INTRODUCTION
It is 20 years since the use of a radioimmunoassay enabled an association to be described between antibodies to the anionic phospholipid cardiolipin and a set of clinical features [1]. These features, which included thrombosis – both venous and arterial – and recurrent foetal loss, subsequently became known as the antiphospholipid syndrome (APS) [2–4]. They had previously been shown to be associated with positive Venereal Disease Reference laboratory (VDRL) and lupus anticoagulant (LA) tests, both of which detect antiphospholipid antibodies [5–8]. However, the radioimmunoassay, and the enzyme-linked assays (ELISA) derived from it [9], are considerably more sensitive, and their use enabled the clinical syndrome to be clearly defined. Many additional clinical features have been described, such as neurological disorders, migraine, livedo reticularis and thrombocytopenia [10–12]. Some of these remain controversial, and may be features of associated connective tissue diseases such as systemic lupus erythematosus (SLE), with which APS frequently coexists. They are not all directly related to thrombosis. APS can occur as an isolated, primary condition [13–15]. It has emerged as the most common cause of acquired thrombophilia, and is a major cause of pregnancy morbidity. Because thrombosis in APS can occur at almost any site, it is a condition that is seen in almost all medical specialities.
Central to our understanding of antiphospholipid syndrome has been the demonstration that the autoantibodies in the condition probably play a direct role in pathogenesis. Strong evidence for this has come from murine models. Passive transfer of antiphospholipid antibodies (aPL) to normal mice can generally [16–19], though not always [20], produce features resembling human APS, notably impairment of foetal development. Similar results have been obtained by immunizing mice with aPL [21]. In a murine model of venous injury the addition of aPL results in increased local clot formation [22].
Another critical discovery was that antibodies to phospholipid appear to represent part of a large family of autoantibodies, many of which recognize phospholipid-binding plasma proteins either alone, or in combination with phospholipid. The first of these proteins to be recognized was beta-2-glycoprotein I (β2GPI) [23,24]. This molecule has number of physiological functions, which include a regulatory role within coagulation pathways. Prothrombin is another important protein recognized by sera of patients with APS. Others include annexin V, high and low molecular weight kininogens, protein C and protein S (see Table 1).
Table 1.
Antigen specificities of antibodies that have been detected in the serum of patients with antiphospholipid syndrome.
| Antigen/antigen complex | References |
|---|---|
| Phospholipids | |
| Anionic phospholipids (e.g. cardiolipin, phosphatidyl serine) | [1] |
| Zwitterionic phospholipids (e.g. phosphatidyl ethanolamine) | [73] |
| Components and regulators of coagulation and fibrinolysis | |
| Beta 2 glycoprotein I | [23,24] |
| Prothrombin | [44–52] |
| Thrombin | [45,54] |
| Anti-thrombin III/thrombin complex | [55] |
| Tissue factor/factor VIIa complex | [36,37] |
| High and low molecular weight kininogen | [73] |
| Prekallikrein | [55] |
| Sulphatides | [74] |
| Protein Z/protein Z protease inhibitor system | [75] |
| Protein C | [30,57] |
| Protein S | [30] |
| Thrombomodulin | [61,62] |
| Tissue plasminogen activator | [67] |
| Annexin V | [70] |
| Lipoproteins | |
| Oxidized low density lipoprotein | [106,107] |
| High density lipoprotein | [111] |
| Apolipoprotein A-I | [111] |
In general, aPL found in patients with antiphospholipid syndrome require the presence of β2GPI for binding to cardiolipin. Indeed, most of these antibodies appear to bind to β2GPI itself (reviewed in [25]). This may be through an epitope on native, unbound β2GPI. However, antibodies to β2GPI are generally of low affinity [25], and it has been proposed that the binding of anionic phospholipid, such as cardiolipin, to β2GPI enhances this affinity: this enhancement may occur through the revealing of a cryptic epitope on the β2GPI molecule [26]. Furthermore, there is evidence for the binding of aPL to different domains of β2GPI, although binding to domain I may predominate (reviewed in [26]). These conflicting results may simply reflect the existence of different subpopulations of aPL within the sera of individuals with APS. What is clear is that, for the majority of so-called antiphospholipid antibodies that are detected by ELISA in the serum of patients with APS, anionic phospholipid such as cardiolipin is not the antigen to which those antibodies are actually binding. By contrast, anticardiolipin antibodies that arise as a consequence of infection – and are not normally associated with the syndrome – do not tend to be dependent on β2GPI for binding to phospholipid [25,27–29].
The picture that has emerged is of an autoimmune condition in which antibodies are expressed in the serum against a variety of phospholipid and protein antigens. Not only are different specificities found in different patients [30], but there may be great heterogeneity within an individual [31,32]. How is it that some of these antibodies appear to cause clinical disease?
HAEMOSTASIS
The central feature of APS is thrombosis. Several theories have emerged to explain the association between raised serum anticardiolipin levels and vascular pathology. Many of these have built on the intimate association between phospholipid-binding proteins and antibodies found in the syndrome. The regulation of haemostasis is complex, and occurs at a number of levels. These include platelet and endothelial cell (EC) activity, as well as the coagulation and fibrinolytic cascades and their regulatory proteins. Intriguingly, there is now considerable evidence for the ability of phospholipid-associated antibodies to interfere with all of these. This may be a reflection of the different populations of such antibodies; or it may be due to the fact that the molecules that these antibodies recognize – such as β2GPI – play important regulatory roles at different levels.
Coagulation
Before the description of the solid-phase assay for anticardiolipin antibodies, the most sensitive way to detect such antibodies was the LA test [33,34]. This is now understood to be an in vitro phenomenon, in which there is a prolongation of the kaolin clotting time (or similar measures of the intrinsic coagulation pathway) that cannot be corrected by the addition of normal plasma (i.e. a source of clotting factors). It probably arises because of an effect of aPL on the prothrombinase complex or on prothrombin itself [34,35]. This does not reflect the overall effect in vivo, where the balance of effects on the clotting cascade is in the direction of increased coagulation.
Many points in the coagulation cascade are phospholipid-dependent, and it was postulated early on that these might be potential sites of action of aPL. Inhibition of action at some of these would be expected to have an anticoagulant effect, while at others it would be predicted to promote clotting. An example of the latter is the complex of factors IXa and VIIIa (the so-called ‘tenase’ complex; see Fig. 1). While it is possible that such mechanisms contribute to the actions of aPL, it now seems more likely that the disturbances of coagulation seen in APS are primarily due to antibodies that recognize phospholipid-binding proteins. These include components of the extrinsic and intrinsic coagulation pathways, respectively the complex of tissue factor and factor VIIa [33–38], and prekallikrein [39].
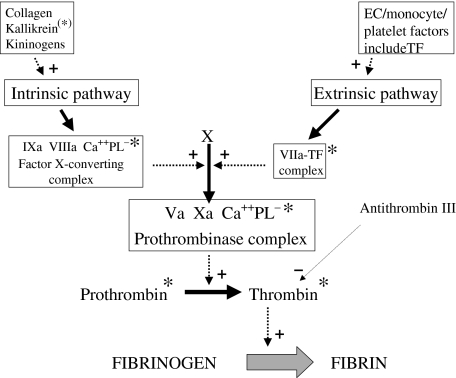
Fig. 1.
A summary of the coagulation pathways. Only a few important stages are shown. Fibrinogen is converted to fibrin by the action of thrombin, which is generated by the action of the prothrombinase complex (containing factor Xa) on prothrombin. Two cascades can lead to the activation of factor X: the intrinsic pathway, promoted by a variety of factors, including collagen (exposed in the vessel wall), kallikrein (derived from prekallikrein*), and kininogens; and the extrinsic pathway, initiated by factors derived from endothelial cells, monocytes or platelets, including tissue factor (TF). The whole process should be envisaged as taking place at the surface of one of these cells, which, when activated, is a source of anionic phospholipid. Solid arrows indicate pathways. Dashed arrows indicate promotion (+) or inhibition (–) of a pathway. Asterisks indicate potential sites of action of antibodies in APS.
