Google Scholar]
- 19.Higashiyama Y, Katamine S, Kohno S, et al. Expression of human T lymphotropic virus type 1 (HTLV-1) tax/rex gene in fresh bronchoalveolar lavage cells of HTLV-1-infected individuals. Clin Exp Immunol. 1994;96:193–201. doi: 10.1111/j.1365-2249.1994.tb06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki M, Higashiyama Y, Mizokami A, et al. Up-regulation of human T lymphotropic virus type 1 (HTLV-1) tax/rex mRNA in infected lung tissues. Clin Exp Immunol. 2000;120:488–98. doi: 10.1046/j.1365-2249.2000.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazato A, Kawakami K, Iwakura Y, Saito A. Chemokine synthesis and cellular inflammatory changes in lungs of mice bearing p40tax of human T lymphotropic virus type 1. Clin Exp Immunol. 2000;120:113–24. doi: 10.1046/j.1365-2249.2000.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama W, Kawabata M, Mizoguchi A, Iwami F, Wakimoto J, Osame M. Influence of human T lymphotrophic virus type I on cryptogenic fibrosing alveolitis − HTLV-I associated fibrosing alveolitis: proposal of a new clinical entity. Clin Exp Immunol. 2003;133:397–403. doi: 10.1046/j.1365-2249.2003.02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki T, Nakagawa M, Osame M. Human T lymphotrophic virus type-I (HTLV-I) seroprevalence in Kagoshima Prefecture. Med J Kagoshima Univ. 1993;45:135–41. [Google Scholar]
- 24.Miyoshi I, Kubonishi I, Yoshimoto S, et al. Type C virus particles in a cord T cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–1. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama W, Mizoguchi A, Iwami F, et al. Clinical investigation of pulmonary Mycobacterium avium complex infection in human T lymphotrophic virus type I carriers. Thorax. 2000;55:388–92. doi: 10.1136/thorax.55.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu LL, McVicar DW, Ben Baruch A, et al. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25:2612–7. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Shimoyama M, Tobinai K, et al. Detection of mRNA for the tax1/rex1 gene of human T cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:5620–4. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Heidenreich O, Kitajima I, et al. Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene. 1996;13:135–42. [PubMed] [Google Scholar]
- 29.Arnulf B, Villemain A, Nicot C, et al. Human T cell lymphotropic virus oncoprotein Tax represses TGF-beta 1 signaling in human T cells via c-Jun activation: a potential mechanism of HTLV-I leukemogenesis. Blood. 2002;100:4129–38. doi: 10.1182/blood-2001-12-0372. [DOI] [PubMed] [Google Scholar]
- 30.Matsuyama W, Kubota R, Hamasaki T, et al. Enhanced inhibition of lymphocyte activation by Mycobacterium avium complex in human T lymphotrophic virus type I carriers. Thorax. 2001;56:394–7. doi: 10.1136/thorax.56.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 32.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 33.Kadota J, Mukae H, Tomono K, Kohno S. High concentrations of beta-chemokines in BAL fluid of patients with diffuse panbronchiolitis. Chest. 2001;120:602–7. doi: 10.1378/chest.120.2.602. [DOI] [PubMed] [Google Scholar]
- 34.Ward SG, Westwick J. Chemokines: understanding their role in T lymphocyte biology. Biochem J. 1998;333:457–70. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gattass CR, King LB, Luster AD, Ashwell JD. Constitutive expression of interferon gamma-inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179:1373–8. doi: 10.1084/jem.179.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanon E, Stinchcombe JC, Saito M, et al. Fratricide among CD8(+) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity. 2000;13:657–64. doi: 10.1016/s1074-7613(00)00065-0. [DOI] [PubMed] [Google Scholar]
- 37.Katoh S, Matsubara Y, Taniguchi H, et al. Characterization of CD44 expressed on alveolar macrophages in patients with diffuse panbronchiolitis. Clin Exp Immunol. 2001;126:545–50. doi: 10.1046/j.1365-2249.2001.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, Suga M, Matsukawa A, et al. Erythromycin attenuates an experimental model of chronic bronchiolitis via augmenting monocyte chemoattractant protein-1. Eur Respir J. 2001;17:360–7. doi: 10.1183/09031936.01.17303600. [DOI] [PubMed] [Google Scholar]
- 39.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 40.Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156:266–71. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 41.Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996;63:42–8. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]
- 42.Yasunaga J, Matsuoka M. Leukemogenesis of adult T cell leukemia. Int J Hematol. 2003;78:312–20. doi: 10.1007/BF02983555. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi K, Watanabe T. Human T lymphotropic virus type-I and adult T cell leukemia in Japan. Int J Hematol. 2002;76(Suppl. 2):240–5. doi: 10.1007/BF03165123. [DOI] [PubMed] [Google Scholar]