Abstract
To clarify immunological differences among patients with Graves’ disease (GD) and Hashimoto's disease (HD) at various levels of severity, we examined the expression of the CD154 molecules on peripheral T cells, which regulate B cell activation, B cell differentiation, and T-cell survival. We found decreases in the intensities of CD154 on peripheral CD4+ cells from euthyroid patients with GD and HD, but we did not find any differences between patients with different disease severities. CD8+ cells did not express CD154 molecules. Thus, CD154 expression on CD4+ cells may be related to the pathogenesis of the autoimmune thyroid diseases, not to the disease severity.
Keywords: disease severity, antigen presenting cell, CD40 ligand, Fas ligand
INTRODUCTION
Patients with Graves’ disease (GD) or Hashimoto's disease (HD) show immunological changes in both the thyroid and the periphery [1–3]. Some patients with GD enter remission through medical treatment; others do not. Some patients with HD develop hypothyroidism; most do not [4]. It is thought that immune factors determine the severity of both of these diseases. We have shown previously that activated cytotoxic T cells, CD30 molecules on T cells and autoantibodies are involved in the disease severity of HD [5,6], and the IgG3-secreting cells are related to the intractability of GD to antithyroid drug therapy [7].
CD154 (CD40 ligand) is a 30–33-kD surface molecule of activation-induced CD4+ T cells. CD154 molecules interact with CD40 molecules expressed on B and T cells [8]. This CD40–CD154 interaction is known to play a key role in B cell activation and differentiation [8,9]. It also appears to be a potent inhibitor of T cell apoptosis [8]. Autoimmune diseases such as thyroiditis, arthritis, lupus nephritis and experimental allergic encephalomyelitis in mice are inhibited by anti-CD154 antibody [10–13]. CD154+ T cells are also reported to play important roles in the autoimmune response [8]. In this study therefore, we examined expression of CD154 by flow cytometry on both peripheral CD4+ and CD8+ T cells in patients with autoimmune thyroid disease (AITD), and investigated the immunological differences among patients with AITD with various levels of severities.
MATERIALS AND METHODS
Subjects
Four groups of patients with GD, two groups with HD, and one group of healthy volunteers (control subjects) recruited by advert were included in the study. The first GD group comprised 4 untreated, thyrotoxic patients (thyrotoxic GD group; 3 Female, 1 Male). The second and third GD groups comprised euthyroid patients who had been under treatment with antithyroid drugs for more than 5 years. One of these two treated groups comprised 26 GD patients who were positive for anti-TSH receptor antibodies (TRAb) (seriously intractable GD group; 24 F, 2 M), and other comprised 14 GD patients who were negative for TRAb (intractable GD group; 13 F, 1 M). The fourth GD group comprised 25 patients in remission who had maintained a euthyroid state and were negative for TRAb for more than 2 years without any treatment (GD in remission group; 25 F). One of the two HD groups comprised 16 untreated, euthyroid patients (mild HD group; 15 F, 1 M), and the other HD group comprised 32 euthyroid patients undergoing thyroxine replacement therapy for hypothyroidism (severe HD group; 25 F, 7 M). All patients in the GD and HD groups were positive for antithyroid microsomal antibodies (MCPA). All patients except those with thyrotoxic GD presented with normal serum concentrations of free thyroxine (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) (Table 1). All GD patients had comprised a clinical history of thyrotoxicosis and elevated levels of TRAb. The control group comprised 16 healthy women, mean age 44·4 ± 8·2 years, who were euthyroid and negative for thyroid-specific autoantibodies. Mean age in the GD groups, the mild HD group (Table 1), and the control group did not differ significantly. Mean ages in the severe HD groups was significantly greater than that in the other groups (Table 1). Venous blood was obtained from all subjects between 1000h and 1200h. Informed consent for the study was obtained from all patients. Protocols were approved by our local Ethical Committee.
Table 1.