Tissue factor acts as a major initiator of the extrinsic coagulation pathway, being a cofactor for the activation of factor VII. Tissue factor activity is up-regulated in patients with APS, probably due to increased expression by endothelial cells and monocytes (see below) [40–43].
The end result of both the extrinsic and intrinsic pathways is the conversion of prothrombin to thrombin (Fig. 1). This has received increasing attention in the context of APS, with antibodies to both molecules being well described, in particular prothrombin [44]. While anti-prothrombin antibodies may be responsible for the in vitro phenomenon of the lupus anticoagulant, in vivo their presence may be associated with a tendency to increased coagulation. This is an area of controversy, with some groups reporting an association between the presence of anti-prothrombin antibodies and clinical or haematological features of APS [45–47], and other groups finding no association [48,49,44]. This may be explained by different detection methods, and by the presence of antibodies with different epitope specificities [50], resulting in different functional properties [51,52]. One group has shown that antibodies that recognize the complex of prothrombin and phosphatidyl serine (an anionic phospholipid) are distinct from those that bind prothrombin alone, and are well correlated with features of APS [53].
Most patients with APS who have anti-prothrombin antibodies also have antibodies to thrombin [45,54]. These may have a procoagulant effect by protecting thrombin from inactivation by the regulatory protein anti-thrombin III. Similarly, antibodies have been described to the complex of anti-thrombin III and thrombin [55].
Protein C pathway
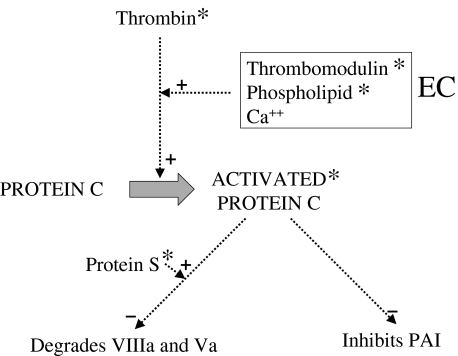
Another area that has received considerable attention is the protein C pathway (Fig. 2). This is an important feedback mechanism for controlling thrombin formation, and thus has an anti-thrombotic effect. Protein C is a vitamin-K-dependent serine proteinase, a heterozygous deficiency of which results in recurrent thrombotic disease [56]. Activated protein C combines with another cofactor, protein S, in the presence of phospholipid to catalyse the degradation of factors Va and VIIIa of the coagulation pathway. For this to take place, protein C is first converted to its active form by thrombin in the presence of thrombomodulin, an EC-derived cofactor.
Fig. 2.
A summary of the protein C pathway. Endothelial cells (EC) are a source of thrombomodulin, and anionic phospholipid. Solid arrows indicate pathways. Dashed arrows indicate promotion (+) or inhibition (–) of a pathway. Asterisks indicate potential sites of action of antibodies in APS.
Protein C is a potential target for antibodies in APS. aPL derived from patients’ serum have been shown in vitro to impair the degradation of factor V by protein C [30,57,58]. This effect has been shown to be phospholipid dependent [59], and may be due to an inhibitory effect on the protein C/protein S complex [60].
The activation of protein C by thrombomodulin could be another target for antibodies in APS. IgG from patients with the lupus anticoagulant have been shown to inhibit the activity of thrombomodulin [61]. Its ability to activate protein C is enhanced by phospholipid; this enhancement was found to be neutralized by an IgM antibody with lupus anticoagulant activity [62].
Fibrinolysis
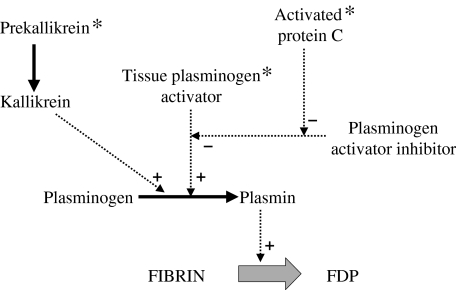
Reduced activitation of protein C could also have an effect on the fibrinolytic system. Fibrin, which is the end product of the coagulation cascade, is degraded by plasmin, which itself is generated as a result of a complex cascade (Fig. 3). It is derived from plasminogen through the action of tissue plasminogen activator (tPA). An important modulator of this process is plasminogen activator inhibitor (PAI), which is another endothelial-derived protein. Activated protein C has been shown to decrease the PAI activity of cultured EC, and may therefore act indirectly as a promoter of fibrinolysis [63,64]. Thus the binding of antibodies to protein C could impair clot degradation.
Fig. 3.
A summary of the fibrinolytic pathway. Solid arrows indicate pathways. Dashed arrows indicate promotion (+) or inhibition (–) of a pathway. Asterisks indicate potential sites of action of antibodies in APS. FDP: fibrin degradation products.
The data on the role of tPA and PAI in APS are conflicting. Some groups have shown a raised level of PAI antigen or activity in APS compared with control patients [65], while others have failed to show any difference [66]. One group has demonstrated the presence of antibodies to tPA in patients with APS [67]. They showed in two cases that these antibodies bind to the catalytic domain of the molecule, suggesting that they could reduce tPA activity, and thus reduce fibrinolysis.
Our own data showed no difference between patients with APS and SLE controls in respect of tPA levels and PAI activity; nor was there any significant correlation between these and levels of anticardiolipin antibodies as measured by ELISA or the lupus anticoagulant [68]. However, we did find a strong positive correlation between levels of von Willebrand factor and IgG anticardiolipin levels, and a strong negative correlation between von Willebrand factor levels and the platelet count. These findings may be explained by an increase in the release of von Willebrand factor from EC. This could lead to enhanced platelet adhesion to vessel walls, resulting in an increased tendency to thrombosis, and a reduction in circulating platelet number.
Kallikrein is another promoter of the conversion of plasminogen to plasmin. Reduced prekallikrein activity has been shown in a group of patients with the lupus anticoagulant, suggesting a further mechanism for impaired fibrinolysis [39].
Annexin V
Another protein that regulates the clotting cascade is annexin V. It has anticoagulant activity, interfering with the binding of procoagulant factors to procoagulant membranes [69]. Notably, it is expressed by endothelial cells in the placenta and the placental precursor, the trophoblast, where it is thought to function as a natural anticoagulant. It probably does so by crystallizing over anionic phospholipids, thus inhibiting them from participating in coagulation reactions. Sera from about half of patients with serum aPL contain antibodies that bind to annexin V [70]. It has been shown that these antibodies can disrupt the annexin shield, allowing increased generation of thrombin [71]. Another group found that IgG anti-annexin antibodies only bind to free annexin, and not when it is associated with phospholipid [72]. Either way, antiannexin V activity could represent part of the mechanism of increased foetal loss in APS (see below).
Other regulatory proteins
In the wealth of literature on antibody specificity in APS, a number of other antigens have been described that are recognized by sera of patients with the condition, binding of which could alter haemostasis. For instance, antibodies have been identified that bind to phospholipid in association with high or low molecular weight kininogens [73]. Antibodies have also been described that bind to sulphatides [74]. These are sulphated glycosphingolipids that are expressed on the surface of erythrocytes, leucocytes and platelets, and that interact with several adhesion molecules involved in haemostasis. Another group has shown an impairment in patients with APS of the protein Z/protein Z protease inhibitor system, another regulatory mechanism that inhibits factor Xa. They also showed that aPL from these patients inhibit this mechanism in vitro[75].
Platelets
Platelets play a central role in coagulation: they provide intrinsic coagulation proteins, and they also form procoagulant membrane surfaces, characterized by the exposure of anionic phospholipids. There is now a growing body of evidence to suggest that some of the pathogenic activity of antibodies in APS may occur through effects on platelets.
