Abstract
B cells that make polyreactive antibodies (PAB+ cells) express polyreactive Ig receptors on their surface and can bind a variety of different antigens. The present study shows that PAB+ cells are widely distributed, are present in varying numbers in different lymphoid organs and that their phenotype varies depending on the organs from which they are isolated. Up to 10 times more cells in PAB+ enriched populations bind antigens as compared to PAB− populations. Comparison of PAB+ with B-1+ cells showed that a high percentage of PAB+ cells are B-1+, but that many PAB+ cells do not express B-1 cell surface markers and, in fact, are B-1−. It is concluded that the B cell population consists of PAB+/B-1+, PAB+/B-1−, PAB−;/B-1+, and PAB−/B-1− cells. The presence of PAB+ cells in the thymus points to the possibility that PAB+ cells may carry endogenous host antigens from peripheral tissues to the thymus where they may contribute to immunological tolerance.
Keywords: B cell receptor, immunological tolerance, innate immunity, natural antibodies, natural immunity
INTRODUCTION
Natural antibodies are present in the sera of newborns and develop in the absence of stimulation by exogenous antigens [1]. They are the outcome of naturally occurring V, D, J gene rearrangement which results in the formation of millions of different immunoglobulin molecules. Although natural antibodies have been known to exist for over 100 years, it was not until the development of hybridoma technology that it was discovered that many of these naturally occurring antibodies are polyreactive, that is, they can bind to a variety of different self and nonself antigens [2–6]. These antibodies (primarily IgM, but also some IgA and IgG) were found to be of low affinity and the genes that encoded many of them were identical or nearly identical to germ-line sequences [1,7]. Gene shuffling experiments and site-directed mutagenesis revealed that the CDR-3 region of the Fab fragment plays a key role in maintaining polyreactivity [8–10].
Precisely how polyreactive antibody molecules bind a variety of different antigens is still not clear, but it is currently believed that low affinity polyreactive molecules are more flexible at their antigen-binding site than high affinity monoreactive molecules which have undergone extensive somatic mutations and affinity maturation [11–17]. Thus, the three-dimensional structure or conformation may explain why certain antibody molecules can accommodate a number of different antigenic configurations. Recent studies suggest that a very high portion of the natural antibody repertoire is made up of polyreactive antibodies [18–20]. Natural antibodies are thought to act as a first line of defence against infectious agents and sera used in standard viral and bacterial neutralization tests often are diluted up to 50 fold to avoid the ‘nonspecific’ background activity of natural antibodies [21,22]. However, the role of polyreactive antibodies in these sera has never been determined.
Studies in our laboratory over the last few years have shown that B cells which make polyreactive antibodies express polyreactive Ig receptors on their surface, and therefore can bind a variety of different antigens [23–25]. By incubating these cells with fluorescein-labelled antigens and measuring antigen-binding by FACS analysis, polyreactive antigen-binding B (PAB+) cells can be identified. PAB+ cells, however, have not been fully characterized nor has their relationship to B-1 cells ever been examined. In contrast, the relationship between B-1 cells and conventional B-2 cells has been studied extensively [18,26,27]. B-1 cells have been shown to differ from B-2 cells both phenotypically and functionally. Phenotypically, B-1 cells are characterized by a number of cell surface markers (e.g. IgMhi, IgDlo, B220lo, Mac-1hi, CD23lo and CD5hi), but variation in the degree of expression of these markers has made identification of these cells difficult. For example, some B cell phenotypes fail to express CD5 protein on their surface, but nonetheless have been assigned to the B-1 population (‘sister’ CD5 or B-1b cells) because they express comparable levels of CD5 mRNA [28]. Functionally, B-1 and B-2 cells have different life spans and respond differently to B cell receptor signalling, receptor-mediated calcium mobilization and signals required for cell cycle differentiation and proliferation [20,26,29]. The origin and development of B-1 cells, as compared to B-2 cells, has been hotly debated with still no resolution [30,31]. One hypothesis holds that B-1 and B-2 cells have a separate lineage, whereas another, the differentiation hypothesis, holds that both types of cells arise from a common precursor. Still a third hypothesis argues that B cell receptor-mediated signalling, as a result of ligation by autoantigen, is required for B-1 cell development [30], but that the threshold and context that leads to B-1 development differs for particular B cell precursors [27]. Recent studies indicate that not all B-1 cells are the same and that B-1 cells from the spleen and peritoneal cavity have different phenotypes and functional properties [27,31].
