Abstract
This report is focused on the functional capacity of Langerhans cells (LC) in the epithelium of skin and oral mucosa, which both meet different antigenic challenges. The capacity of LC from human oral and skin epithelium to provide co-stimulatory signals to T cells in vitro was compared. LC in a crude suspension of oral epithelial cells had a significantly enhanced T cell co-stimulatory capacity compared to skin epithelial cells. This applied both to cultures with concanavalin A (con-A)-stimulated syngeneic T cells and to a mixed epithelial cell lymphocyte reaction involving allogeneic T cells. The co-stimulatory capacity of oral and skin epithelial cells was reduced by >70% if monoclonal antibodies against HLA-DR, -DP and -DQ were added to the cultures with allogeneic T cells, indicating the involvement of HLA class II expressing LC. Immunohistochemistry revealed that 6% of the epithelial cells were CD1a + LC in sections from both oral and skin epithelium. Interleukin (IL)-8 production was higher in cultures of oral epithelial cells and con-A stimulated T cells than in corresponding cultures with skin epithelial cells as accessory cells. The results suggest that LC in human oral epithelium are more efficient at stimulating T cells than those of skin.
Keywords: co-stimulation, dendritic cells, human, MHC, T lymphocytes
INTRODUCTION
The oral mucosa and intestine are exposed to large amounts of antigens emanating from food, bacteria, viruses, fungi and their by-products. In skin, not harbouring such a diverse microbiota, the quantity of antigenic exposure assumable is less. This difference in antigenic load may demand different immune responses.
Langerhans cells (LC) are a subpopulation of the bone marrow-derived dendritic cells (DC). They are antigen-presenting cells (APC), capable of internalizing and processing antigens [1]. Because they reside in epithelium of skin and mucosal membranes they may be the primary target cell for antigens entering oral mucosa and skin. Human LC express CD1a, human leucocyte antigen (HLA) -DR, -DP, -DQ, CD80, CD83, CD86, CD40, Langerin/CD207 and Birbeck's granulae [2–6]. After antigen uptake they migrate to regional lymph nodes where peptides, in the context of major histocompatibility complex (MHC) class II molecules, are presented to T cells with appropriate T cell receptors. This first signal to the T cell, together with a second signal, delivered in part by the interaction between CD80 and CD86 molecules on the LC and CD28 and CTLA4 (CD152) ligands on the T cell, results in expansion, but also regulation of T cell clones [7,8]. During the migration from the epithelium LC mature functionally with increased expression of MHC class II molecules, CD80, CD86 and adhesion molecules but decreased expression of CD1a and Birbeck's granulae [9–11]. Freshly isolated LC from skin are inefficient as APC, while DC found in draining lymph nodes are highly immunostimulatory [10]. Freshly isolated epidermal LC are also poor stimulators of allogeneic T cells in a mixed epithelial cell lymphocyte reaction (MELR) in comparison with cultured epidermal LC [12,13]. Recent knowledge on DC maturation has been gained from DC generated from human CD34-positive progenitor cells [3,14]. Expression and up-regulation of CD83 enhance allogenic T cell response, thus indicating maturation of dendritic cells [15]. Cytokines, especially granulocyte-macrophage colony stimulating factor (GM-CSF) and tumour necrosis factor (TNF), are essential in LC/DC maturation [14,16,17].
Keratinocytes and LC produce interleukin (IL)-8, which are chemotactic for T cells and neutrophils [18,19]. LC carry receptors for this chemokine [20]. Proinflammatory cytokines such as IL-1β and TNF induce IL-8 secretion [21]. Thus LC both produce and are influenced by these substances.
Studies on DC/LC derived from skin, oral mucosa, airways, peripheral blood and gut show differences in maturation relating to the origin of the cells, manifested as a variation in the capacity to induce a T cell response [12,22–25]. To mount an appropriate response to antigen at various body compartments it is most likely that DC/LC are adapted to the local environment. We have reported earlier that in rodents, freshly isolated LC from oral epithelium have potent T cell co-stimulatory capacity compared to skin LC [25]. The present study was undertaken in order to determine whether there is a similar functional difference between human oral and skin LC.
MATERIALS AND METHODS
Subjects
Healthy volunteers (six men, age range 29–46 years, mean 41 years, median 44 years) without medical histories of allergies, chronic diseases or ongoing medication were asked to participate in the experiments.
Biopsies
Biopsy areas were washed with 0·1% chlorhexidine solution (Ipex Medical AB, Helsingborg, Sweden). Punch biopsies (5 mm and 3 mm in diameter) of oral mucosa (bucca) and skin (ventral side of lower leg) were obtained under local anaesthesia (prilocaine hydrochloride 30 mg/ml, felypressin 0·54 µg/ml; Citanest Octopressin®, Astra, Södertälje, Sweden). Biopsy specimens with 5 mm diameter were transferred immediately to 0·9% saline for use in cell cultures, while 3 mm specimens were transferred to Histocon® (Histolab, Bethlehem Trading Ltd, Göteborg, Sweden), and snap-frozen in embedding medium (OCT Compound, Miles, IN, USA) for use in immunohistochemical analysis. Venous blood was drawn from each volunteer. The Ethical Committee at Göteborg University approved the study.