Increased platelet activation can be been demonstrated in patients with APS [76], and there is evidence that aPL can directly promote this. Activation of platelets has been shown in an in vitro model, using polyclonal and monoclonal aPL from patients with APS [77]. It has also been shown in a different model that antiβ2GPI antibodies can promote platelet binding to vascular subendothelium [78]. Another group has found that complexes of aPL and β2GPI can increase the production from platelets of thromboxane A2, an eicosanoid that promotes vasoconstriction and clotting [79]. This appears to occur through an increase in the activity of platelet cyclic AMP [80].
Recently there has been more direct evidence for the role of antiβ2GPI antibodies in promoting platelet adhesion and aggregation. Using an in vitro flow system, de Groot and colleagues [81] have shown that dimerized β2GPI (which mimics the effects of β2GPI–antiβ2GPI complexes) can increase adhesion of platelets to collagen, and their aggregation. They have further demonstrated, by coimmunoprecipitation, that this activity is probably mediated by the apolipoprotein E receptor 2′, which is a member of the low density lipoprotein (LDL) receptor family [81]. Since many types of cell express members of this receptor family on their surface, such a mechanism could mediate the activation of other cells in APS.
However, not all groups have confirmed the ability of aPL or antiβ2GPI antibodies to activate platelets [82]. The picture is complicated by the presence of specific antiplatelet antibodies in the serum of patients with APS and associated connective tissue diseases.
There has been much controversy as to whether thrombocytopenia is a manifestation of APS. If it occurs it is generally mild, and does not usually lead to problems with bleeding. What is clear is that the administration of aPL to experimental animals generally results in a lowering of the platelet count [17,19]. The mechanism is uncertain. It may be due to platelet consumption, or may result from the presence of antibodies to platelet glycoproteins [83,84].
Endothelial cells
In the study of APS pathogenesis, the area that has received perhaps the greatest attention in recent years has been the endothelial cell. In its normal state, the endothelial lining of blood vessels plays a central part in homeostasis, helping to maintain blood fluidity via a number of mediators that inhibit coagulation. However, certain stimuli can alter the phenotype of EC, allowing them to act as a surface that promotes coagulation. There has been accumulating evidence that aPL may have a direct effect on these cells, helping to promote the switch to the pro-coagulant phenotype. This state parallels the ‘pro-adhesive’ or pro-inflammatory phenotype.
A relatively early observation was that aPL may interfere with the release from endothelial cells of prostacyclin [85]. This is an eicosanoid that has actions broadly opposed to those of thromboxane. It was suggested at the time that this action of aPL might occur through an effect on cell surface phospholipid. Although the finding was controversial [86,87], it was soon recognized that the sera of patients with APS frequently contain antibodies that bind to the surface of endothelial cells [88]. However, there did not appear to be a close relationship between antiendothelial cell and antiphospholipid binding. For instance, antiendothelial activity could only be poorly absorbed by preincubation with phospholipid micelles [88–91]. This is consistent with the resting state of the endothelial cell membrane, in which anionic phospholipids are not exposed on the outside. However, even when endothelial cells are activated, the binding of aPL-positive sera is not necessarily enhanced.
These findings can be explained by the observation that β2GPI may be the chief molecule involved in the binding of aPL-positive sera to endothelial surfaces [92,93]. Sera that contained anticardiolipin and antiβ2GPI antibodies were found to have reduced antiendothelial cell activity when the EC had been cultured in serum-free medium. The antiendothelial cell activity was restored when purified human β2GPI was added. It was postulated that β2GPI in the culture medium adhered to EC, and was recognized by antiβ2GPI antibodies in the test sera; when serum-free medium was used, this source of β2GPI was not available.
These observations are supported by the finding that β2GPI can bind to EC in vitro, and can be demonstrated on trophoblast EC in vivo (reviewed in [88]). The binding of β2GPI to EC appears to occur through the cationic, phospholipid-binding site in the fifth domain of the molecule.
If β2GPI is indeed present on the surface of EC in vivo, this could suggest a potential pathway for the action of antibodies in APS. The pro-inflammatory, procoagulant phenotype can be induced in EC in vitro by incubation with antiβ2GPI antibodies [42,92–97]. This has been shown with both monoclonal and polyclonal antibodies. Characteristics of this change in phenotype include the up-regulation of adhesion molecules such as E-selectin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM −1), and the increased secretion of pro-inflammatory cytokines, including interleukin 1 (IL-1) and interleukin 6 (IL-6). It can also result in the expression of tissue factor. This activator of the extrinsic coagulation pathway can be expressed by a variety of cells, including EC, in response to inflammatory cytokines (e.g. IL-1β and tumour necrosis factor α) or endotoxin. Tissue factor production by EC can be up-regulated by antiβ2GPI antibodies in vitro[42]; and raised levels of tissue factor have been demonstrated in patients with APS [41]. Anti-β2GPI antibodies can induce the production of other procoagulant proteins by EC [97]. In this way, antibodies that bind to β2GPI – and are generally present in the sera of patients with APS – could promote a hypercoagulable state.
These effects of antiβ2GPI antibodies on EC appear to be mediated by nuclear factor kappa B [98]. Annexin II may also play a role [99].
Other cells
Monocytes can contribute directly to coagulation under physiological conditions, and this also appears to be the case in APS. Serum from patients with APS can induce a procoagulant, tissue factor-like activity in monocytes in vitro[40,42]; and monocytes taken from such patients also show an enhanced production of tissue factor, which correlates with a history of thrombosis [100]. This may be due to binding of β2GPI at the cell surface [77]: there is a correlation between the expression on monocytes of β2GPI and of tissue factor in patients with APS [43].
Although serum antibodies in APS can be found to bind to surface molecules on a variety of cells, it is unlikely that this involves anionic phospholipid, which is not exposed on the surface of intact cell membranes. aPL do, however, bind to apoptotic cells, where the membrane is disrupted [101]. This may provide a stimulus for sustaining or enhancing the autoimmune process, but it is uncertain whether it contributes directly to the pathogenic action of the antibodies.
VESSEL TONE
Another way in which endothelial cells could mediate the effects of aPL is through their control of vessel tone. EC release a number of factors that can alter the tone of the vessel wall, including vasoconstrictors such as endothelin and platelet activating factor, and vasodilators such as nitric oxide and prostacyclin. As noted above, aPL may reduce the release of prostacyclin from EC [85]. It has also been shown that levels of the vasoconstrictor endothelin I peptide are raised in patients with thrombosis due to APS [102]. A perturbation of the balance of vessel wall regulation towards vasoconstriction could explain the increased frequency of Raynaud's phenomenon and livedo reticularis seen in APS, and could also contribute to thrombosis.
ATHEROSCLEROSIS
Over the last few years it has become increasingly recognized that many autoimmune conditions carry with them an increased risk for the development or progression of atherosclerosis. In the case of APS there are no prospectve studies to confirm this; and the picture is confused by the close association with SLE, in which there may be many risk factors for the enhancement of atheroma formation, including the inflammatory disease and corticosteroid treatment [103]. Nevertheless, it does appear that APS is an independent risk factor for an increased development or progression of atherosclerosis [104].
There are a number of potential mechanisms through which antibodies found in APS could promote atherogenesis (reviewed in [105]). Perhaps the most compelling is through binding to β2GPI. This molecule (also known as apolipoprotein H) is present in various lipoprotein fractions, including oxidized LDL. It is the uptake of oxidized LDL by macrophages in the blood vessel wall to form foam cells that is thought to initiate atheroma plaque formation. Antibodies to oxidized LDL are well described in APS, although there has been some controversy as to whether they cross-react with β2GPI [106,107]. One group has isolated the ligand on oxidized LDL to which β2GPI binds. They have shown in an in vitro model that liposomes containing this ligand are taken up by macrophages, and that this process is enhanced by β2GPI and anti-β2GPI antibodies [108]. Furthermore, LDL-receptor-deficient mice immunized with β2GPI show accelerated atherosclerosis [109]. If macrophages are activated by the uptake of oxidized LDL, this could result in damage to endothelial cells, and subsequent promotion of thrombosis [110].