The actual biological role of PAB+ cells and B-1 cells is still not clear but, they are thought to be involved in the production of natural antibodies and serve as a first line of immunological defense [1,32–36]. Other functions have been attributed to these cells including the production of autoantibodies and precursors of CD5+ chronic lymphatic leukaemia cells [20]. The present experiments were initiated to identify and separate PAB+ from PAB–, determine their tissue distribution and phenotypic properties, and define their relationship to B-1 cells.
MATERIALS AND METHODS
Mice
Six to 12 week-old BALB/c, C57BL/6 and FVB mice were obtained from Charles River Laboratories (Raleigh, NC, USA). Eight to 12 week-old C57BL/10-Igh-6tmlCgn (IgM–/–, µMT) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were housed under specific pathogen-free conditions.
Single cell preparations
Tissue from PerC, SPL, BM and thymus were prepared as described previously [25] and passed through a 100 µm nylon Cell Strainer (BD Labware, Franklin Lakes, NJ, USA) to obtain single cell suspensions. Some tissues, for example, Peyer's patches (PP) and lamina Propria (LP), were first dissociated with collagenase type V (Sigma, St Louis, MO, USA) at a concentration of 1 mg/ml at 37°C in PBS for 15–30 min, followed by passage through a 100 µm nylon Cell Strainer. In other experiments (e.g. LP), discontinuous percoll gradients (Amersham Pharmacia Biotech Inc, Piscataway, NJ, USA) were used and cells at the interface between the 40 and 75% layers were collected to obtain mononuclear cells.
Antigens and antibodies
Biotin-conjugated β-gal, FITC-conjugated insulin, FITC-conjugated anti-actin antibody, purified thyroglobulin and actin were purchased from Sigma (St Louis, MO, USA). Thyroglobulin was FITC-conjugated using a FluoroTag™ FITC conjugation kit purchased from Sigma. FITC-conjugated tetanus toxin C fragment was purchased from Roche Diagnostics Corporation (Indianapolis, IN, USA). FITC-conjugated lipopolysaccharide (LPS, Escherichia coli 0111:B4) was obtained from Sigma. Antibodies specific for mouse B220, CD19, CD21, CD23, IgM, IgD, IgA, CD5, Mac-1, rat IgG and streptavidin that had been biotinylated or FITC- or PE- or PerCP- conjugated were purchased from PharMingen (San Diego, CA, USA). Magnetic beads-conjugated with anti-mouse B220 antibody, anti-biotin antibody or streptavidin were purchased from Miltenyi Biotec (Auburn, CA, USA).
Sorting of cells with magnetic beads
To enrich for B cells, single cell suspensions from different mouse tissues were incubated with anti-B220 magnetic beads followed by two-rounds of positive selection with a MACS system (Miltenyi Biotec) according to the manufacturer's protocols. B220 positive cells from PerC, SPL, BM, LP, PP and thymus were obtained at 95–99% purity.
To separate B220 positive cells into PAB+ and PAB– subsets, single cell suspension from various organs were first incubated with biotin-conjugated β-gal (25 µg/107 cells). PAB+ cells were positively separated by streptavidin magnetic beads or anti-biotin magnetic beads using MACS. The PAB+ depleted cell suspension then was further selected by anti-B220 magnetic beads to yield PAB– cells. All the selections underwent two passages through MACS. By this method, approximately 98% of the separated PAB+ and PAB– cells were B220 positive. Red blood cell contamination was excluded by their smaller size, compared with B cells, during FACS analysis.
