Abstract
Pemphigus foliaceus is an autoimmune blistering skin disease mediated by autoantibodies directed against desmoglein 1 and occurs as a sporadic form throughout the world, or as an endemic form called fogo selvagem in Brazil. Healthy subjects living in Brazilian endemic areas produce antidesmoglein 1 antibodies, suggesting the role of environmental factors in the initiation of the autoimmune response. Tunisia was described recently as an endemic area where the disease is characterized by its high rate among young people, especially women. An enzyme-linked immunosorbent assay using recombinant desmoglein 1 as antigen was used to detect antibodies against desmoglein 1 and calibrated with sera from 67 French healthy blood donors, 20 French pemphigus foliaceus patients and patients with other bullous skin diseases. When sera from 179 healthy Tunisian blood donors were tested, 31 (17%) were found positive. The desmoglein 1 binding activity of these 31 sera was confirmed in 10 cases by indirect immunofluorescence analysis and/or immunoblotting using human epidermal extract. Subclass analysis of antidesmoglein 1 antibodies showed that they were almost exclusively of the IgG2 subclass in positive normal sera and of IgG4 subclass in patients with PF. Thus, antibodies against desmoglein 1 are prevalent in normal subjects living in Tunisia which, along with their IgG2 isotype, suggests the role of the environment in the pathogenesis of this endemic type of pemphigus foliaceus and the need for additional factors to switch from a subclinical to a clinical form of the disease.
Keywords: autoimmunity, desmoglein 1, ELISA, pemphigus foliaceus
INTRODUCTION
Pemphigus foliaceus (PF) is an organ-specific autoimmune cutaneous disease characterized clinically by the development of superficial blisters and erosions of skin, histologically by loss of cell adhesion of keratinocytes in the upper part of epidermis, and immunologically by in vivo bound and circulating IgG autoantibodies directed against desmoglein 1 (Dsg1), a transmembrane desmosomal glycoprotein to the cadherin family [1,2]. In the absence of therapy, PF can be lethal as a result of skin loss and sepsis. Cadherins are transmembrane cell adhesion molecules that mediate Ca2+-dependent cell–cell interactions and are important for maintenance of epithelial cell integrity [3,4]. Anti-Dsg1 autoantibodies produced in the course of the disease are pathogenic, as demonstrated by passive transfer of IgG purified from PF patient sera to neonatal BALB/c mice [5,6], and cause loss of cell adhesion by an as yet not elucidated mechanism [7].
PF occurs throughout the world as sporadic cases and in Brazil as an endemic form termed fogo selvagem [8]. Another endemic area of pemphigus was described recently in Tunisia [9,10]. Epidemiological studies showed that the incidence rate of endemic Tunisian pemphigus is significantly higher (6·7 cases per million per year) than in France, which is related mainly to cases of PF occurring in young adult women [10]. Indeed, among women aged 25–34 years, the incidence rate of pemphigus reaches 20 per million per year [10], a rate approaching that reported in Brazilian endemic areas [8]. However, the absence of cases among genetically related household members and during childhood contrasts with the familial clustering of cases observed in fogo selvagem.
Recently, it has been demonstrated that circulating autoantibodies directed against Dsg1 are present in 10–39% of normal subjects living in fogo selvagem endemic areas, suggesting the role of environmental factors in the initiation of the autoimmune process [11].
To determine the prevalence of anti-Dsg1 antibodies in the Tunisian population, sera obtained from a large number of healthy individuals originating from North or South Tunisia were analysed by enzyme-linked immunosorbent assay (ELISA) using a recombinant fragment of Dsg1 encompassing the extracellular domains of the protein as antigen.
METHODS
Patients and controls
Serum samples were obtained from 28 Tunisian and 20 French patients with PF who fulfilled clinical, histological and immunological criteria of the disease. Serum samples were also obtained from 179 Tunisian healthy blood donors aged 17–55 years, originating from Tunis (North Tunisia) and Gabès (South Tunisia). The demographic characteristics of the population are given in Table 1. Sera were also obtained from 26 pemphigus vulgaris (PV) patients, 25 patients with other bullous skin diseases and 67 French healthy blood donors (Rouen, France). Informed consent was obtained from healthy blood donors.