Another possible mechanism is interference with the protective effect of high-density lipoprotein (HDL) and apolipoprotein A-I (apo A-I). HDL helps to prevent the oxidation of LDL, while apo A-I stabilizes paraoxonase, an antioxidant enzyme within the HDL particle. Patients with APS have a high frequency of antibodies to HDL and apo A-I, a large percentage of which cross-react with cardiolipin [111].
At this stage the link between specific antibodies and atherogenesis in APS is less strong than for thrombosis, although clearly the two processes are related [112].
FOETAL LOSS
It was long thought that miscarriage in APS could largely be explained by impaired foetal blood supply caused by placental thrombosis and infarction. Placental infarcts have been described in cases of foetal loss due to APS (reviewed in [113]). Any of the potential mechanisms for increased coagulation outlined above could play a role, notably antiannexin V activity. However, placental infarction is not always present, and it is thought likely that other mechanisms are equally or even more important [114,115].
It is known that the spiral arteries of the placenta show abnormal development in APS [116]. This could be due to an effect on endothelial function, as outlined above. However, it has also been shown that purified aPL can bind specifically to placental antigens [117], providing a potential mechanism for nonthrombotic placental damage and impaired foetal blood supply. aPL and antibodies to β2GPI have also been found to modify trophoblast proliferation and differentiation [118,119]. aPL may bind directly to trophoblast cell membranes through exposed anionic phospholipid and adhered β2GPI: this may result in altered gonadotrophin secretion [119]. One group has shown a direct effect of aPL on embryonic implantation in a murine model [18]. Using elegant embryo transfer experiments, they have demonstrated that defects in both the embryo and the mother contribute to pregnancy failure [120].
There is emerging evidence that the complement pathway may also mediate foetal damage in APS. Salmon and colleagues have shown in a mouse model of APS that activation of the C3 component of complement is needed for foetal loss to occur [121]; in the same model they have also demonstrated a requirement for complement C5 as a mediator of foetal injury [122]. It has been suggested that local complement activation could be a mechanism for damage to tissues such as vascular endothelium and the trophoblast [121]. This would fit with the observation that local complement inhibition appears to be a requirement for normal murine pregnancy [123]. A drawback to such animal experiments is the uncertainty that remains about how relevant murine models of APS are to the human disease, particularly those that involve the transfer of heterologous antibodies, which could result in immune complex formation and complement activation. However, a number of findings in humans do support these initial conclusions: inflammatory changes have been described in placentae from women with APS [124,125]; elevated levels of complement split products have been demonstrated in the serum of patients with cerebral thrombotic events due to APS [126]; and the complement-fixing ability of aPL has been shown to be associated with foetal loss (and indeed thrombosis) [127].
NEUROLOGICAL DAMAGE
A wide variety of neurological disorders have been reported in APS [3,10]. Many of these, such as stroke and mononeuritis, can be explained by thromboembolism. Even here there remains some controversy about the precise relationship between aPL and such events. For instance, a large American study has recently found that the presence of aPL in patients with ischaemic stroke does not predict an increased risk for subsequent vascular occlusive events [128]. Unfortunately animal models are unhelpful here: among the many that have been reported, thrombosis outside the placenta is not a characteristic feature, and nor are specific neurological abnormalities.
There are other neurological features seen in human APS that are less readily explained by thrombosis: examples include cerebral dysfunction (for instance poor concentration or forgetfulness) and multiple sclerosis-like lesions. Although such features could be due to microthrombi, there is increasing evidence that aPL can in fact cause direct damage to neurones. Antibodies to the anionic phospholipid phosphatidylserine have been shown to bind directly to neuronal tissue [129], as have antibodies to β2GPI [130]. Subsequent experiments have indicated that there may be functional effects of such antibodies on neuronal cells. For instance, it has been demostrated that aPL can cause depolarization of synaptoneurosomes in an in vitro preparation, suggesting that these antibodies could disrupt neuronal function by a direct action on nerve terminals [131]. Shoenfeld and colleagues performed in vivo experiments, administering purified IgG from patients with APS into the cerebral ventricles of normal mice: they found impairment of learning and memory, again suggesting a direct antineuronal effect [132].
SECOND HIT PHENOMENON
There seem, therefore, to be multiple ways in which aPL and related antibodies could cause pathology. Yet many individuals with high IgG aPL levels do not develop features of APS. This may be due to the particular pattern of antibody specificities in their serum. However, it appears that for many people other factors may be needed for the expression of APS, i.e. a ‘second hit’ is required. Thus pregnancy (a hypercoagulable state) can lead to the development of thrombosis in patients with raised aPL levels [133,134]. Other promoters of thrombosis in APS include the presence of factor V Leiden [135], vascular injury and infection [136].
CONCLUSIONS
In the last 20 years a wealth of information has emerged about the potential action of autoantibodies in APS. It seems very likely that at least some of these antibodies are directly pathogenic. A large number of mechanisms have been proposed, most of which involve disturbance of coagulation pathways, their regulatory systems, and the cells that control them. It is improbable that they all have a significant role in vivo: much of the evidence comes from in vitro experiments; and in some areas it depends on single reports. Although many of these putative mechanisms are closely related, it may well be that their multiplicity reflects the wide heterogeneity of antibody specificities within individuals and between different people with the condition. It may indeed be that thrombosis represents the final common pathway of many disease processes, each of which is dependent on its own particular autoantibody profile. The same could apply to foetal loss and neuronal disease. One of the chief aims of research over the next few years will be to establish which of these many mechanisms are truly central to the disease process, so that specific therapies can be designed for this unusual and often devastating condition.