Clinical characteristics of each AITD study group
| n | Age (years) | Free T4 (ng/dl) | Free T3 (pg/ml) | TSH (mU/ml) | TRAb (%) | |
|---|---|---|---|---|---|---|
| Normal range | 0·97–1·79 | 2·47–4·34 | 0·34–3·5 | <15 | ||
| Graves’ disease groups | ||||||
| Thyrotoxic | ||||||
| Untreated | 4 | 46·3 ± 8·2 | 2·87 ± 0·79** | 5·75 ± 3·72** | – | 44·3 ± 27·4† |
| Euthyroid | ||||||
| Seriously intractable | 26 | 44·1 ± 15·4 | 1·45 ± 0·68 | 3·51 ± 1·53 | 1·54 ± 2·04 | 32·7 ± 27·9† |
| Intractable | 14 | 39·8 ± 10·8 | 1·14 ± 0·23 | 2·97 ± 0·29 | 2·75 ± 2·42 | 8·3 ± 5·9 |
| In remission | 25 | 45·5 ± 13·4 | 1·20 ± 0·19 | 3·03 ± 0·35 | 1·61 ± 0·99 | 2·5 ± 4·4 |
| Hashimoto's disease groups | ||||||
| Euthyroid | ||||||
| Severe | 32 | 58·0 ± 8·9* | 1·50 ± 0·37 | 3·15 ± 0·44 | 1·44 ± 2·74 | ND |
| Mild | 16 | 49·8 ± 13·1 | 1·00 ± 0·24 | 3·11 ± 0·31 | 2·57 ± 1·67 | ND |
AITD, Autoimmune thyroid disease; - Not detected; ND, Not done;
P < 0·05 vs. other groups;
P < 0·01 vs. other groups;
P < 0·01 vs. intractable GD, GD in remission, and HD groups
Monoclonal antibodies
We used fluorescein-isothiocyanate (FITC)-conjugated anti-CD8 (Becton Dickinson, Mountain View, CA, USA), phycoerythrin (PE)-conjugated anti-CD154 (Coulter Co., Marseille Cedex, France), and Peridinin Chlorophyll Protein (PerCP)-conjugated anti-CD4 monoclonal antibodies (Becton Dickinson).
Lymphocyte subsets
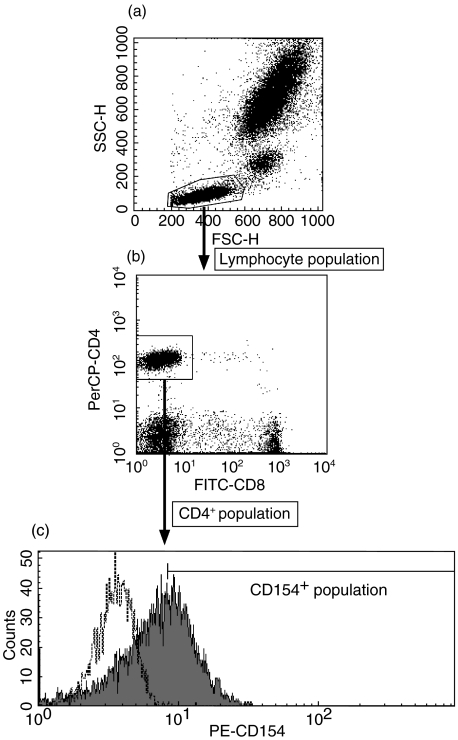
Samples EDTA-treated whole blood 100 µl were incubated for 30 min at 4 degree C with 20 µl of each antibody. The samples were then haemolysed and fixed with lysing reagent (FACS Lysing Solution; Becton Dickinson). They were then washed once and subjected to three-colour flow cytometry in FACSCalibur (Becton Dickinson) to determine the percentages of lymphocyte subsets. The typical profile of flow cytometry in analysing CD4+CD154+ T cells is shown in Fig. 1. At first we gated lymphocyte population on forward- and side-scatter plot (Fig. 1a), then we gated CD4+ population on FITC-CD8 and PerCP-CD4 fluorescence plot (Fig. 1b). Finally, we measured the proportion and MFI of CD154+ cells, using the isotype-control to determine cut-off (Fig. 1c). Mean fluorescence intensities (MFIs) of PE-CD154 were standardized by the QuantiBRITE PE (Becton Dickinson) and shown as the number of PE molecules per cell.