Cell suspensions
T cells
Peripheral blood mononuclear cells (PBMC) were isolated on Lymphoprep® from heparinized venous blood (20 ml) according to the manufacturer's instructions (Nycomed, Pharma AS, Oslo, Norway). Cells were then seeded in culture flasks (Nunc AS, Roskilde, Denmark) and incubated at 37°C for 45 min in Dulbecco's modification of Eagle's medium (DMEM) containing 10% heat-inactivated fetal calf serum (FCS), 1%l-glutamine, gentamycin (25 mg/l), penicillin (100 U/ml) and streptomycin (100 µg/ml; DMEM + +). Non-adherent cells were recovered and residual class II molecule-expressing cells were removed by the following procedures: (i) incubation of recovered cells (5 × 106) with mouse antihuman HLA-DR, -DP, -DQ monoclonal antibody (100 µg/ml; clone Tü39, Pharmingen, San Diego, CA, USA) in 4°C for 30 min; (ii) repeated washes in cold medium to remove free antibodies; (iii) incubation for 60 min at 37°C under gentle stirring with immunomagnetic beads coated with a sheep antimouse IgG according to the manufacturer's instructions (Dynabeads® M-280, Dynal AS, Oslo, Norway); (iv) separation of HLA-DR, -DP and -DQ molecule-expressing cells with a magnet. The viability of the recovered cells was estimated by trypan blue exclusion and consistently exceeded 95%.
Oral and skin epithelial cells
Dissected oral mucosal and skin specimens were put into 0·2% chlorhexidine solution (Hibitane Dental, ICI-Pharma, Stockholm, Sweden) for 10 s and rinsed in phosphate buffered saline (PBS) for 10 s. Specimens were then floated on Dispase type II (Boehringer-Mannheim, GmBH, Mannheim, Germany) diluted to a concentration of 1·2 U/ml in DMEM + + for 2 h. After incubation, epithelia were separated easily from the underlying connective tissue, placed with the subepithelial side up in Petri dishes, covered with 0·5% trypsin (Trypsine, Life Technologies, Paisley, UK) and incubated further at 37°C for 30 min in 5% CO2 atmosphere. Immediately after this incubation procedure, tissue sheets were covered with DMEM + + supplemented with 20% FCS to inactivate the trypsin. To release oral epithelial and epidermal cells, sheets were first transferred to a vial containing fresh DMEM + + supplemented with 20% FCS and then subjected to pipetting. After sedimentation a supernatant containing released cells was harvested. The cell suspensions were washed twice in DMEM + + and cells were counted. Cell viability was assessed by trypan blue exclusion.
Immunohistochemistry
Immunohistochemistry to determine the number of CD1a- and CD83 molecule-expressing cells in oral and skin epithelium was performed on frozen sections (4 µm) prepared and air-dried for 15 min. The sections were then fixed in acetone for 10 min at 4°C and air-dried for 20 min. All incubations were followed by extensive rinsing in Tris-HCL buffer (pH 7·6) containing 0·9% NaCl (TBS) for at least 15 min. Endogenous peroxidase activity was inhibited by incubating the sections in 0·3% H2O2 in TBS for 5 min. After this the sections were incubated with 4% bovine serum albumin (BSA) in TBS for 30 min at room temperature. Incubation with a mouse anti-CD1a monoclonal antibody (NA1/34; Serotec Ltd, Oxon, UK) diluted in 4% BSA in TBS was performed at 4°C overnight. Sections were then incubated with biotinylated F(ab)2 IgG rabbit antimouse antibodies (Dako AS, Glostrup, Denmark) diluted in 4% BSA in TBS for 30 min. Incubation with the secondary antibody was followed by incubation with avidin–biotin–peroxidase complex (Dakopatts AS) for 30 min in room temperature.
Incubation with a mouse anti-CD83 monoclonal antibody (HB15e; Serotec Ltd) diluted in 4% BSA in TBS was performed for 30 min at room temperature. This was followed by incubation for 30 min at room temperature with Dako EnVision™ (peroxidase, antimouse, Dakopatts AS).
Sections were then developed for 10 min in 3-amino-9-ethyl-carbaxol (10 mg) dissolved in dimethyl-sulphoxide (6 ml) in sodium acetate (50 ml, 0·02 m, pH 5·5) and 4 µl H2O2 (30%) and counterstained with Mayer's haematoxylin. The monoclonal antibody and secondary antibody were diluted in 4% BSA in TBS. Negative controls were incubated with 4% BSA in TBS instead of monoclonal antibody (MoAb). Quantitative analysis was conducted on 1–2 consecutive sections of each biopsy. Two or three high-power fields (HPF × 200) in the periphery and the middle of the sections were selected for cell counting. In a light microscope with a chilled colour CCD camera, computerized images of the HPF were obtained. The sections were then analysed with the NIH Image Software (National Institute of Health, Bethesda, USA). Within the epithelium all nucleated positively and negatively stained cells were counted. Results were expressed as the mean percentage of positively stained cells (CD1a) or number of positive cells/mm2 (CD83).