References
- 1.Harris EN, Gharavi AE, Boey ML, et al. Anticardiolipin antibodies: detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;ii:1211–4. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon-Segovia D, Deleze M, Oria CV, et al. Antiphospholipid antibodies and the antiphospholipid syndrome in systemic lupus erythematosus. A prospective analysis of 500 consecutive patients. Medicine (Baltimore) 1989;68:353–65. doi: 10.1097/00005792-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cuadrado MJ, Hughes GRV. Hughes (antiphospholipid) syndrome. clinical features. Rheum Dis Clin North Am. 2001;27:507–24. doi: 10.1016/s0889-857x(05)70217-9. [DOI] [PubMed] [Google Scholar]
- 4.Lockshin MD, Druzin ML, Goei S, et al. Antibody to cardiolipin as a predictor of fetal distress or death in pregnant patients with systemic lupus erythematosus. N Eng J Med. 1985;313:152–6. doi: 10.1056/NEJM198507183130304. [DOI] [PubMed] [Google Scholar]
- 5.Laurell B-B, Nilsson IM. Hypergammaglobulinaemia, circulating anticoagulant, and biologic false positive Wassermann reaction. J Laboratory Clin Med. 1957;49:694–707. [PubMed] [Google Scholar]
- 6.Bowie EJW, Thompson JH, Jr, Pascuzzi CA, Owen CA., Jr Thrombosis in systemic lupus erythematosus despite circulating anticoagulants. J Laboratory Clin Med. 1963;62:416–30. [PubMed] [Google Scholar]
- 7.Firkin BG, Howard MA, Radford N. Possible relationship between lupus inhibitor and recurrent abortion in young women. Lancet. 1980;ii:366. doi: 10.1016/s0140-6736(80)90361-x. [DOI] [PubMed] [Google Scholar]
- 8.Gastineau DA, Kazmier FJ, Nichols WL, Bowie EJW. Lupus anticoagulants. an analysis of the clinical and laboratory features of 219 cases. Am J Hematol. 1985;19:265–75. doi: 10.1002/ajh.2830190308. [DOI] [PubMed] [Google Scholar]
- 9.Loizou S, McCrea JD, Rudge AC, et al. Measurement of anticardiolipin antibodies by enzyme linked immunosorbent assay: standardisation and quantitation of results. Clin Exp Immunol. 1985;62:738–44. [PMC free article] [PubMed] [Google Scholar]
- 10.Navarrete MG, Brey RL, Levine SR. Cerebral disease in the antiphospholipid syndrome. In: Khamashta MA, editor. Hughes Syndrome, Antiphospholipid Syndrome. London: Springer Verlag; 2000. pp. 43–58. [Google Scholar]
- 11.Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36:970–82. doi: 10.1016/s0190-9622(97)80283-6. [DOI] [PubMed] [Google Scholar]
- 12.Cuadrado MJ, Mujic F, Munoz E, et al. Thrombocytopenia in the antiphospholipid syndrome. Ann Rheum Dis. 1997;56:194–6. doi: 10.1136/ard.56.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackworth-Young CG, David J, Loizou S, Walport MJ. Primary antiphospholipid syndrome: features of patients with raised anticardiolipin antibodies and no other disorder. Ann Rheum Dis. 1987;26:94. doi: 10.1136/ard.48.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alarcon-Segovia D, Sanchez-Guerrero J. Primary antiphospholipid syndrome. J Rheumatol. 1989;42:1309–11. [PubMed] [Google Scholar]
- 15.Asherson RA, Khamashta MA, Ordi-Ros J, et al. The ‘primary’ antiphospholipid syndrome: major clinical and serological features. Medicine (Baltimore) 1989;68:366–74. [PubMed] [Google Scholar]
- 16.Branch DW, Dudley DJ, Mitchell MD, et al. Immunoglobulin G fractions from patients with antiphospholipid antibodies cause fetal death in BALB/c mice: a model for autoimmune fetal loss. Am J Obstet Gynecol. 1990;163:210–6. doi: 10.1016/s0002-9378(11)90700-5. [DOI] [PubMed] [Google Scholar]
- 17.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of antiphospholipid syndrome by passive transfer of anti-cardiolipin antibodies. Proc Natl Acad Sci USA. 1991;88:3069–73. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sthoeger ZM, Mozes E, Tartakovsky B. Anti-cardiolipin antibodies induce pregnancy failure by impairing embryonic implantation. Proc Natl Acad Sci USA. 1993;90:6464–7. doi: 10.1073/pnas.90.14.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason AN, Mageed RA, Mackworth-Young CG. The effects of a human IgM monoclonal anticardiolipin antibody on pregnancy in a transgenic mouse model. Lupus. 2001;10:289–94. doi: 10.1191/096120301680416986. [DOI] [PubMed] [Google Scholar]
- 20.Silver RM, Smith LA, Edwin SS, et al. Variable effects on murine pregnancy of immunoglobulin G fractions from women with antiphospholipid antibodies. Am J Obstet Gynecol. 1997;177:229–33. doi: 10.1016/s0002-9378(97)70466-6. [DOI] [PubMed] [Google Scholar]
- 21.Bakimer R, Fishman P, Blank M, et al. Induction of primary antiphospholipid syndrome in mice by immunisation with a human monoclonal anticardiolipin antibody (H-3) J Clin Invest. 1992;89:1558–63. doi: 10.1172/JCI115749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierangeli SS, Harris EN. Antiphospholipid antibodies in an in vivo thrombosis model in mice. Lupus. 1994;3:247–51. doi: 10.1177/096120339400300408. [DOI] [PubMed] [Google Scholar]
- 23.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 24.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: β2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roubey RAS. Immunology of the antiphospholipid syndrome. Arthritis Rheum. 1996;39:1444–54. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 26.Giles IP, Isenberg DA, Latchman DS, Rahman A. How do antiphospholipid antibodies bind β2-glycoprotein I? Arthritis Rheum. 2003;48:2111–21. doi: 10.1002/art.11101. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JE, McNeil HP, Morgan GJ, et al. A phospholipid-beta 2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmue disease but not with infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 28.Forastiero RR, Martinuzzo ME, Kordich LC, et al. Reactivity to beta 2 glycoprotein I clearly differentiates anticardiolipin antibodies from antiphospholipid syndrome and syphilis. Thrombosis Haemostasis. 1996;75:717–20. [PubMed] [Google Scholar]
- 29.Gharavi AE, Sammaritano LR, Wen J, et al. Characteristics of HIV and chlorpromazine induced antiphospholipid antibodies: effect of β2-glycoprotein I on binding to phospholipid. J Rheumatol. 1984;21:94–9. [PubMed] [Google Scholar]
- 30.Oosting JD, Derksen RH, Bobbink IW, et al. Antiphospholipid antibodies directed against a combination of phospholipids with prothrombin, protein C, or protein S. an explanation for their pathogenic mechanism? Blood. 1993;81:2618–25. [PubMed] [Google Scholar]
- 31.Lieby P, Soley A, Levallois H, et al. The clonal analysis of anticardiolipin antibodies in a single patient with primary antiphospholipid syndrome reveals an extreme antibody heterogeneity. Blood. 2001;97:3820–8. doi: 10.1182/blood.v97.12.3820. [DOI] [PubMed] [Google Scholar]
- 32.Shoenfeld Y, Krause I, Kvapil F, et al. Prevalence and clinical correlations of antibodies against six beta2-glycoprotein-I-related peptides in the antiphospholipid sydrome. J Clin Immunol. 2003;23:377–83. doi: 10.1023/a:1025321617304. [DOI] [PubMed] [Google Scholar]
- 33.Lee SL, Saunders M, Kahny HMA. A disorder of blood coagulation in systemic lupus erythematosus. J Clin Invest. 1955;34:1814–22. doi: 10.1172/JCI103237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derksen RH, Hasselar P, Blokzijl L, et al. Coagulation screen is more specific than the anticardiolipin aantibody ELISA in defining a thrombotic subset of lupus patients. Ann Rheum Dis. 1998;47:364–71. doi: 10.1136/ard.47.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RF. Lupus anticoagulant IgGs (LA) are not directed to phosholipids only, but to a complex of lipid-bound human prothrombin. Thrombosis Haemostasis. 1991;66:629–32. [PubMed] [Google Scholar]