Fig. 1.
The typical profile of flow cytometry in analysing CD4+CD154+ T cells. (a) Forward- and side-scatter dot plot (b) FITC-CD8 and PerCP-CD4 fluorescence dot plot, and (c) PE-CD154 fluorescence histogram in control sample are shown. Examined sample (——) and isotype-matched IgG control (——) are plotted in (c). Fluorescence is shown as value before standardization.
Thyroid function test and thyroid autoantibodies
Commercially available kits were used to measure serum concentrations of FT4 (Eiken Immunochemical Laboratory, Tokyo, Japan), FT3 (Japan Kodak Diagnostic Co., Tokyo, Japan), and TSH (Daiichi Radioisotope Laboratories, Tokyo, Japan). The serum titres of antithyroglobulin antibodies (TGPA) and MCPA were determined with commercially available passive agglutination kits (Fuji Rebio, Tokyo, Japan). Serum levels of TRAb were determined by radioreceptor assay with a commercial kit (Cosmic Co., Tokyo, Japan).
Statistical analysis
Data were analysed by Mann–Whitney U-test. Correlation between the autoantibody titres and the expression of surface antigens was established by Spearman's correlation coefficient by ranks test. Correlation between the serum concentration of FT4 or FT3 and the expression of surface antigens was also established by Spearman's correlation coefficient by ranks test. Probability values less than 0·05 were considered significant.
RESULTS
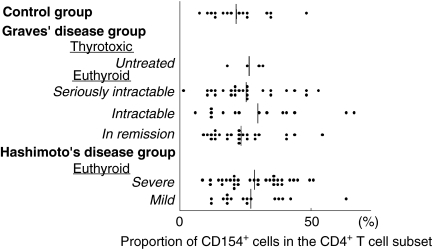
In the control group, there was no correlation between CD154 expression and age. The proportions of CD154+ cells in CD4+ cells did not differ significantly between the two HD groups or among the four GD groups (Fig. 2). Neither did the proportion of CD154+ cells in CD4+ cells in the control group differ from that in any of the other study groups. The MFIs of CD154 molecules on CD4+ cells, which reflects the density of the CD154 molecules on the cells, was significantly decreased in CD4+ cells in all euthyroid GD groups and in all HD groups in comparison to that of the controls group (Fig. 3). CD154 molecules were not expressed on CD8+ cells from AITD patients or control subjects.
Fig. 2.
The proportions of CD154+ cells in the CD4+ T cell subset from patients with autoimmune thyroid diseases of various levels of severity and from normal subjects. Vertical bars indicate mean values.
Fig. 3.
The intensities of CD154 expression on the CD4+CD154+ T cell subset from patients with autoimmune thyroid diseases of various levels of severity and from normal subjects. Vertical bars indicate mean values. Probability values versus normal intensity are shown.
The proportion of CD154+ cells in CD4+ cells and the MFI of CD154 expression on CD4+ cells did not correlate with the concentration of FT4 or FT3 in untreated patients with GD, including thyrotoxic GD patients, euthyroid patients with GD in remission, and euthyroid patients with mild HD. Neither was there any correlation between these proportions or MFI and the dose of antithyroid drug in GD patients under treatment. There was not also any correlation between these proportions or MFI and the levels of thyroid-specific autoantibodies in untreated patients with AITD.