Cell staining and flow cytometry analysis
Three to 10 × 105 cells were preincubated with 0·5 µg Mouse Fc Block™ (PharMingen) in 90 µl PBS containing 0·5% BSA and 2 mm EDTA at 4°C for 10 min to minimize the effect of nonspecific FcR binding sites. Biotin-, FITC-, PE- or PerCP- conjugated antigens (2·5 µg in 10 µl) and/or rat anti-mouse monoclonal antibodies (1–2·5 µg in 5–10 µl) then were added, incubated for 30 min and washed. Actin binding was detected by FITC-conjugated anti-actin antibody. If biotin-conjugated antigen or antibody was used, cells were further stained with FITC-, PE-, or PerCP- conjugated streptavidin for 30 min, washed and/or fixed with 4% paraformaldehyde and analysed by the FACSCalibur system (Becton Dickinson, San Jose, CA, USA). FITC-, PE- and PerCP- conjugated streptavidin or rat IgG were used as negative controls for antigen-binding to surface polyreactive antibodies on PAB+ cells or rat anti-mouse monoclonal antibodies binding to their specific targets (surface antigens expressed by mouse B cells), respectively. FACS results were analysed by CELLQuest software (BD, San Jose, CA, USA).
Bacteria particle-binding assay
Fluorescein conjugated Staphylococcus aureus and Escherichia coli K12 (BioParticles) were obtained from Sigma. The particles were suspended in PBS at 1 mg/ml and were disaggregated into single cell suspensions by ultrasound at low energy. PAB+ and PAB– cells from BALB/c mice were incubated with a suspension of single bacterial particles (100 µg/106cell/200 µl) at 4°C for 45 mins, washed, and then analysed by the FACSCalibur system. Unbound particles were excluded by their smaller size as compared with B cells.
RESULTS
Distribution of PAB+ cells
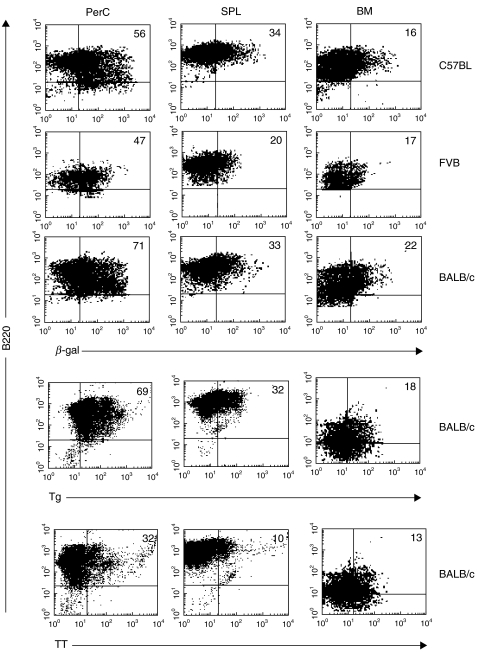
To determine the relative distribution of PAB+ cells in different lymphoid organs, B cells were isolated from the peritoneal cavity (PerC), spleen (SPL) and bone marrow (BM) by anti-mouse B220 magnetic beads. The cells then were incubated with β-galactosidase (β-gal), thyroglobulin (Tg) or tetanus toxin (TT) and antibody to B220. As seen in Fig. 1, 56% of the B cells from the PerC, 34% from the SPL and 16% from the BM of C57BL mice bound β-gal. Similarly, for FVB and BALB/c mice, the percentage of PAB+ cells, as measured by β-gal binding, was highest in the PerC, followed by SPL and BM. The percentage of PAB+ cells in BALB/c mice that bound Tg was nearly the same as that for β-gal, but TT binding was considerably lower. In the case of both Tg and TT, binding to B cells in the PerC was higher than in the SPL or BM.
Fig. 1.
Distribution of PAB+ cells. B cells were isolated from the PerC, SPL and BM of C57BL, FVB and BALB/c mice using anti-B220 magnetic beads. The positively selected cells then were incubated with β-gal, Tg or TT, stained with antibody to B-220, and analysed by FACS. The percentage of double-positive cells (Ag-binding B cells) is indicated.