- 36.Skjonsberg OH, Wisloff F, Godal HC. Inhibition of the tissue factor-factor VII mediated activation of coagulation factor X by a monoclonal antibody expressing lupus anticoagulant activity. Thrombosis Res. 1990;58:349–52. doi: 10.1016/0049-3848(90)90104-k. [DOI] [PubMed] [Google Scholar]
- 37.Bidot CJ, Horstman LL, Huisheng H, et al. Factor VII/VIIa: a new antigen in the antiphospholipid antibody syndrome. Br J Haematol. 2003;120:618–26. doi: 10.1046/j.1365-2141.2003.04161.x. [DOI] [PubMed] [Google Scholar]
- 38.Angles-Cano E, Guillin M-C. Antiphospholipid antibodies and the coagulation cascade. Rheum Dis Clin North Am. 2001;27:573–86. doi: 10.1016/s0889-857x(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 39.Sanfelippo MJ, Drayna CJ. Prekallikrein inhibition assocoated with the lupus anticoagulant. Am J Clin Pathol. 1982;77:275–9. doi: 10.1093/ajcp/77.3.275. [DOI] [PubMed] [Google Scholar]
- 40.Kornberg A, Blank M, Kaufman S, Shoenfeld Y. Induction of tissue factor-like activity in monocytes by anti-cardiolipin antibodies. J Immunol. 1994;153:1328–32. [PubMed] [Google Scholar]
- 41.Atsumi T, Khamashta MA, Amengual O, Hughes GRV. Up.-regulated tissue factor expression in antiphospholipid syndrome. Thrombosis Haemostasis. 1997;77:222–3. [PubMed] [Google Scholar]
- 42.Amengual O, Atsuni T, Khamashta MA, Hughes GRV. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thrombosis Haemostasis. 1998;79:276–81. [PubMed] [Google Scholar]
- 43.Conti F, Sorice M, Circella A, et al. Beta-2-glycoprotein I expression on monocytes is increased in antiphospholipid antibody syndrome and correlates with tissue factor expression. Clin Exp Immunol. 2003;132:509–16. doi: 10.1046/j.1365-2249.2003.02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amengual O, Atsumi T, Koike T. Specificities, properties and clinical significance of antiprothrombin antibodies. Arthritis Rheum. 2003;48:886–95. doi: 10.1002/art.10831. [DOI] [PubMed] [Google Scholar]
- 45.Sorice M, Pittone V, Circella A, et al. Anti-thrombin but not ‘pure’ anti-cardiolipin antibodies are associated with the clinical features of the antiphospholipid antibody syndrome. Thrombosis Haemostasis. 1998;80:16–22. [PubMed] [Google Scholar]
- 46.Nojima J, Kuratsune H, Suehisa E, et al. Anti-prothrombin antibodies combined with lupus anti-coagulant activity is an essential risk factor for venous thromboembolism in patients with systemic lupus erythematosus. Br J Heamatol. 2001;114:647–54. doi: 10.1046/j.1365-2141.2001.02950.x. [DOI] [PubMed] [Google Scholar]
- 47.Nojima J, Kuratsune H, Suehisa E, et al. Acquired protein C resistance is associated with the co-existence of anti-prothrombin antibodies and lupus anticoagulant activity in patients with systemic lupus erythematosus. Br J Haematol. 2002;118:577–83. doi: 10.1046/j.1365-2141.2002.03642.x. [DOI] [PubMed] [Google Scholar]
- 48.Pengo V, Biasiolo A, Brocco T, et al. Autoantibodies to phospholipid-binding proteins in patients with thrombosis and phospholipid-reactive antibodies. Thrombosis Haemostasis. 1996;75:721–4. [PubMed] [Google Scholar]
- 49.Forastiero R, Martinuzzo M, Cerrato G, et al. Relationship of anti-beta2-glycoprotein I and anti prothrombin antibodies to thrombosis and pregnancy loss in patients with antiphospholipid antibodies. Thrombosis Haemostasis. 1997;78:1008–114. [PubMed] [Google Scholar]
- 50.Horbach D, van Oort E, Derksen R, de Groot P. The contribution of anti-prothrombin antibodies to lupus anticoagulant activity – discrimination between functional and non-functional anti-prothrombin antibodies. Thrombosis Haemostasis. 1998;79:790–5. [PubMed] [Google Scholar]
- 51.Galli M, Beretta G, Daldossi M, et al. Different anticoagulant and immunological properties of anti-prothrombin antibodies in patients with antiphospholipid antibodies. Thrombosis Haemostasis. 1997;77:486–91. [PubMed] [Google Scholar]
- 52.Horbach D, van Oort E, Donders R, et al. Lupus anticoagulant is the strongest risk factor for both venous and arterial thrombosis in patients with systemic lupus erythematosus: comparison between different assays for the detection of antiphospholipid antibodies. Thrombosis Haemostasis. 1996;76:916–24. [PubMed] [Google Scholar]
- 53.Atsumi T, Ieko M, Bertolaccini ML, et al. Association of autoantibodies against the phosphatidylserine-prothrombin complex with manifestations of the antiphospholipid syndrome and with the presence of lupus anticoagulant. Arthritis Rheum. 2000;43:1982–93. doi: 10.1002/1529-0131(200009)43:9<1982::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Hwang K-K, Grossman JM, Visvanathan S, et al. Identification of anti-thrombin antibodies in the antiphospholipid syndrome that interfere with the inactivation of thrombin by antithrombin. J Immunol. 2001;167:7192–8. doi: 10.4049/jimmunol.167.12.7192. [DOI] [PubMed] [Google Scholar]
- 55.Shibata S, Harpel PC, Gharavi AE, et al. Autoantibodies to heparin from patients with antiphospholipid antibody syndrome inhibit formation of antithrombin III-thrombin complexes. Blood. 1994;83:2532–40. [PubMed] [Google Scholar]
- 56.Gonias SI, Pizzo SV. The biochemistry of haemostasis. Clin Laboratory Haematol. 1986;8:281–305. doi: 10.1111/j.1365-2257.1986.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 57.Marciniak E, Romond EH. Impaired catalytic function of activated protein C. a new manifestation of lupus anticoagulant. Blood. 1989;74:2426–32. [PubMed] [Google Scholar]
- 58.Borrell EM, Sala N, de Castellarnau C, et al. Immunoglobulin fractions isolated from patients with antiphospholipid antibodies prevent the inactivation of factor Va by activated protein C on human endothelial cells. Thrombosis Haemostasis. 1992;68:268–72. [PubMed] [Google Scholar]
- 59.Smirnov MD, Triplett DT, Comp PC, et al. On the role of phosphtidylethanolamine in the inhibition of activated protein C activity by antiphospholipid antibodies. J Clin Invest. 1995;95:309–16. doi: 10.1172/JCI117657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malia RG, Kirchens S, Greaves M, Preston FE. Inhibition of activated protein C and its cofactor protein S by antiphospholipid antibodies. Br J Haematol. 1990;76:101–7. doi: 10.1111/j.1365-2141.1990.tb07843.x. [DOI] [PubMed] [Google Scholar]
- 61.Comp PC, DeBault LE, Esmon NL, Esmon CT. Human thrombomodulin is inhibited by IgG from two patients with non-specific anticoagulants. Blood. 1983;62:299a. [Google Scholar]
- 62.Freyssinet JM, Wiesel M-L, Gauchy J, et al. An IgM lupus anticoagulant that neutralizes the enhancing effect of phospholipid on purified endothelial thrombomodulin activity. A mechanism for thrombosis. Thrombosis Haemostasis. 1986;55:309–13. [PubMed] [Google Scholar]
- 63.Sakata Y, Curriden S, Lawrence D, et al. Activated protein C stimulates the fibrinolytic activity of cultured endothelial cells and decreases anti-activator activity. Proc Natl Acad Sci USA. 1985;82:1121–5. doi: 10.1073/pnas.82.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hinsberg VWM, Bertina RM, van Wijngaarden A, et al. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood. 1986;65:444–51. [PubMed] [Google Scholar]
- 65.Violi F, Ferro D, Valesini G, et al. Tissue plasminogen activator inhibitor in patients with systemic lupus erythematosus and thrombosis. Br Med J. 1990;300:1099–102. doi: 10.1136/bmj.300.6732.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keeling DM, Campbell SJ, Mackie IJ, et al. The fibrinolytic response to venous occlusion and the natural anticoagulants in patients with antiphospholipid antibodies both with and without systemic lupus erythematosus. Br J Haematol. 1991;77:354–9. doi: 10.1111/j.1365-2141.1991.tb08583.x. [DOI] [PubMed] [Google Scholar]