Table 1. Demographic characteristics of the healthy blood donors whose sera were tested in the Rec1–5 Dsg1 ELISA.
| Geographical origin | Males | Females |
|---|---|---|
| North (Tunis) | 33 | 25 |
| South (Gabès) | 75 | 46 |
Production of recombinant desmoglein 1 (Rec1–5 Dsg1)
The extracellular domain of Dsg1 was produced as a baculoprotein. The plasmid constructs are described in detail elsewhere (H. Mouquet, submitted). Briefly, the cDNA corresponding to the entire extracellular domain, the signal peptide and the pro-sequence was amplified with appropriate primers, then cloned into pGEM4Z vector (Promega, Madison, WI, USA). Synthetic oligonucleotides corresponding to restriction sites, FLAG tag (coding for DYKDDDDK), hexahistidine tag and stop codon were inserted to construct cassette vector, pGEM-PS-TAG. The final construct cloned in p119 transfer vector (pB Rec 1/5) was sequenced totally and showed no base change. Sf9 insect cells were co-transfected with viral DNA AcSLp10 and pB Rec1/5 to obtain recombinant viruses. For large scale expression, 5 × 108 Sf9 cells were infected with recombinant viruses and maintained in culture for 96 h. Supernatants were collected and stored at −80°C until purification.
Purification of Rec1–5 Dsg1
Supernatants were applied to a 5 ml column of anti-FLAG affinity gel equilibrated with 50 mm Tris-HCl pH 7·4, 150 mm NaCl, 30 µg/ml E64 (Roche, Mannheim, Germany), 10 µg/ml leupeptine and 10 µg/ml pepstatine (TS). The column was washed with 40 ml of Tris-buffered saline (TBS) and the bound proteins were eluted with 15 ml of TS containing FLAG octapeptide (0·1 mg/ml). One-ml fractions were collected and analysed by SDS-PAGE. Positive fractions were pooled and concentrated with Ultrafree-15 centrifugal filter device (Millipore, Billerica, MA, USA). The purity was controlled by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis followed by Western blot with anti-FLAG monoclonal antibody (MoAb) (Sigma, Saint-Quentin Fallavier, France).
ELISA
Ninety-six-well microtitre plates (Maxisorb Nunc, Roskilde, Denmark) were coated with 100 µl of 10 µg/ml purified Rec1–5 Dsg1 at 4°C overnight. After washing in TBS (25 mm Tris-HCl pH 7·4, 136 mm NaCl, 2·6 mm KCl) with Ca2+ 1 mm and 0·05% Tween (TBS- Ca2+/T), the wells were blocked with sucrose 5% (Eurobio, Paris, France) and casein 3% (Duchefa, Haarlen, the Netherlands) in TBS-Ca2+/T. Sera were diluted (1/100) and incubated for 1 h at room temperature. After washing three times with TBS-Ca2+/T, the plates were incubated with 2000-fold diluted alkaline phosphatase-conjugated goat antihuman IgG antibodies (Caltag Laboratories, Burlingame, CA, USA) for 1 h at room temperature. Colour development was achieved by adding 100 µl of p-nitrophenyl phosphate (pNpp) substrate (Sigma) solution for 20 min and OD at 450 nm was determined using an ELISA reader.
For IgG subclass studies, sera were used at the dilution of 1/20 and the plates were incubated with antihuman IgG1, IgG2, IgG3 or IgG4 murine MoAb (Sigma), then with a 10 000-fold diluted biotinylated-goat anti-mouse immunoglobulin (Caltag Laboratories). After three washes, streptavidin–phosphatase (Caltag Laboratories) was added at the dilution of 1/10 000 into TBS-T. Colour development was achieved as described above. Each plate contained a negative control serum and four dilutions of an anti-Dsg1 positive serum sample that were used to correct for plate-to-plate variability. The cut-off value was determined by using receiving-operating-characteristic (ROC) analysis curves of values observed in serum samples obtained from French PF patients and French healthy blood donors.
Immunoblotting of normal human epidermal extracts
Human epidermal extract. Mammary surgery specimens of normal human skin were used as source of epidermis. To separate dermis from epidermis, samples were incubated in 1 m NaCl at 4°C overnight. Epidermis was extracted with ice-cold 65 mm Tris HCl pH 6·8, 1·5% SDS, 1 mm dithiotreitol and 10 ng/ml protease inhibitors. The suspension was sonicated, boiled for 10 min and centrifuged at 15 000 r.p.m. for 30 min at 4°C. Samples were frozen at −70°C.
Immunoblot procedure.