Comparison of PAB+ and PAB– cells: antigen binding and cell surface markers
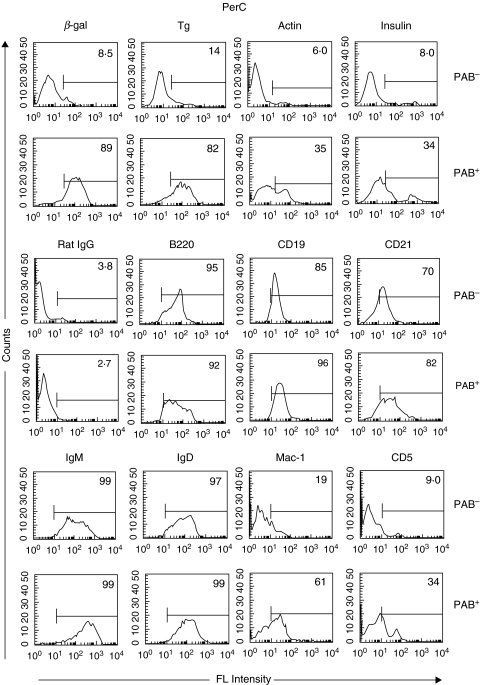
B cells from the PerC or SPL were separated into PAB+ and PAB– populations. In brief, this was done by incubating single cell suspensions from the PerC or SPL with biotin-labelled β-gal and positively selecting the cells that bound β-gal (PAB+) with strepavidin or antibiotin magnetic beads. The B cells that did not bind β-gal (PAB–) were further selected by anti-B220 magnetic beads. Approximately 95% of the PAB+ and PAB– cells were B220 positive. PAB+ and PAB– cells then were incubated with different antigens (i.e. β-gal, Tg, actin, insulin). In the case of PerC cells (Fig. 2), less than 10% of the PAB– cells, whereas close to 90% of the PAB+ cells, bound β-gal. Similarly, 4–6 times more of the PAB+ cells bound Tg, actin and insulin than the PAB– cells. The antigens that bound best to PAB+ cells were β-gal and Tg followed by actin and insulin.
Fig. 2.
PAB+ and PAB– cells in the PerC. PAB+ and PAB– cells from the PerC of BALB/c mice were positively selected as described in Materials and methods. The binding of Ags (β-gal, Tg, actin and insulin) and the expression of cell surface markers was determined by FACS analysis. Rat IgG served as a negative control. The percentage of positively stained cells is indicated.
Staining for surface markers showed that the percentage of PAB+ and PAB– cells expressing B220, CD19, CD21, IgM and IgD was nearly the same, although the intensity of staining for IgM was stronger in the PAB+ than the PAB– cells. Similarly, 61% of the PAB+ cells expressed Mac-1 as compared to only 19% of the PAB– cells and 34% of the PAB+ cells expressed CD5 as compared to 9% of the PAB– cells.
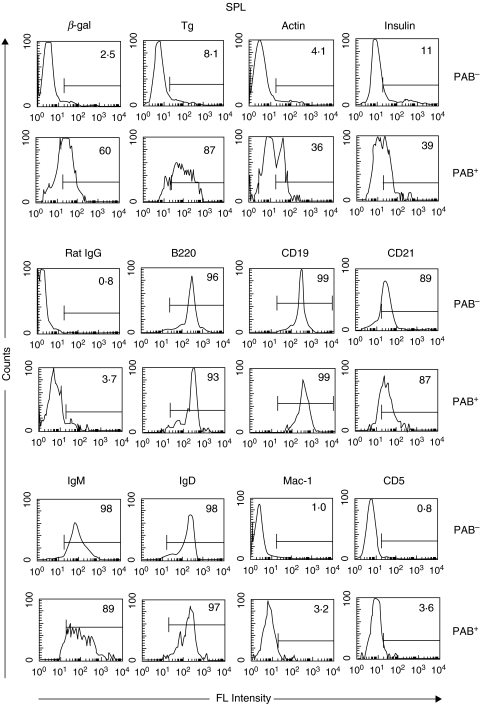
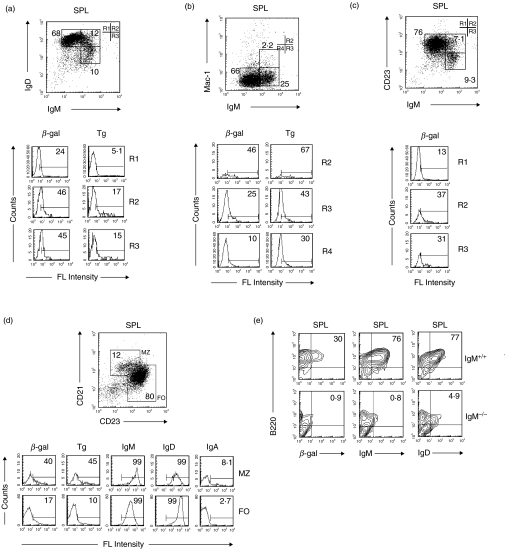
The binding of antigens to PAB+ and PAB– cells from the SPL yielded results similar to that found with cells from the PerC (Fig. 3). Between 10 and 24 times more of the PAB+ cells bound β-gal and Tg than the PAB– cells. A somewhat lower percentage of PAB+ cells bound actin and insulin. As in the case with PAB+ cells from the PerC, the percent of PAB+ and PAB– cells from the SPL expressing the cell surface markers B220, CD19, CD21, IgM and IgD was nearly the same. In contrast to PerC cells, only a very low percentage of either PAB+ or PAB– cells from the SPL expressed Mac-1 or CD5.