Interleukin-8 (IL-8) enzyme-linked immunosorbent assay (ELISA)
The presence of IL-8 in supernatants from cell cultures, derived from two subjects, was examined by ELISA. Briefly, 96-well plates (Immunoplate, Maxisorp, Nunc, Denmark) were coated with goat antihuman IL-8 antibody (10 µg/ml; clone AB-208-NA, R&D Systems, UK) diluted in carbonate buffer (pH 9·0) overnight at 4°C. This was followed by repeated washing in PBS and the plates were blocked with 2% BSA for 2 h. Recombinant IL-8 (32 ng/ml−0·5 ng/ml; R&D systems, UK) was used as a standard. The standard and duplicate samples, in serial dilutions, were incubated for 3 h at room temperature. This was followed by overnight incubation at 4°C with a rabbit antihuman IL-8 antibody (1 µg/ml; clone P-801, Endogen, Boston, MA, USA) followed by incubation for 2 h with an alkaline phosphatase conjugated goat antirabbit IgG antibody (1 : 1000; clone D487, Dakopatts, Copenhagen, Denmark). Captured IL-8 was visualized with the substrate P-nitrophenyl phosphate (Sigma, Stockholm, Sweden). Absorbance was measured at 405 nm with an ELISA counter (Spectra MAX, Molecular Devices Corp., CA, USA).
Functional assays
T cells (2·5 × 105 cells/well) were incubated with 2·5 µg/ml concanavalin A (con A; Pharmacia LKB Biotechnology AB, Uppsala, Sweden) together with syngeneic oral or skin epithelial cells (1 × 104 cells/well). In some cultures mouse anti-HLA-DR, -DP, -DQ MoAbs were added (25 µg/ml; clone Tü39; Pharmingen). As controls, T cells and epithelial cells were incubated separately. All incubations were performed in duplicate or triplicate at 37°C for 72 h in 5% CO2 atmosphere in 96-well U-bottomed culture plates (Nunc AS) containing 0·2 ml DMEM + + 0. Following 48 h of incubation, 5 µCi/ml of [3H]-thymidine (methyl-[3H]-thymidine; 24 Ci/nMol, Amersham, Buckinghamshire, UK) was added to each well. After 72 h the cells were harvested onto glass fibre filters in a Skatron harvester (Skatron, Flow Laboratories, Oslo, Norway) and counted in a liquid scintillator.
Supernatants from cell cultures from two subjects, as described above, were harvested after 72 h. Culture plates were centrifuged at 200 g for 10 min and 150 µl aliquots of supernatants were collected and frozen in −70°C for analysis of IL-8 production.
Allogeneic T cells (2·5 × 105 cells/well) from one subject served as responder cells in a mixed epithelial cell lymphocyte reaction (MELR). Oral or skin epithelial cells (1 × 104 cells/well) from another subject served as stimulator cells. Some cultures received a mouse antihuman HLA-DR, -DP, -DQ monoclonal antibody (25 µg/ml; Tü39, Pharmingen). As controls, T cells and epithelial cells were incubated separately. All incubations were performed in duplicate or triplicate at 37°C for 72 h in 5% CO2 atmosphere in 96-well U-bottomed culture plates (Nunc AS) containing 0·2 ml DMEM + + and following 72 h of incubation, 5 µCi/ml of [3H]-thymidine (methyl-[3H]-thymidine; 24 Ci/nMol, Amersham, Buckinghamshire, UK) was added to each well. After 96 h the cells were harvested onto glass-fibre filters in a Skatron harvester (Skatron) and counted in a liquid scintillator.
In a mixed lymphocyte reaction, peripheral blood mononuclear cells (total 2·5 × 105 cells/well) from two subjects were mixed in equal numbers in triplicate cultures at 37°C in 5% CO2 atmosphere in 96-well U-bottomed culture plates (Nunc AS) containing 0·2 ml DMEM + + 0. Following 24, 48, 72 and 96 h of incubation, 5 µCi/ml of [3H]-thymidine (methyl-[3H]-thymidine; 24 Ci/nMol, Amersham) was added to each well and cells were harvested 24 h later and counted as described above. In some cultures mouse anti-HLA-DR, -DP, -DQ MoAbs were added (25 µg/ml; Tü39; Pharmingen). Following 72 h of incubation, the cells were pulsed with 5 µCi/ml of [3H]-thymidine harvested and counted as described above. Control cultures received an irrelevant MoAb of the same isotype (25 µg/ml; mouse IgG2α,κ; Sigma, St Louis, MO, USA), when cell yield permitted.