DISCUSSION
When T cells are activated, CD154 expression increases on the cell surface [8]. Blockade of CD154 function suppresses experimental autoimmune thyroiditis [10] and other autoimmune diseases [11–13] in mice by inhibiting the priming of T cells. The proportion of CD154+ cells and CD154 mRNA expression are increased in patients with systemic autoimmune disease such as systemic sclerosis [14] and systemic lupus erythematosus [15]. Thus we expected the proportion of CD154+ cells to be increased in AITD patients. Contrary to our expectation, the proportion of CD154+ cells in CD4+ cells did not change in any group of patients with AITD, and the MFIs of CD154 molecules on CD4+ cells were decreased. These MFIs did not relate to the concentration of thyroid hormone or the dose of antithyroid drug. Therefore, thyroid hormone and antithyroid drug may have minor effect to CD154 expression in AITD. Moreover, we found no differences in CD154 expression on T cells between patients with AITD at different levels of severity.
Certain cytokines are known to affect CD154 expression. IL-12 and IL-18 up-regulate CD154 on peripheral T cells [16,17], and IL-15 up-regulates CD154 on both peripheral and synovial T cells from patients with rheumatoid arthritis [18]. Therefore, it is expected that a decrease in the production of these cytokine would cause a decrease in the CD154 intensity on T cells. However, the serum concentration of IL-12 is reported to increase in GD patients [19,20], and both IL-12 and IL-15 are suggested to play a role in triggering the onset of thyroiditis in animals [21,22] Therefore, changes in these cytokines may have little effect on the decreases in CD154 intensities in GD.
Another possibility for the decrease of CD154 intensity is that a fundamental abnormality exists in the regulation of CD154 expression in AITD. In other words, CD154 expression on CD4+ cells may be primarily suppressed in AITD, and this decrease in CD154 expression may permit autoreactive CD4+ T cells to survive. Interestingly, it has been reported that the CD154–CD40 interaction activates CD40+ antigen presenting cells (APCs) to express FasL on their surface, and then CD40+ FasL+ APCs induce apoptosis of activated Fas+ T cells [23]. CD40+ APCs are reported to be colocalized with activated CD4+ T cells in the thyroid gland of GD patients [24]. Furthermore, it has been reported that CD154–CD40 interaction has been shown to contribute to negative regulation of T cell autoreactivity, and a defect in this interaction can lead to autoimmunity [25]. We concluded therefore that the decreased CD154 intensity on CD4+ T cells observed in AITD patients, despite the disease severity, may be related to the pathogenesis, but not the severity of the disease. It would be important to apply CD154 MFI test prospectively to a new population of patients/controls, and also to examined CD154 expression in patients with other autoimmune disorders, such as rheumatoid arthritis, insulin-dependent diabetes mellitus, and systemic lupus erythematosus to clarify the significance of reduced CD154 expression in autoimmune disease.
Acknowledgments
This study was supported by The Originative Study Result Fostering Project from The Japan Science and Technology Corporation, and Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
References
- 1.Iwatani Y, Amino N, Kaneda T, et al. Marked increase of CD5+ B cells in hyperthyroid Graves’ disease. Clin Exp Immunol. 1989;78:196–200. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatani Y, Amino N, Hidaka Y, et al. Decreases in αβ T cell receptor negative T cells and CD8 cells, and an increase in CD4+CD8+ cells in active Hashimoto's disease and subacute thyroiditis. Clin Exp Immunol. 1992;87:444–9. doi: 10.1111/j.1365-2249.1992.tb03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwatani Y, Hidaka Y, Matsuzuka F, et al. Intrathyroidal lymphocyte subsets, including unusual CD4+CD8+ cells and CD3loTCRαβlo/–CD4–CD8– cells, in autoimmune thyroid disease. Clin Exp Immunol. 1993;93:430–6. doi: 10.1111/j.1365-2249.1993.