Fig. 3.
PAB+ and PAB– cells in the SPL. PAB+ and PAB– cells from the SPL of BALB/c mice were positively selected as described in Materials and Methods and analysed as indicated in Fig. 2 for Ag-binding and expression of cell surface markers.
Relationship of PAB+ cells from the PerC to B-1 cells
Although there is no unanimity as to how to identify B-1 cells based on cell surface markers, we employed several of the widely used and generally accepted B-1 cell surface markers (e.g. IgMhi, IgDlo, B220lo, Mac-1hi, CD5hi, and CD23lo) to study the relationship of PAB+ cells to B-1 cells. B cells, isolated from the PerC of BALB/c mice by anti-B220 magnetic beads, were incubated with antibodies to different cell surface markers to identify B-1 cells and with different antigens to evaluate antigen binding.
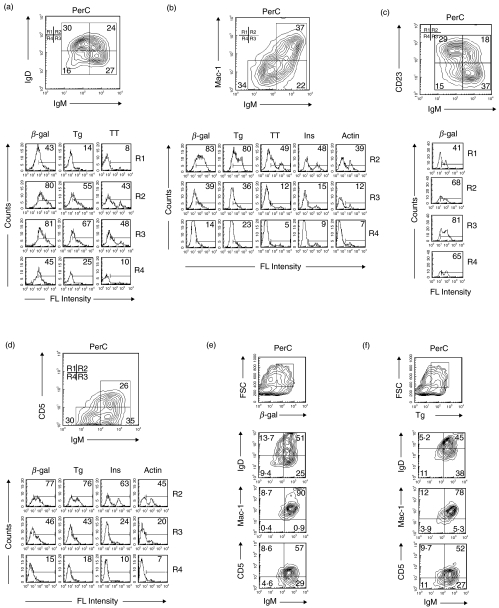
In earlier studies, IgDloIgMhi was used to identify B-1 cells [37,38]. As seen in Fig. 4a, between 48 and 81% of the IgDloIgMhi cells (R3) bound β-gal, Tg and TT. However, approximately the same number of IgDhiIgMhi cells (R2) also bound these antigens. Moreover, lower but nonetheless a substantial percentage of IgDhiIgMlo (R1) and IgDloIgMlo (R4) cells bound antigens, especially β-gal. Thus, PAB+ cells are more widely distributed than B-1 cells as characterized by the IgDloIgMhi phenotype.
Fig. 4.
Relationship of PAB+ cells from the PerC to B-1 cell surface markers. B cells from the PerC of BALB/c mice were positively selected with anti-B220 magnetic beads. (a–f) Cells then were incubated with antibodies to cell surface markers (anti-IgM, anti-IgD, anti-Mac-1, anti-CD23 and anti-CD5) and different antigens. The percentage of gated cells binding antigens in each region (R) is shown in the histograms. (e, f) Based on high antigen-binding and high FSC, gated cells were analysed to determine their relationship to B-1 cell surface markers.
Mac-1hi also has been used to identify B-1 cells [38]. As seen in Fig. 4b, approximately 80% of the Mac-1hiIgMhi cells (R2) bound β-gal and Tg and between 39% and 49% bound TT, Ins and actin. Although, considerably less, 39% and 36% of the Mac-1lo IgMhi cells (R3) bound β-gal and Tg, respectively, and 12–15% bound TT, Ins and actin. Mac-1, however, is not required for antigen binding since Mac-1 knockout mice bound as much or more β-gal as wild type mice (data not shown).