Statistical analysis
Statistical analyses of differences between groups were performed using the Wilcoxon signed-rank test. A P-value <0·05 was considered as a significant difference.
RESULTS
CD1a molecule-expressing Langerhans cells and CD83 molecule-expressing cells in oral and skin epithelium
CD1a-positive cells in oral and skin epithelium were located preferentially in the suprabasal portion of the epithelium and showed CD1a-positive cells with dendritic morphology. Enumeration of positive and negative cells revealed a mean frequency of 5·8% (range: 1·0–10·5) CD1a-positive LC in oral epithelium and 5·7% (range: 3·8–8·0) CD1a-positive LC in skin epithelium (Table 1).
Table 1.
CD1a-positive Langerhans cells (LC) in biopsies from oral and skin epithelium
| Subject no. | Frequency of LC in oral epithelium (%) | Frequency of LC in skin epithelium (%) |
|---|---|---|
| 1 | Not done | Not done |
| 2 | 6·6 ± 3·1 | 8·0 ± 3·2 |
| 3 | 10·5 ± 1·4 | 3·8 ± 2·0 |
| 4 | Not done | Not done |
| 5 | 5·0 ± 3·2 | 6·3 ± 0·4 |
| 6 | 1·0 ± 0·1 | 4·6 ± 3·0 |
Frequency of CD1a-positive Langerhans cells in sections from subjects. Data are expressed as the percentage (%) of positive cells ± standard deviation.
CD83-positive cells in oral and skin epithelium were located preferentially in the suprabasal portion of the epithelium. In oral epithelium some CD83-positive cells showed the characteristic dendritic morphology of LC, while dendritic appearance was less pronounced in skin epithelium. Enumeration of the number of positive cells revealed a mean number of 40 positive cells/mm2 (range: 3–120) in oral epithelium and seven positive cells/mm2 (range: 0–15) in skin epithelium (Table 2).
Table 2.
CD83-positive cells in biopsies from oral and skin epithelium
| Subject no. | CD83-positive cells in oral epithelium (no. of cells/mm2) | CD83-positive cells in skin epithelium (no. of cells/mm2) |
|---|---|---|
| 1 | 18 ± 2 | 14 ± 0·4 |
| 2 | 3 ± 1 | 0 |
| 3 | 120 ± 8 | 0 |
| 4 | Not done | Not done |
| 5 | 20 ± 3 | 15 ± 1 |
| 6 | Not done | Not done |
CD83-positive cells in sections from subjects. Data are expressed as the number of positive cells/mm2± standard deviation.
Cell yield
Oral biopsies yielded a mean of 2·6 × 105 cells (range: 1–3·8 × 105 cells), while skin biopsies gave a mean of 4·7 × 105 cells (range: 1–6·1 × 105). Cell viability exceeded 95%. Dead cells were large cells with keratinocyte morphology and without nucleus.
Co-stimulatory capacity of oral and skin LC incubated with con-A-stimulated T cells
Incubation of oral and skin epithelial cell suspensions containing LC, with syngeneic T cells in the presence of con-A, resulted in a mean T cell proliferation response 1·5 times higher in cultures with oral epithelial cells than in cultures with skin epithelial cells (P < 0·05; Table 3). In all cultures containing oral epithelial cells and con-A-stimulated T cells proliferation was higher than in cultures containing skin epithelial cells and T cells, although two subjects (nos 3 and 5) showed little difference (Table 3). Con-A stimulation of peripheral blood mononuclear cells showed substantial interindividual variation (Table 3). Cultures of oral and skin epithelial cells without T cells or con-A never gave counts per million (cpm) values above 500, confirming insignificant proliferation of epithelial cells alone.
Table 3.
Co-stimulatory activity of human oral and skin epithelial cells, including Langerhans cells, incubated with syngeneic T cells and con-A
| Subject no. | Oral epithelial cells + T cells + con-A | Skin epithelial cells + T cells + con-A | Oral epithelial cells + T cells | Skin epithelial cells + T cells | T cells + con A | T cells | Peripheral blood mononuclear cells + con-A | Peripheral blood mononuclear cells |
|---|---|---|---|---|---|---|---|---|
| 1 | 13573 ± 84 | 6011 ± 2738 | 330a | 127a | 2204 ± 363 | 63a | 19991 ± 3087 | 10734 ± 532 |
| 2 | 36950 ± 9537 | 29716 ± 19082 | 1060 ± 89 | 853 ± 306 | 13518 ± 1285 | 482 ± 42 | 91534 ± 8701 | 2938 ± 528 |
| 3 | 8828 ± 2520 | 6109 ± 1579 | 473 ± 101 | 499 ± 343 | 1243 ± 613 | 339 ± 41 | 26043 ± 1096 | 3323 ± 467 |
| 4 | 41748 ± 10816 | 21113 ± 11080 | 640 ± 297 | 725 ± 391 | 1856 ± 567 | 780 ± 373 | 110014 ± 25251 | Not done |
| 5 | 17295 ± 3967 | 16741 ± 3099 | 597 ± 106 | 474 ± 159 | 547 ± 41 | 476 ± 70 | 13501 ± 3144 | Not done |
| P < 0·05 | Not significant |
Accessory cell capacity of fresh oral and skin epithelial cells (1 × 104 cells/well) in con-A stimulation of syngeneic T cells (2·5 × 105 cells/well) and con-A-stimulated peripheral blood mononuclear cells (2·5 × 105 cells/well). The data are expressed as mean cpm ± standard deviation of triplicate culture wells (except as noted) in five experiments on five subjects;
cpm from one well.