SDS-polyacrylamide gel electrophoresis was performed with a 4% separating gel. The proteins were transferred electrophoretically to nitrocellulose filters. Longitudinal strips of the filters were cut and sites were blocked by immersion for 1 h in phosphate-buffered saline (PBS) containing 5% milk powder then incubated for 2 h with a 1/50 dilution of the test serum. After washing, strips were incubated with a 500-fold diluted antihuman IgG murine MoAb (Sigma). Strips were then washed and exposed to biotinylated goat antimouse immunoglobulin (Caltag Laboratories) for 1 h at room temperature. After washing, the strips were incubated with alkaline phosphatase-conjugated streptavidin (Caltag Laboratories). Colour development was achieved by incubation with 5-bromo-4, 3-indoxyl phosphate and nitroblue tetrazolium (Sigma).
Indirect immunofluorescence staining using monkey oesophagus
Sections of monkey oesophagus (The Binding Site, Birmingham, UK) were incubated with a 1/20 dilution of tested sera. After washing, sections were incubated with fluorescein/isocyanate-conjugated goat antihuman IgG antibodies (The Binding Site). The sections were examined under a fluorescence microscope equipped with standard filters.
Statistical analysis
The χ2 test was used to compare the frequency of anti-Dsg1 positive values between males and females, southern and northern individuals and healthy blood donors > or ≤30 years of age.
RESULTS
Rec1–5 Dsg1 ELISA
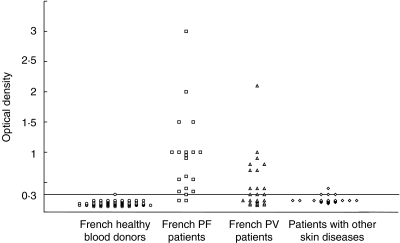
In a first series of experiments, sera from 67 French healthy blood donors and 20 French PF patients were tested in an ELISA using our Dsg1 baculoprotein as antigen. Figure 1 shows the values observed in French healthy blood donors and PF patients. The cut-off value was determined from ROC analysis of French sera. For a cut-off value of 0·2, 100% of PF patient sera were positive versus 13% of healthy controls; for a cut-off value of 0·3, only 1·4% of blood donors were positive while anti-Dsg1 IgG antibodies were detected in 90% of PF patients. Thus, we chose a cut-off value of 0·3 which represents the mean value (0·16) given by French normal sera plus 3 s.d. (0·046).
Fig. 1.
Analysis of sera from French patients with pemphigus foliaceus (PF), pemphigus vulgaris (PV) or other skin diseases and from French normal blood donors in a solid phase ELISA, using the recombinant ectodomain of desmoglein 1 as antigen. The horizontal line shows the cut-off value for a positive result (0·3 OD).
Among the 26 sera from pemphigus vulgaris patients, 13 gave positive values (50%), as expected and observed in other studies [12–14]. Only one serum from the 22 patients with other skin diseases gave a positive OD, while three of them were at the threshold value.
In a second series of experiments, we used our solid phase ELISA with the same cut-off value to detect anti-Dsg1 IgG antibodies in Tunisian PF patients. Among sera obtained from 28 individuals, 25 (89%) gave an OD above the cut-off value. The three patients whose sera did not give an OD above the cut-off value were in clinical remission. The mean OD value given by sera obtained from Tunisian PF patients (0·738 ± 0·38) was very similar to that given by French patients (0·917 ± 0·68). The overall sensitivity of the assay for both French and Tunisian PF sera was 87% (42 of 48 total PF).
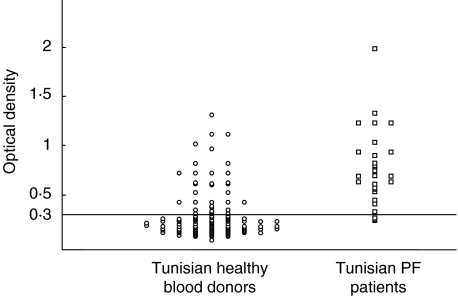
In parallel, 179 sera obtained from normal subjects living in Tunisia were analysed in our ELISA (Fig. 2). The mean value of the OD given by these sera was 0·329 with a range of 0·116–1·3 and a standard deviation of 0·27. With the cut-off value of 0·3, 31 Tunisian healthy blood donor sera were positive (17%) and had OD values ranging from 0·3 to 1·3. Half of them had OD in the range of those given by PF patient sera obtained during the active phase of the disease. Among the 31 donors, 16 were males and 15 females; eight (25%) originated from Tunis and 23 (75%) from Gabès. Although the frequency of anti-Dsg1 positive values was found to be higher in females (16·9%) than in males (14·8%) and in southern (18·2%) than in northern individuals (13·7%) the differences were not significant. However, and interestingly, the proportion of positive sera was significantly higher in females originating from the South than in northern females (P < 0·0016).