Over the last several years CD23lo has joined the ranks of important B-1 cell surface markers [39,40]. As seen in Fig. 4c, 81% of the CD23loIgMhi cells (R3) bound beta-gal, but 68% of the CD23hiIgMhi (R2) also bound β-gal showing that PAB+ cells are more broadly distributed than B-1 cells as characterized by the CD23lo marker.
CD5+ initially was thought to be an important marker for B-1 cells [37,38]. It was soon recognized, however, that both CD5+ and CD5− B cells could make polyreactive antibodies [41]. The ability of CD5+ and CD5− B cells to bind antigens is illustrated in Fig. 4d. A high percentage of CD5hiIgMhi cells (R2) bound β-gal, Tg, Ins and actin. CD5loIgMhi cells (R3) bound less, but nonetheless substantial amounts of the same antigens.
To analyse in more detail the extent to which the overall PAB+ population consists of B-1 cells or at least cells with B-1 cell surface markers, high β-gal- and Tg-binding B cells with high forward scatter (FSC) were gated and analysed for the B-1 cell surface markers IgDlo, Mac-1 hi and CD5hi. As seen in Fig. 4e, of the high β-gal- binding cells, 25% were IgDloIgMhi, 90% were Mac-1hiIgMhi and 57% were CD5hiIgMhi. Of the high Tg-binding cells, 38% were IgDloIgMhi, 78% were Mac-1hiIgMhi and 52% were CD5hiIgMhi (Fig. 4f). This showed that in the PerC the majority of the PAB+ cells were Mac-1hi, but IgDhi and CD5lo cells also bound substantial amounts of antigens further demonstrating that the PAB+ population in the PerC consists of both B-1+ and B-1– phenotypes.
Relationship of PAB+ cells from the SPL to B-1 cells
Although B-1 cells are present in both the PerC and SPL, there are less of them in the SPL and both phenotypic and functional differences have been reported [27,31,39]. The relationship of PAB+ cells to B-1 cells in the SPL, as evaluated by cell surface markers, is illustrated in Fig. 5a–c. Cells which displayed B-1 cell surface markers bound β-gal and Tg (e.g. IgDloIgMhi(R3), Mac-1hiIgMhi(R2), and CD23loIgMhi(R3)), but cells which did not have the B-1 phenotype (e.g. IgDhiIgMhi(R2), Mac-1loIgMhi(R3) and CD23hiIgMhi(R2)) also bound β-gal and Tg. The percentage of the cells in the spleen that bound antigen and the intensity of antigen binding generally was less than that of cells with the same phenotype in the PerC (Fig. 4).
Fig. 5.
Relationship of PAB+ cells from the SPL to B-1 cells surface markers. (a–c) B cells from the SPL of BALB/c mice were positively selected with anti-B220 magnetic beads. Cells then were incubated with antibodies to cell surface markers (anti-IgM, anti-IgD, anti-Mac-1, and anti-CD23) and different antigens. Percentage of gated cells binding antigens in each region (R) is shown in the histograms. (d) Binding of antigens to cells from the MZ (CD21hiCD23int) and FO (CD21intCD23hi) of the SPL. (e) B cells from the SPL of C57BL/6 IgM+/+ and IgM–/– mice were isolated with anti-B220 magnetic beads and the expression of cell surface markers and the binding of β-gal determined.
PAB+ cells in the MZ and FO of the SPL
To determine which region of the SPL contains the highest percentage of PAB+ cells, B cells were isolated with anti-B220 magnetic beads. The cells then were incubated with β-gal or Tg and stained with anti-CD21 and anti-CD23 to identify MZ cells (CD21hiCD23int) and FO (CD21intCD23hi) cells [32,42–46]. As seen in Fig. 5d, considerably more PAB+ cells as measured by the binding of β-gal and Tg were found in the MZ than in the FO. The majority of the cells in both the MZ and FO expressed IgM and IgD with very few cells expressing IgA. The intensity of staining for IgM was somewhat higher in the MZ than in the FO and for IgD somewhat higher in the FO than in the MZ.