Capacity of oral and skin LC to stimulate allogeneic T cells
Oral and skin epithelial cells were incubated with allogeneic T cells. The oral epithelial cells induced 1·3 times higher mean response in the allogeneic T cells than did the skin epithelial cells (P < 0·05; Table 4). T cells incubated with syngeneic oral and skin epithelial cells did not show any significant difference in proliferative response (Table 4, P > 0·05). In all subjects oral epithelial cells induced higher allogeneic T cell responses than did skin epithelial cells, although a considerable interindividual variation was registered (Table 4). Also, three subjects (nos 3, 5 and 6) showed little difference between oral and skin epithelial cells in allogeneic T cells response, but in all three subjects the allogeneic T cells response induced by oral epithelial cells was higher than when skin epithelial cells were used for stimulation (Table 4). One subject (no. 2) had a marked increase in autoproliferation of syngeneic and allogeneic T cells (Table 4), which may be caused by incomplete purification of T cells. However, any remaining accessory cells in the T cell suspension would have affected the oral and skin epithelial cell cultures equally.
Table 4.
Co-stimulatory activity of human oral and skin epithelial cells incubated with allogeneic or syngeneic T cells in the mixed epithelial cell lymphocyte reaction
| Subject no. | Oral epithelial cells + allogeneic T cells | Skin epithelial cells + allogeneic T cells | Oral epithelial cells + syngeneic T cells | Skin epithelial cells + syngeneic T cells | Oral epithelial cells + allogeneic T cells + antibodies against HLA DR, DP, DQ | Skin epithelial cells + allogeneic T cells + antibodies against HLA DR, DP, DQ | Allogeneic T cells | Syngeneic T cells |
|---|---|---|---|---|---|---|---|---|
| 2 | 58169 ± 5626 | 44055 ± 2149 | 29516 ± 343 | 22229 ± 1955 | 4576 ± 1116 | 2390 ± 9 | 16532 ± 1245 | 26114 ± 556 |
| 3 | 10682 ± 3692 | 9273 ± 1595 | 1158 ± 158 | 3340 ± 2437 | 1905 ± 642 | 2126 ± 570 | 443 ± 28 | 234 ± 50 |
| 4 | 5627 ± 2025 | 2248 ± 1211 | 1841 ± 1704 | 426 ± 107 | 2146 ± 1324 | 1293 ± 249 | 1214 ± 72 | 262a |
| 5 | 2846 ± 906 | 2450 ± 491 | 697 ± 202 | 369 ± 168 | 1119a | 632 ± 44 | 740 ± 309 | 418 ± 21 |
| 6 | 9546 ± 330 | 8456 ± 492 | 2545 ± 190 | 2547 ± 328 | 3899 ± 693 | 2496 ± 222 | 443 ± 28 | 300 ± 45 |
| P < 0·05 | Not significant |
Accessory cell capacity of fresh oral and skin epithelial cells (1 × 104 cells/well) in stimulation of allogeneic T cells (2·5 × 105 cells/well). The data are expressed as mean cpm ± standard deviation of triplicate culture wells in five experiments on five subjects except those noted;
cpm from one well.
The mean T cell response in the five MELR assays was reduced by >70% when anti-HLA-DR, -DP, -DQ monoclonal antibodies were added to the cultures of both oral and skin epithelial cells and allogeneic T cells (Table 4). Cultures of oral and skin epithelial cells without T cells or con-A never gave cpm values above 500, confirming insignificant proliferation of epithelial cells alone. In mixed lymphocyte reactions, the anti-HLA -DR, -DP, -DQ monoclonal antibodies reduced the proliferation of peripheral blood mononuclear cells by >50% compared to cultures with isotype-matched monoclonal antibodies present (data not shown).
IL-8 production in cultures of con-A stimulated T cells and oral or skin epithelial cells
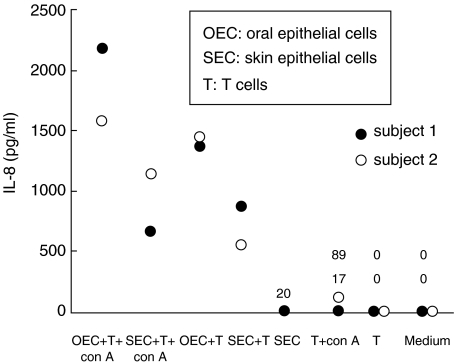
Oral or skin epithelial cells were cultured with con-A-stimulated syngeneic T cells and supernatants were collected after 72 h. In the cultures containing oral epithelial cells there was a higher concentration of IL-8 than in cultures containing skin epithelial cells (Fig. 1). Also, without con-A stimulation cultures of oral epithelial cells and T cells produced IL-8 (Fig. 1). In cultures of skin epithelial cells or T cells alone IL-8 production was diminutive (Fig. 1). Cell yields did not allow assay of oral epithelial cells separately.