- 67.Cugno M, Cabibbe M, Galli M, et al. Antibodies to tissue-type plasminogen activator (tPA) in patients with antiphospholipid syndrome: evidence of interaction between the antibodies and the catalytic domain of tPA in two patients. Blood. 2004;00:000–000. doi: 10.1182/blood-2003-07-2422. in press. [DOI] [PubMed] [Google Scholar]
- 68.Mackworth-Young CG, Andreotti F, Harmer IJ, et al. Endothelium-derived haemostatic factors and the antiphospholipid syndrome. Br J Rheumatol. 1995;34:201–6. doi: 10.1093/rheumatology/34.3.201. [DOI] [PubMed] [Google Scholar]
- 69.Andree HA, Stuart MC, Hermens WT, et al. Clustering of lipid-bound annexin V may explain its anticoagulant effect. J Biol Chem. 1992;267:17907–12. [PubMed] [Google Scholar]
- 70.Matsuda J, Saitoh N, Gohchi K, et al. Anti-annexin V antibody in systemic lupus erythematosus patients with lupus anticoagulant and/or anticardiolipin antibody. Am J Hematol. 1994;47:56–8. doi: 10.1002/ajh.2830470112. [DOI] [PubMed] [Google Scholar]
- 71.Rand JH, Wu XX, Quinn AS, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am J Pathol. 2003;163:1193–200. doi: 10.1016/S0002-9440(10)63479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arai T, Matsubayashi H, Sugi T, et al. Anti-annexin A5 antibodies in reproductive failures in relation to antiphospholipid antibodies and phosphatidylserine. Am J Reprod Immunol. 2003;50:202–8. doi: 10.1034/j.1600-0897.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 73.Sugi T, McIntyre JA. Autoantibodies to phosphatidylethanolamine (PE) recognize a kininogen-PE complex. Blood. 1995;86:3083–9. [PubMed] [Google Scholar]
- 74.Merten M, Motamedy S, Ramamurthy S, et al. Sulfatides: targets for anti-phospholipid antibodies. Circulation. 2003;108:2082–7. doi: 10.1161/01.CIR.0000095030.44185.6A. [DOI] [PubMed] [Google Scholar]
- 75.Forastiero RR, Martinuzzo ME, Lu L, Broze GJ. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thrombosis Haemostasis. 2003;1:1764–70. doi: 10.1046/j.1538-7836.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 76.Joseph JE, Harrison P, Mackie IJ, et al. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001;115:451–9. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- 77.Reverter JC, Tassies D, Font J, et al. Effects of human monoclonal anticardiolipin antibodies on platelet function and on tissue factor expression on monocytes. Arthritis Rheum. 1998;41:1420–7. doi: 10.1002/1529-0131(199808)41:8<1420::AID-ART11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 78.Font J, Esinosa G, Tassies D, et al. Effects of beta2-glycoprotein I and monoclonal anticardiolipin antibodies in platelet interaction with subendothelium under flow conditions. Arthritis Rheum. 2002;46:3283–9. doi: 10.1002/art.10634. [DOI] [PubMed] [Google Scholar]
- 79.Robbins DL, Leung S, Miller-Blair DJ, et al. Effect of anticardiolipin/beta2-glycoprotein I complexes on production of thromboxane A2 by platelets from patients with antiphospholipid syndrome. J Rheumatol. 1998;25:51–6. [PubMed] [Google Scholar]
- 80.Opara R, Robbins DL, Ziboh VA, Cyclic AMP. agonists inhibit antiphospholipid/beta2-glycoprotein I induced synthesis of human platelet thromboxane A2 in vitro. J Rheumatol. 2003;30:55–9. [PubMed] [Google Scholar]
- 81.Lutters BCH, Derksen RHWM, Tekelenburg WL, et al. Dimers of β2-glycoprotein I increase platelet deposition to collagen via interaction with phospholipids and the apolipoprotein E receptor 2′. J Biol Chem. 2003;278:33831–8. doi: 10.1074/jbc.M212655200. [DOI] [PubMed] [Google Scholar]
- 82.Reverter JC, Tassies D. Mechanisms of thrombosis in the antiphospholipid syndrome: binding to platelets. In: Khamashta MA, editor. Hughes Syndrome, Antiphospholipid Syndrome. Springer Verlag; 2000. pp. 290–8. [Google Scholar]
- 83.Galli M, Daldossi M, Barbui T. Anti-glycoprotein IIb/IX and IIb/IIIa antibodies in patients with antiphospholipid antibodies. Thrombosis Haemostasis. 1994;71:571–5. [PubMed] [Google Scholar]
- 84.Godeau B, Piette J-C, Fromont P, et al. Specific antiplatelet glycoprotein autoantibodies are associated with the thrombocytopenia of primary antiphospholipid syndrome. Br J Haematol. 1997;98:873–9. doi: 10.1046/j.1365-2141.1997.3063123.x. [DOI] [PubMed] [Google Scholar]
- 85.Carreras LO, Defreyn G, Machin SJ, et al. Arterial thrombosis, intrauterine death and ‘lupus’ anticoagulant: detection of immunoglobulin interfering with prostacyclin formation. Lancet. 1981;i:244–6. doi: 10.1016/s0140-6736(81)92087-0. [DOI] [PubMed] [Google Scholar]
- 86.Rustin MHA, Bull HA, Machin SJ, et al. Effects of the lupus anticoagulant in patients with systemic lupus erythematosus in endothelial cell prostacyclin release and procoagulant activity. J Invest Dermatol. 1988;90:744–8. doi: 10.1111/1523-1747.ep12560947. [DOI] [PubMed] [Google Scholar]
- 87.Coade SB, van Haaren E, Loizou S, et al. Endothelial prostacyclin relaese in systemic lupus erythematosus. Thrombosis Haemostasis. 1989;61:97–100. [PubMed] [Google Scholar]
- 88.Riboldi P, Gerosa M, Raschi E, et al. Endothelium as a target for antiphospholipid antibodies. Immunobiology. 2003;207:29–36. doi: 10.1078/0171-2985-00211. [DOI] [PubMed] [Google Scholar]
- 89.Rosenbaum J, Pottinger BE, Woo P, et al. Measurement and characterisation of circulating anti-endothelial cell IgG in connective tissue diseases. Clin Exp Immunol. 1988;72:450–6. [PMC free article] [PubMed] [Google Scholar]
- 90.Vismara A, Meroni PL, Tincani A, et al. Relationship between anti-cardiolipin and anti-endothelial cell antibodies in systemic lupus erythematosus. Clin Exp Immunol. 1988;74:247–53. [PMC free article] [PubMed] [Google Scholar]
- 91.Hassalaar P, Derksen RHWM, Blokzijl L, de Groot PG. Cross reactivity of antibodies directed against cardiolipin, DNA, endothelial cells and blood platelets. Thrombosis Haemostasis. 1990;63:169–73. [PubMed] [Google Scholar]
- 92.Del Papa N, Guidali L, Spatola L, et al. Relationship between anti-phospholipid and anti-endothelial cell antibodies III. β2 glycoprotein I mediates the antibody binding to endothelial membranes and induces the expression of adhesion molecules. Clin Exp Rheumatol. 1995;13:179–85. [PubMed] [Google Scholar]
- 93.Simantov R, LaSala JM, Lo SK, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–9. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gharavi AE, Pierangeli SS, Colden-Stanfield M, et al. GDKV-induced antiphospholipid antibodies enhance thrombosis and activate endothelial cells in vivo and in vitro. JImmunol. 1999;163:2922–7. [PubMed] [Google Scholar]
- 95.Pierangeli SS, Colden-Stanfield M, Liu JH, et al. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99:1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 96.George J, Blank Y, Levy P, et al. Differential effects of anti-beta 2 glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation. 1998;97:900–6. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 97.Meroni PL, Raschi E, Camera M, et al. Endothelial activation by aPL. a potential pathogenetic mechanism for the clinical manifestations of the syndrome. J Autoimmun. 2000;15:237–40. doi: 10.1006/jaut.2000.0412. [DOI] [PubMed] [Google Scholar]
- 98.Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteix B, et al. NFκB is an essential intermediate in the activation of endothelial cells by anti-β2-glycoprotein I antibodies. Thrombosis Haemostasis. 2002;88:851–7. [PubMed] [Google Scholar]
- 99.Ma K, Simantov R, Jing-Chuan Z, et al. High affinity binding of β2-glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem. 2000;275:15541–8. doi: 10.1074/jbc.275.20.15541. [DOI] [PubMed] [Google Scholar]