Fig. 2.
Analysis of sera from Tunisian patients with endemic pemphigus foliaceus and from Tunisian normal blood donors in the solid phase ELISA described in Fig. 1. The same cut-off value (0·3 OD) was chosen.
Anti-Dsg1 IgG subclasses
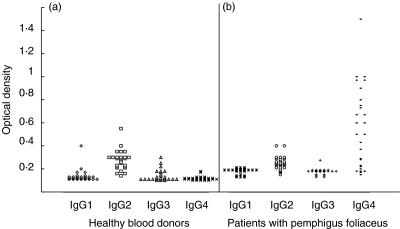
Twenty-seven sera from the 31 Tunisian healthy blood donors who were positive for anti-Dsg1 antibodies were available for the analysis of IgG subclass distribution of anti-Dsg1 antibodies using the ELISA (Fig. 3). In each IgG subclass ELISA, the cut-off value represented the mean value given by French normal sera plus 2 s.d. (IgG1: 0·110; IgG2: 0·193; IgG3 : 0·130; IgG4: 0·104). All anti-Dsg1-positive sera also gave positive results above the cut-off value when anti-IgG subclass murine MoAb were used as the tracers. In particular, anti-Dsg1 antibodies were IgG2 in all cases, associated with IgG1 in two or IgG3 in three cases. Anti-Dsg1 IgG4 were never found in these sera. In contrast, in Tunisian PF patients all anti-Dsg1 antibodies were of the IgG4 subclass.
Fig. 3.
Analysis of IgG subclass distribution of antibodies to desmoglein 1 detected in Tunisian normal blood donors (a) and patients with pemphigus foliaceus (b). Antigen-coated microtitre plates were incubated with tested sera, washed and then incubated with antihuman IgG1, IgG2, IgG3 or IgG4 murine MoAb, then with biotinylated goat antimouse Ig.
Indirect immunofluorescence and Western blot analyses
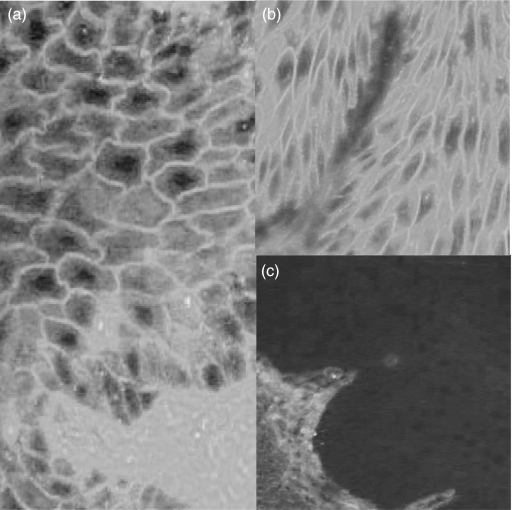
Indirect immunofluorescence and Western blot analyses were performed with 25 of the 31 healthy blood donor sera that were positive in the ELISA. By indirect immunofluorescence analysis using monkey oesophagus as substrate, eight sera gave an intercellular staining of the whole epidermis and 17 were negative (Fig. 4). By Western blot analysis of human epidermal extract, five sera reacted with the 160 kDa band of Dsg1 (data not shown).
Fig. 4.
Indirect immunofluorescence analysis on monkey oesophagus sections of an antidesmoglein 1 positive normal serum from Tunisian normal blood donor (a), an anti-Dsg1 positive pemphigus foliaceus patient serum (b), and an anti-Dsg1 negative normal serum (c) (magnification ×400).
DISCUSSION
To detect antibodies against Dsg1, the ectodomain of Dsg1 was produced as a recombinant protein in a baculovirus expression system and used as antigen in a solid phase ELISA. The assay was set up by screening sera obtained from French patients with PF or other skin diseases and French normal individuals. This test showed a high sensitivity, as 90% of patients with PF had a positive test. Using a similar assay, in particular a recombinant ectodomain of Dsg1 as antigen, others [12,13] have reported previously that 92–97·9% of PF sera contained Dsg1-binding activity. The 90% sensitivity of our assay is due probably to the disease activity heterogeneity of PF patients included in the study; some of them, in particular those whose sera did not react with Dsg1, were in complete clinical remission.