Fig. 1.
IL-8 production, measured by ELISA, in supernatants from cultures of oral and skin epithelial cells (1 × 104 cells/well) and T cells (2·5 × 105 cells/well) with or without con-A present following 4 days of incubation. Data are from two subjects, cultures of skin epithelial cells only from one subject.
DISCUSSION
The present study indicates clearly that human oral epithelial cells provided T cells with more efficient co-stimulation than cells from skin epithelium in a MELR or during con-A stimulation. The co-stimulatory capacity in the MELR was dependent on HLA-DR, -DP and -DQ-expressing cells.
These findings are in line with a previous study in which we report that rat oral epithelial cells cultured with allogeneic T cells or syngeneic con-A-stimulated T cells are superior co-stimulatory cells compared to skin epithelial cells [25]. The allogeneic T cell stimulating capacity of epithelial cells was reliant on MHC class II molecule-expressing cells, as depletion of such cells resulted in abolished T cell proliferation [25]. In healthy human epidermis LC are the only cells expressing HLA-DR, -DP -DQ, and even if keratinocytes during pathological conditions and in culture can express class II molecules they are not able to deliver co-stimulatory help to T cells in a MELR [26–28]. Thus, the T cell stimulation is conditional of LC.
Topographically, LC are the first professional APC that may encounter a certain antigen [2]. The two-step signalling process, necessary to induce a T cell response, starts with presentation of peptides by professional APC to a T cell with appropriate specificity, followed by a second signal, delivered in part by CD80 and CD86 present on the APC [29]. Difference in signal strength and temporal sequence between CD80 and CD86 give either co-stimulation or suppression of the T cell response or induction of regulatory T cell [8,30]. Thus, LC are part of the intricate system for regulation of the immune responses both to foreign and self antigens.
It is conceivable that keratinocytes produce soluble factors necessary for LC maturation, as in vitro culturing of purified LC or generation of DC from progenitor cells demand the addition of extrinsic factors [12]. Key cytokines in LC maturation are GM-CSF together with TNF, which can be used to generate DC/LC from CD34-positive progenitor cells [3]. Keratinocytes can produce GM-CSF and TNF [18]. Thus, a suspension of epithelial cells containing both LC and keratinocytes constitute a good environment for LC. The experimental design in this study, using whole suspensions of epithelial cells in cultures, ensures keratinocyte-derived factors vital for LC viability.
Analysis of human epidermal cells with flow cytometry has shown that LC constitute 2–5% of the epithelial cells [31]. Our findings of approximately 6% CD1a-expressing cells with dendritic morphology in sections of oral and skin epithelium is close to that figure. The interindividual range in frequency of CD1a-expressing cells is high and most subjects have a slightly higher frequency of CD1a-expressing cells in skin than in oral epithelium. Thus, the superior co-stimulatory capacity of oral epithelial cells is not due to higher numbers of LC in oral epithelium compared to skin epithelium.
In healthy human epidermis LC are the only cells expressing MHC class II molecules [26,27]. However, during pathological conditions and in culture keratinocytes can be stimulated to express class II molecules [27,32,33]. Keratinocytes can also express CD80 and CD86, which has been shown in epithelium of patients with atopic dermatitis [34]. IFN-γ treatment of keratinocytes does not seem to induce CD80 and CD86 expression [35]. However, even if keratinocytes do express CD80 and CD86 they fail to stimulate T cells in MELR [28]. Consequently, it is reasonable to assume that the cells responsible for the T cell proliferation in our assays were the HLA-DR, -DP, -DQ and CD80-, CD86-expressing LC. Cell yield did not permit concomitant culture of irrelevant isotype matched monoclonal antibodies in the MELR, but the anti-HLA DR, -DP, -DQ monoclonal antibodies inhibited cell proliferation in MELR and mixed lymphocyte reactions [36]. The incomplete blocking of T cell proliferation by the anticlass II antibodies could be explained by CD8-positive T cell proliferation.
In the experiments with both syngeneic and allogeneic T cells as responder cells and oral or skin epithelial cells as stimulator cells there was a considerable interindividual difference in allogeneic T cell proliferation. However, there was always the same relation in the T cell response between cultures with oral and skin epithelial cells, i.e. oral epithelial cells induced higher T cell proliferation in each individual.