Antigens bind primarily to IgM expressing cells in the SPL
Evidence that much of the binding of β-gal is to IgM-expressing cells within the B220 cell population comes from comparing the binding of β-gal to B220 positive cells obtained from IgM+/+ and IgM–/– mice which lack mature B cells, but may possess some early precursor B cells. As seen in Fig. 5e, few if any of the B220 positive cells from the SPL of IgM–/– mice bound β-gal (0·9%) as compared to IgM+/+ mice (30%). Similarly, very few of the B220 positive cells from the PerC of IgM–/– mice bound β-gal (7·0%) as compared to IgM+/+ mice (57%) (data not shown). B220 positive cells from the SPL of IgM–/– mice expressed no IgM and very little IgD.
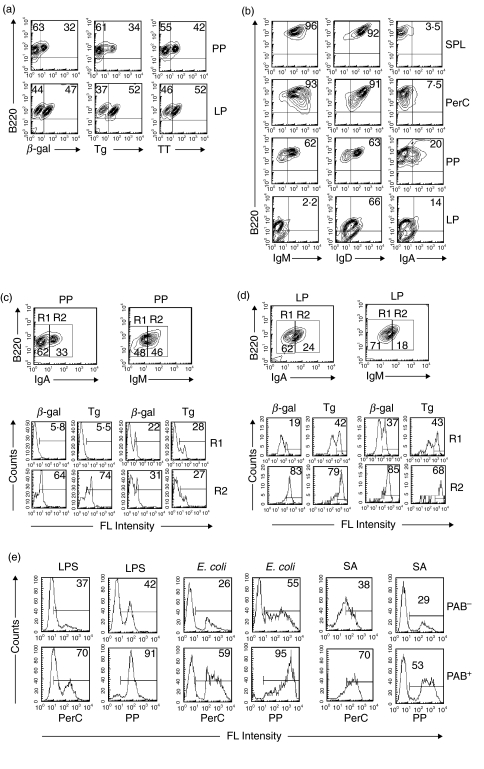
PAB+ cells in PP and LP
B cells from PP and LP were isolated with anti-B220 magnetic beads and incubated with β-gal, Tg or TT. As seen in Fig. 6a, 32% and 47%, respectively, of the B220 cells from PP and LP bound β-gal, 34% and 52% Tg and 42% and 52% TT. These findings demonstrate the presence of PAB+ cells in both PP and LP.
Fig. 6.
PAB+ cells in PP and LP. (a) B cells from the PP and LP were positively selected by anti-B220 magnetic beads, and two-colour stained with various combination of anti-B220, β-gal, Tg, or TT. The percentage of B220 cells that bound antigens was determined. (b) B cells from the SPL, PerC, PP and LP of BALB/c mice were positively selected with anti-B220 magnetic beads and two-colour stained with various combinations of anti-B220, anti-IgM, anti-IgD or anti-IgA. (c, d) B cells from PP and LP were positively isolated with anti-B220 magnetic beads, incubated with β-gal or Tg and three-colour stained with various combinations of anti-B220, anti-IgM or anti-IgA to determine the percentage of IgM and IgA cells that bound β-gal or Tg. (e) PAB+ and PAB– cells from the PerC and PP of C57BL/6 mice were positively selected as described in Materials and Methods. The cells then were incubated with LPS, E. coli or Staph. aureus (SA) and percent binding determined.
The percentage of IgM, IgD and IgA expressing B220 cells in PP and LP, as compared to SPL and PerC, is seen in Fig. 6b. Whereas over 90% of the B220+ cells in SPL and PerC and 62% of the cells in PP expressed IgM, less than 3% of the cells in LP expressed IgM. Curiously, and still unexplained, despite the low percentage of IgM-expressing cells in the LP, 66% of them expressed IgD. In the case of IgA, the percentage of these cells in PP and LP was 2–6 times higher than in SPL or PerC.