This ELISA was used to perform the same study in Tunisia where pemphigus is endemic. Among a large panel of sera obtained from Tunisian healthy subjects, 31 had Dsg1 binding activity above the cut-off value. This observation is very similar to that made recently in Brazilian regions, where 39% of normal subjects living in endemic areas of folgo selvagem and 10–28% living in proximity of endemic areas had anti-Dsg1 antibodies [11]. Indirect immunofluorescence assay and immunoblotting analysis of human epidermal extract were used to confirm the specificity of the binding activity. Only 10 anti-Dsg1 positive sera were positive by indirect immunofluorescence and/or immunoblotting (32%). Again, our findings are close to those found in Brazilian normal subjects with anti-Dsg1 antibodies detected by ELISA and living in endemic areas of fogo selvagem as only 46% of them were positive with an immunoprecipitation assay [11]. Several explanations may account for the discrepancy between results given by ELISA and other immunological techniques. First, anti-Dsg1 antibodies present in these sera may be directed against particular epitopes that are either not well exposed on the native protein or, in contrast, denatured by the immunoblotting procedure. Secondly, the solid phase ELISA may be more sensitive than other assays for detecting anti-Dsg1 antibodies, in particular indirect immunofluorescence assay, as reported previously [13].
Because Tunisian endemic pemphigus is more frequent in young adults, especially women, and because the disease is more prevalent in the south of the country [10], the prevalence of antibodies against Dsg1 was compared in healthy subjects according to sex and geographical origin. No statistically significant differences in the frequency or titre of anti-Dsg1 antibodies were observed between males and females, individuals above or under 30 years of age or individuals from Gabès or Tunis. However, the proportion of anti-Dsg1-positive sera and among females of southern origin was significantly higher as compared to females originating from the North, respectively. The increased frequency of anti-Dsg1 antibodies in this age group and in females of southern Tunisia might correspond to and explain the high prevalence of PF in this geographical area and suggests, on one hand, that the general population is exposed frequently to a factor(s) triggering the autoantibody response and, on the other hand, that additional endogenous or exogenous factors are required to induce the transition from the subclinical to the clinical status of pemphigus. Further epidemiological studies of a large number of Tunisian women, including the prevalence study of anti-Dsg1 antibodies in Tunisian normal individuals living in a world area where the incidence of PF is low (for example France), are needed to confirm the role of environmental factors.
The second finding of this study is that anti-Dsg1 autoantibodies found in Tunisian healthy blood donors belong almost exclusively to the IgG2 subclass. IgG4 anti-Dsg1 autoantibodies, which were highly prevalent in patients with PF, were never detected in healthy individuals. The demonstration that IgG4 constitutes the predominant antibody subclass of the anti-Dsg1 autoimmune response in PF patients is similar to that made previously by others in PV patients [14] and more recently in Brazilian endemic pemphigus [15]. However, the distribution of IgG subclasses of anti-Dsg1 antibodies in Tunisian healthy individuals was different from that observed previously in normal subjects living in areas of endemic PF or relatives of PV patients. Indeed, anti-Dsg1 antibodies detected in Tunisian healthy subjects belong predominantly to the IgG2 subclass, while anti-Dsg1 antibodies detected in normal individuals living in Brazilian endemic areas or in healthy relatives of PV patients belong to IgG1 [15], which may be explained by the participation of different environmental factors in the initiation the autoimmune response. It is known that the switch to IgG2 subclass is induced by polysaccharidic antigens borne by various microbial agents [16,17]. One thus could hypothesize that the production of IgG2 anti-Dsg1 antibodies in healthy subjects is triggered by infection with unknown microorganisms which could induce (i) anti-Dsg1 autoimmunity through antigen mimicry or release of self-antigens by damaged epidermal cells and (ii) the production of IgG2 subclass autoantibodies in an appropriate inflammatory and cytokine environment.