The oral cavity, being the first part of the gastrointestinal tract, is constantly exposed to antigens from microbes and food. This antigenic influx may cause an up-regulation of the maturation stage of LC. LC isolated from skin epithelium show low co-stimulatory potential in vitro, while culturing them for 2–5 days before adding T cells increase the co-stimulatory capacity [10,37]. In contrast, fresh oral LC have high co-stimulatory potential in vitro, without prior culturing [25]. Similarly, Liu et al. have shown that fresh DC derived from intestinal epithelium have a functional capacity resembling mature DC [23]. The intestine, just as the oral cavity, is loaded with microbes and food antigens. It could be expected that this would bestow oral LC with an enhanced capacity to induce a T cell response. Zhou et al. report that in vitro-generated CD1a+ CD83+ cells are potent stimulatory cells in allogeneic mixed leucocyte reactions [15]. In the present study CD83-positive cells were found in higher numbers in oral epithelium than in skin epithelium, thus supporting the finding that oral DC have higher T cell stimulating capacity than skin DC. The difference in activity between oral and skin DC may be a result of difference in maturation stimuli, e.g. bacterial products. If this is the case we do not know, but a recent study showed that bacterial stimuli had a greater effect on oral than on skin keratinocytes [38]. This might also be the case for oral and skin DC.
Several studies emphasize the importance of proinflammatory cytokines, such as IL-8, in local inflammatory responses to exogenous antigens [39–43]. IL-8 is a potent chemoattractant and activator of inflammatory response [44], which is produced by epithelial cells provoked by bacteria and other stimuli [39,41]. In this study IL-8 was assessed in cultures of oral and skin epithelial cells and con-A-stimulated T cells in two subjects. The cultures with oral epithelial cells had a higher IL-8 concentration than cultures with the skin epithelial cells. The higher concentration of IL-8 in the oral epithelial cell suspension might reflect the presence of various danger signals in the oral mucosa which are not present in the skin. It may also be that the increased immunogenicity of the oral DC is caused by the increased production of IL-8. We will try investigate this possibility by neutralizing IL-8 with monoclonal antibodies. Several investigations have shown that production of IL-8 by epithelial cells is dependent on both the type of bacterial stimulation and time of provocation [21,41,43].
In conclusion, we show that fresh oral LC have a T cell co-stimulating capacity superior to that of cultured skin LC. The results suggest that fresh oral LC have a phenotype, which resemble mature DC. It is likely that local environmental factors in the oral cavity are responsible for this. It can also be speculated that the clinical behaviour of immunomediated skin and mucosal diseases is influenced by this fact.
Acknowledgments
The authors acknowledge the excellent technical assistance of Ms Christina Eklund BSc. We also acknowledge the valuable assistance of Ms Stina Olsson, Ms Helena Kahu, Dr Kerstin Bäckman and Ms Elisabeth Sandberg BSc. This study was supported by the Swedish Medical Research Council (grant no. 13073), Swedish Dental Society and Adlerbertska Forskningsfonden.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Lappin MB, Kimber I, Norval M. The role of dendritic cells in cutaneous immunity. Arch Dermatol Res. 1996;288:109–21. doi: 10.1007/BF02505819. [DOI] [PubMed] [Google Scholar]
- 3.Caux C, Massacrier C, Vanbervliet B, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- 4.Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Dieu-Nosjean MC, Dezutter C, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–30. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jotwani R, Palucka AK, Al-Quotub M, et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167:4693–700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman R, Inaba K. Immunogenicity: role of dendritic cells. Bioessays. 1989;10:145–52. doi: 10.1002/bies.950100503. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 9.Streilein JW, Grammer SF. In vitro evidence that Langerhans cells can adopt two functionally distinct forms capable of antigen presentation to T lymphocytes. J Immunol. 1989;143:3925–33. [PubMed] [Google Scholar]
- 10.Dai R, Grammer SF, Streilein JW. Fresh and cultured Langerhans cells display differential capacities to activate hapten-specific T cells. J Immunol. 1993;150:59–66. [PubMed] [Google Scholar]
- 11.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans’ cell frequency by tumour necrosis factor-alpha. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 12.Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700–5. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witmer-Pack M, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–98. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Dezutter-Dambuyant C, et al. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+ T cells and process soluble antigen. J Immunol. 1995;155:5427–35. [PubMed] [Google Scholar]