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajjan RA, Watson PF, McIntosh RS, et al. Intrathyroidal cytokine gene expression in Hashimoto's thyroiditis. Clin Exp Immunol. 1996;105:523–8. doi: 10.1046/j.1365-2249.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M, Yamamoto N, Maruoka H, et al. Independent involvement of CD8+CD25+ cells and autoantibodies in disease severity of Hashimoto's disease. Thyroid. 2002;12:801–8. doi: 10.1089/105072502760339370. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Yamamoto N, Maruoka H, et al. Relation of CD30 molecules on T-cell subsets to the severity of autoimmune thyroid disease. Thyroid. 2003;13:259–63. doi: 10.1089/105072503321582051. [DOI] [PubMed] [Google Scholar]
- 7.Nakamoto Y, Niki M, Watanabe M, et al. Increase in immunoglobulin G3-secreting cells in intractable Graves’ disease. Thyroid. 2003;13:325–31. doi: 10.1089/105072503321669794. [DOI] [PubMed] [Google Scholar]
- 8.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Iwatani Y, Watanabe M. Normal mechanisms for self-tolerance. In: Volpe R, editor. Autoimmune Endocrinopathies. Totawa: Humana Press Inc.; 1999. pp. 1–30. [Google Scholar]
- 10.Carayanniotis G, Masters SR, Noelle RJ. Suppression of murine thyroiditis via blockade of the CD40–CD40L interaction. Immunology. 1997;90:421–6. doi: 10.1111/j.1365-2567.1997.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durie FH, Fava RA, Foy TM, et al. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–30. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 12.Gerritse K, Laman JD, Noelle RJ, et al. CD40–CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan C, Shi Y, Laman JD, et al. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154:1470–80. [PubMed] [Google Scholar]
- 14.Valentini G, Romano MF, Naclerio C, et al. Increased expression of CD40 ligand in activated CD4+ T lymphocytes of systemic sclerosis patients. J Autoimmun. 2000;15:61–6. doi: 10.1006/jaut.2000.0387. [DOI] [PubMed] [Google Scholar]
- 15.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98:826–37. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X, Remacle JE, Kasran A, et al. IL-12 up-regulates CD40 ligand (CD154) expression on human T cells. J Immunol. 1998;160:1166–72. [PubMed] [Google Scholar]
- 17.Hoshino T, Yagita H, Ortaldo JR, et al. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Mottonen M, Isom]aki P, Luukkainen R, et al. Interleukin-15 up-regulates the expression of CD154 on synovial fluid T cells. Immunol. 2000;100:238–44. doi: 10.1046/j.1365-2567.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamaru M, Matsuura B, Onji M. Increased levels of serum interleukin-12 in Graves’ disease. Eur J Endocrinol. 1999;141:111–6. doi: 10.1530/eje.0.1410111. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka Y, Okumura M, Fukata S, et al. Increased serum concentration of interleukin-12 in patients with silent thyroiditis and Graves’ disease. Thyroid. 1999;9:149–53. doi: 10.1089/thy.1999.9.149. [DOI] [PubMed] [Google Scholar]
- 21.Chen K, Wei Y, Sharp GC, et al. Induction of experimental autoimmune thyroiditis in IL-12-/- mice. J Immunol. 2001;167:1720–7. doi: 10.4049/jimmunol.167.3.1720. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser P, Rothwell L, Vasicek D, et al. A role for IL-15 in driving the onset of spontaneous autoimmune thyroiditis? J Immunol. 2002;168:4216–20. doi: 10.4049/jimmunol.168.8.4216. [DOI] [PubMed] [Google Scholar]
- 23.Shibaki A, Katz SI. Activation through CD40 ligation induces functional Fas ligand expression by Langerhans cells. Eur J Immunol. 2001;31:3006–15. doi: 10.1002/1521-4141(2001010)31:10<3006::aid-immu3006>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Quadbeck B, Eckstein AK, Tews S, et al. Maturation of thyroidal dendritic cells in Graves’ disease. Scand J Immunol. 2002;55:612–20. doi: 10.1046/j.1365-3083.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumanogoh A, Wang X, Lee I, et al. Increased T cell autoreactivity in the absence of CD40–CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001;166:353–60. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]