Finally, the high prevalence of anti-Dsg1 antibodies in the Tunisian general population suggests that autoreactive B cell clones secreting anti-Dsg1 antibodies are present in the normal B cell repertoire and that expansion of these autoreactive B cells might be triggered by endogenous and exogenous factors. Because anti-Dsg1 antibodies are considered to be pathogenic [5], the question arises why only a few subjects develop the disease. It is widely accepted that autoimmune diseases are multifactorial. Other genetic and/or environmental factors are probably required to trigger the production of pathogenic antibodies, such as those regulating isotype switching and epitope spreading. In this regard, certain HLA class II alleles and a single nucleotide polymorphism of the Dsg1 gene have been shown to confer susceptibility to PF [18] and repeated administration of the triggering agent may promote the production of IgG4, the highly suspected pathogenic subclass [19].
Acknowledgments
This work was supported by a grant from INSERM/DGRST (France/Tunisia).
REFERENCES
- 1.Koulu L, Kusumi A, Steinberg MS, et al. Human autoantibodies against a desmosomal core protein in pemphigus foliaceus. J Exp Med. 1984;160:1509–18. doi: 10.1084/jem.160.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa MM, Hashimoto T, Konohana A, et al. Immunoblot analyses of Brazilian pemphigus foliaceus antigen using different antigen sources. Arch Dermatol Res. 1990;282:84–8. doi: 10.1007/BF00493463. [DOI] [PubMed] [Google Scholar]
- 3.Buxton RS, Cowin P, Franke WW, et al. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993;121:481–3. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxton RS, Magee AI. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol. 1992;3:157–67. doi: 10.1016/s1043-4682(10)80012-1. [DOI] [PubMed] [Google Scholar]
- 5.Anhalt GJ, Labib RS, Voorhees JJ, et al. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–96. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Patel HP, Labib RS, et al. Experimentally induced pemphigus vulgaris in neonatal BALB/c mice: a time–course study of clinical, immunologic, ultrastructural, and cytochemical changes. J Invest Dermatol. 1985;84:41–6. doi: 10.1111/1523-1747.ep12274679. [DOI] [PubMed] [Google Scholar]
- 7.Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- 8.Diaz LA, Sampaio SA, Rivitti EA, et al. Endemic pemphigus foliaceus (fogo selvagem). II. Current and historic epidemiologic studies. J Invest Dermatol. 1989;92:4–12. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- 9.Morini JP, Jomaa B, Gorgi Y, et al. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch Dermatol. 1993;129:69–73. doi: 10.1001/archderm.129.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Bastuji-Garin S, Souissi R, Blum L, et al. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol. 1995;104:302–5. doi: 10.1111/1523-1747.ep12612836. [DOI] [PubMed] [Google Scholar]
- 11.Warren SJ, Lin MS, Giudice GJ, et al. Cooperative Group on Fogo Selvagem Research. The prevalence of antibodies against desmoglein 1 in endemic pemphigus foliaceus in Brazil. N Engl J Med. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 12.Harman KE, Gratian MJ, Seed PT, et al. Diagnosis of pemphigus by ELISA: a critical evaluation of two ELISAs for the detection of antibodies to the major pemphigus antigens, desmoglein 1 and 3. Clin Exp Dermatol. 2000;25:236–40. doi: 10.1046/j.1365-2230.2000.00624.x. [DOI] [PubMed] [Google Scholar]
- 13.Amagai M, Komai A, Hashimoto T, et al. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–7. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- 14.Kricheli D, David M, Frusic-Zlotkin M, et al. The distribution of pemphigus vulgaris IgG subclasses and their reactivity with desmoglein 3 and 1 in pemphigus patients and their first degree relatives. Br J Dermatol. 2000;143:337–42. doi: 10.1046/j.1365-2133.2000.03659.x. [DOI] [PubMed] [Google Scholar]
- 15.Warren SJP, Arteaga LA, Rivitti EA, et al. The role of subclass switching in the pathogenesis of endemic pemphigus foliaceus. J Invest Dermatol. 2003;120:104–8. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]
- 16.Riesen WF, Skvaril F, Braun DG. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5:383–90. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Martel P, Gilbert D, Busson M, et al. Epistasis between DSG1 and HLA class II genes in pemphigus foliaceus. Genes Immun. 2002;3:205–10. doi: 10.1038/sj.gene.6363839. [DOI] [PubMed] [Google Scholar]
- 19.Maran R, Dueymes M, Le Corre R, et al. IgG subclass of human autoantibodies. Ann Int Med (Paris) 1997;148:29–38. [PubMed] [Google Scholar]