- 15.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch F, Heufler C, Kampgen E, Schneeweiss D, Bock G, Schuler G. Tumor necrosis factor alpha maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colony-stimulating factor, without inducing their functional maturation. J Exp Med. 1990;171:159–71. doi: 10.1084/jem.171.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canque B, Camus S, Dalloul A, et al. Characterization of dendritic cell differentiation pathways from cord blood CD34(+) CD7(+) CD45RA(+) hematopoietic progenitor cells. Blood. 2000;96:3748–56. [PubMed] [Google Scholar]
- 18.Feliciani C, Gupta AK, Sauder DN. Keratinocytes and cytokine/growth factors. Crit Rev Oral Biol Med. 1996;7:300–18. doi: 10.1177/10454411960070040101. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa S, Koomen CW, Bos JD, Teunissen MB. Differential modulation of human epidermal Langerhans cell maturation by ultraviolet B radiation. J Immunol. 1999;163:5192–200. [PubMed] [Google Scholar]
- 20.Larregina A, Morelli A, Kolkowski E, Fainboim L. Flow cytometric analysis of cytokine receptors on human Langerhans’ cells. Changes observed after short-term culture. Immunology. 1996;87:317–25. doi: 10.1046/j.1365-2567.1996.451513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68:31–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Holt PG, Oliver J, Bilyk N, et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu LM, MacPherson GG. Antigen processing: cultured lymph-borne dendritic cells can process and present native protein antigens. Immunology. 1995;84:241–6. [PMC free article] [PubMed] [Google Scholar]
- 24.O'Doherty U, Steinman RM, Peng M, et al. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte- conditioned medium. J Exp Med. 1993;178:1067–76. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasseus B, Jontell M, Bergenholtz G, Eklund C, Dahlgren UI. Langerhans cells from oral epithelium are more effective in stimulating allogeneic T-cells in vitro than Langerhans cells from skin epithelium. J Dent Res. 1999;78:751–8. doi: 10.1177/00220345990780030701. [DOI] [PubMed] [Google Scholar]
- 26.Klareskog L, Tjernlund U, Forsum U, Peterson PA. Epidermal Langerhans cells express Ia antigens. Nature. 1977;268:248–50. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- 27.Rowden G, Lewis MG, Sullivan AK. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977;268:247–8. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- 28.Gaspari AA, Ferbel B, Chen Z, Razvi F, Polakowska R. Accessory and alloantigen-presenting cell functions of A431 keratinocytes that stably express the B7 antigen. Cell Immunol. 1993;149:291–302. doi: 10.1006/cimm.1993.1156. [DOI] [PubMed] [Google Scholar]
- 29.Lanier LL, O'Fallon S, Somoza C, et al. CD80 (B7) and CD86 (B70) provide similar co-stimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 30.Nuriya S, Yagita H, Okumura K, Azuma M. The differential role of CD86 and CD80 co-stimulatory molecules in the induction and the effector phases of contact hypersensitivity. Int Immunol. 1996;8:917–26. doi: 10.1093/intimm/8.6.917. [DOI] [PubMed] [Google Scholar]
- 31.Ashworth J, Kahan MC, Breathnach SM. Flow cytometric analysis and sorting of HLA-DR+CD1+ Langerhans cells. Br J Dermatol. 1989;121:11–8. doi: 10.1111/j.1365-2133.1989.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 32.Basham TY, Nickoloff BJ, Merigan TC, Morhenn VB. Recombinant gamma interferon differentially regulates class II antigen expression and biosynthesis on cultured normal human keratinocytes. J Interferon Res. 1985;5:23–32. doi: 10.1089/jir.1985.5.23. [DOI] [PubMed] [Google Scholar]
- 33.Breathnach SM, Katz SI. Keratinocytes synthesize Ia antigen in acute cutaneous graft-vs-host disease. J Immunol. 1983;131:2741–5. [PubMed] [Google Scholar]
- 34.Ohki O, Yokozeki H, Katayama I, Umeda T, Azuma M, Okumura K, Nishioka K. Functional CD86 (B7–2/B70) is predominantly expressed on Langerhans cells in atopic dermatitis [see comments] Br J Dermatol. 1997;136:838–45. [PubMed] [Google Scholar]
- 35.Grousson J, Concha M, Schmitt D, Peguet-Navarro J. Effects of CD40 ligation on human keratinocyte accessory function. Arch Dermatol Res. 1998;290:325–30. doi: 10.1007/s004030050312. [DOI] [PubMed] [Google Scholar]
- 36.Hessle C, Hanson LA, Wold AE. Interleukin-10 produced by the innate immune system masks in vitro evidence of acquired T-cell immunity to E. coli. Scand J Immunol. 2000;52:13–20. doi: 10.1046/j.1365-3083.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 37.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–46. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han DC, Huang GT, Lin LM, Warner NA, Gim JS, Jewett A. Expression of MHC class II, CD70, CD80, CD86 and pro-inflammatory cytokines is differentially regulated in oral epithelial cells following bacterial challenge. Oral Microbiol Immunol. 2003;18:350–8. doi: 10.1046/j.0902-0055.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 39.Madianos PN, Papapanou PN, Sandros J. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect Immun. 1997;65:3983–90. doi: 10.1128/iai.65.10.3983-3990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol. 2001;280:L493–502. doi: 10.1152/ajplung.2001.280.3.L493. [DOI] [PubMed] [Google Scholar]
- 41.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest. 2000;105:1769–77. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clin Sci (Colch) 1999;97:385–90. [PubMed] [Google Scholar]
- 43.Huang GT, Kim D, Lee JK, Kuramitsu HK, Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001;69:1364–72. doi: 10.1128/IAI.69.3.1364-1372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–74. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]