- 100.Dobado-Berrios PM, Lopez-Pedrera C, Velasco F, et al. Increased levels of tissue factor mRNA in mononuclear blood cells of patients with primary antiphospholipid syndrome. Thrombosis Haemostasis. 1999;82:1578–82. [PubMed] [Google Scholar]
- 101.Price BE, Jauch J, Shia MA, et al. Anti-phospholipid antibodies bind to apoptotic, but not viable, thymocytes in a β2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–8. [PubMed] [Google Scholar]
- 102.Atsumi T, Khamashta MA, Haworth RS, et al. Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin I. Arthritis Rheum. 1998;41:800–7. doi: 10.1002/1529-0131(199805)41:5<800::AID-ART5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 103.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 104.MacGregor AJ, Dhillon VB, Binder A, et al. Fasting lipids and anticardiolipin antibodies as risk factors for vascular disease in systemic lupus erythematosus. Ann Rhem Dis. 1992;38:529–34. doi: 10.1136/ard.51.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuura E, Kobayashi K, Kasahara J, et al. Anti-β2-glycoprotein I autoantibodies and atherosclerosis. Intern Rev Immunol. 2002;21:51–66. doi: 10.1080/08830180210414. [DOI] [PubMed] [Google Scholar]
- 106.Vaarala O, Alfthan M, Jauhiainen M, et al. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 2003;341:923–5. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 107.Tinahones FJ, Cuadrado MJ, Khamashta MA, et al. Lack of cross-reaction between antibodies to β2-glycoprotein I and oxidised low-density lipoprotein in patients with antiphospholipid syndrome. Br J Rheumatol. 1998;37:746–9. doi: 10.1093/rheumatology/37.7.746. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi K, Matsuura E, Liu Q, et al. A specific ligand for β2-glycoprotein I mediates autoantibody-dependent uptake of oxidised low density lipoprotein by macrophages. J Lipid Res. 2001;42:697–709. [PubMed] [Google Scholar]
- 109.George J, Harats D, Gilburd B, et al. Induction of early atherosclerosis in LDL-receptor-deficient mice immunised with β2-glycoprotein I. Circulation. 1988;98:1108–15. doi: 10.1161/01.cir.98.11.1108. [DOI] [PubMed] [Google Scholar]
- 110.Ames PRJ. Antiphospholipid antibodies, thrombosis and atherosclerosis in systemic lupus erythematosus: a unifying ‘membrane stress syndrome’ hypothesis. Lupus. 1994;3:371–7. doi: 10.1177/096120339400300503. [DOI] [PubMed] [Google Scholar]
- 111.Delgado Alves J, Kumar S, Isenberg DA. Cross-reactivity be-tween anti-cardiolipin, anti-high-density lipiprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology. 2003;42:893–9. doi: 10.1093/rheumatology/keg248. [DOI] [PubMed] [Google Scholar]
- 112.Sherer Y, Shoenfeld Y. Antiphospholipid antibodies: are they pro-atherogenic or an epiphenomenon of atherosclerosis? Immunobiology. 2003;207:13–6. doi: 10.1078/0171-2985-00212. [DOI] [PubMed] [Google Scholar]
- 113.Mackworth-Young CG, Loizou S, Walport MJ. Antiphospholipid antibodies and disease. Quart J Med. 1989;72:767–77. [PubMed] [Google Scholar]
- 114.Stone S, Khamashta MA, Poston L. Placentation, antiphospholipid syndrome and pregnancy outcome. Lupus. 2001;10:67–74. doi: 10.1191/096120301667486047. [DOI] [PubMed] [Google Scholar]
- 115.Gharavi AE, Pierangeli SS, Levy RA, Harris EN. Mechanisms of pregnancy loss in antiphospholipid syndrome. Clin Obstet Gynecol. 2001;44:11–9. doi: 10.1097/00003081-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 116.Parke AL. Placental pathology in antiphospholipid antibody syndrome. In: Khamashta MA, editor. Hughes Syndrome, Antiphospholipid Syndrome. London: Springer Verlag; 2000. pp. 281–9. [Google Scholar]
- 117.Donohoe S, Kingdom JCP, Mackie IJ. Affinity purified human antiphospholipid antibodies bind normal term placenta. Lupus. 1999;8:525–31. doi: 10.1191/096120399678840756. [DOI] [PubMed] [Google Scholar]
- 118.Chamley LW, Duncalf AM, Mitchell MD, et al. Action of anticardiolipin and antibodies to beta 2 glycoprotein I on trophoblast proliferation as a mechanism for fetal death. Lancet. 1998;352:1037–8. doi: 10.1016/s0140-6736(05)60080-3. [DOI] [PubMed] [Google Scholar]
- 119.Di Simone N, Meroni PL, Del Papa N, et al. Antiphospholipid antibodies affect trophoblast gonadotrophin secretion and invasiveness by binding directly and through adhered beta 2 glycoprotein I. Arthritis Rheum. 2000;43:140–53. doi: 10.1002/1529-0131(200001)43:1<140::AID-ANR18>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tartakovski B, Bermas BL, Sthoeger Z, et al. Defective maternal–fetal interaction in a murine autoimmune model. Human Reprod. 1996;11:2408–11. doi: 10.1093/oxfordjournals.humrep.a019125. [DOI] [PubMed] [Google Scholar]
- 121.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–20. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu C, Mao D, Holers VM, et al. A critical role for the murine complement receptor Crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 124.Out HJ, Kooijman CD, Bruinse HW, Derksen RH. Histopathological findings in placentae from patients with intra-uterine fetal death and antiphospholipid antibodies. Eur J Obstet Gynecol Reprod Biol. 1991;41:179–86. doi: 10.1016/0028-2243(91)90021-c. [DOI] [PubMed] [Google Scholar]
- 125.Magid MS, Kaplan C, Sammaritano LR, et al. Placental pathology in systemic lupus erythematosus: a prospective study. Am J Obstet Gynecol. 1998;179:226–34. doi: 10.1016/s0002-9378(98)70277-7. [DOI] [PubMed] [Google Scholar]
- 126.Davis WD, Brey RL. Antiphospholipid antibodies and complement activation in patients with cerebral ischaemia. Clin Exp Immunol. 1992;10:455–60. [PubMed] [Google Scholar]
- 127.Munakata Y, Saito T, Seino J, et al. Detection of complement-fixing antiphospholipid antibodies in association with thrombosis. Thrombosis Haemostasis. 2000;83:728–31. [PubMed] [Google Scholar]
- 128.Levine SR, Brey RL, Tilley BC, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. J Am Med Assoc. 2004;291:576–84. doi: 10.1001/jama.291.5.576. [DOI] [PubMed] [Google Scholar]
- 129.Kent M, Vogt E, Rote NS. Monoclonal antiphosphatidylserine antibodies react directly with feline and murine central nervous system. J Rheumatol. 1997;24:1725–33. [PubMed] [Google Scholar]
- 130.Caronti B, Calderaro C, Alessandri C, et al. Serum anti-beta2-glycoprotein I antibodies from patients with antiphospholipid antibody syndrome bind central nervous system cells. J Autoimmun. 1998;11:425–9. doi: 10.1006/jaut.1998.0214. [DOI] [PubMed] [Google Scholar]
- 131.Chapman J, Cohen-Armon M, Shoenfeld Y, Korczyn AD. Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus. 1999;8:127–33. doi: 10.1191/096120399678847524. [DOI] [PubMed] [Google Scholar]
- 132.Shoenfeld Y, Nahum A, Korczyn AD, et al. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus. 2003;12:436–42. doi: 10.1191/0961203303lu409oa. [DOI] [PubMed] [Google Scholar]
- 133.Lima F, Khamashta MA, Buchanan NMM, et al. A study of sixty pregnancies with the antiphospholipid syndrome. Clin Exp Rheumatol. 1996;14:131–6. [PubMed] [Google Scholar]
- 134.Ringrose DK. Anaesthesia and antiphospholipid syndrome – a review of 20 obstetric patients. Int J Obstet Anaesth. 1997;6:107–11. doi: 10.1016/s0959-289x(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 135.Brenner B, Vulfons SL, Lanir N, Nahir M. Coexistence of familial antiphospholipid syndrome and factor V Leiden: impact on thrombotic diathesis. Br J Haematol. 1996;94:166–7. doi: 10.1046/j.1365-2141.1996.d01-1757.x. [DOI] [PubMed] [Google Scholar]
- 136.Rosendaal F. Thrombosis in the young: epidemiology and risk factors. A focus on venous thrombosis. Thrombosis Haemostasis. 1997;78:1–6. [PubMed] [Google Scholar]