Because of the relatively high percentage of IgA-bearing cells in PP and LP as compared to SPL and PerC, the binding of antigens to IgA-bearing cells in PP and LP was analysed. B cells from both of these tissues was isolated with anti-B220 magnetic beads, incubated with β-gal or Tg, stained with antibodies to IgM, IgA and B220, gated and by three colour fluorescence the percentage of cells that bound antigens determined. As seen in Fig. 6c, 64% and 74%, respectively, of the B220hiIgAhi cells (R2) from the PP bound β-gal and Tg, whereas only a little over 5% of the B220hiIgAlo cells (R1) bound these antigens. In contrast to the high binding of β-gal and Tg to IgA-bearing cells, only 22% to 31% of the IgM-bearing cells (R1 or R2) bound β-gal and Tg. In the case of the LP (Fig. 6d) both B220hiIgAhi (R2) and B220hiIgMhi (R2) cells bound β-gal and Tg. These findings show that a high percentage of IgA-expressing PAB+ are present in PP and LP.
PAB+ cells from PerC and PP bind endotoxin and bacteria
The role of PAB+ cells in natural defense is still unresolved. To see whether PAB+ cells could bind LPS or whole bacteria, PAB+ and PAB– cells were isolated as described in Materials and Methods from the PerC and PP and incubated with LPS, E. coli, or S. aureus. Figure 6e shows that approximately twice as many PAB+ cells bound LPS, E. coli and S. aureus as PAB– cells.
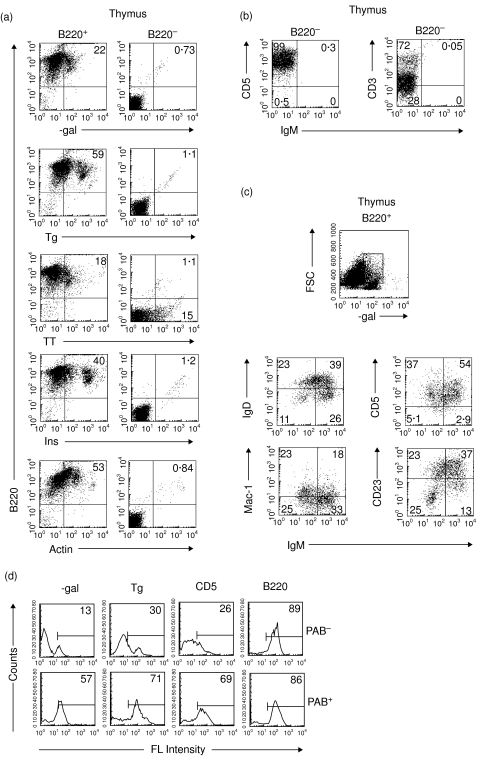
PAB cells are present in the thymus
The percentage of B cells in the thymus, as compared to peripheral lymphoid organs, is low. Some thymic B cells are known to have B-1 cell surface markers [47], but the proportion of thymic B cells that are PAB+ has never been determined. Thymic B cells were positively and negatively selected with anti-B220 magnetic beads. As seen in Fig. 7a, 22% of the positively selected B220+ cells bound β-gal, 59% Tg, 18% TT, 40% Ins and 53% actin. In contrast, essentially none of the B220– cells, which were primarily T cells (Fig. 7b), bound any of these antigens. Examination of the high β-gal-binding cells with antibody to B-1 cell surface markers (Fig. 7c) showed that except for CD5hiIgMhi cells, most of the cells that bound β-gal were not typical B-1 cells based on surface markers. High β-gal binders were IgDhi, Mac-llo and CD23hi. Thus, PAB+ cells are present in the thymus, but many of them are B-1–. For direct comparison, PAB+ and PAB– cells were isolated from the thymus as described in Materials and Methods. As seen in Fig. 7d, 2·5 to 4·0 times more PAB+ cells bound β-gal and Tg than PAB– cells.
Fig. 7.
PAB cells in the thymus. (a) B cells from the thymus were positively and negatively selected by 2 passages with anti-220 magnetic beads. The B220+ and B220– populations then were incubated with antibody to B220 and one of several different antigens (i.e. β-gal, Tg, TT, Ins, Actin) and analysed by FACS. (b) B220– cells stained with antibodies to the T cell markers CD5 and CD3 and the B cell marker IgM. (c) To determine their phenotypes, high β-gal binding-cells with high FSC were gated and incubated with antibodies to IgM, IgD, Mac-1, CD5 and CD23. (d) PAB+ and PAB– cells from the thymus of BALB/c mice were positively selected as described in Materials and Methods and analysed as indicated in Fig. 2 for Ag-binding and expression of cell surface markers.
DISCUSSION
Earlier studies on the fine specificity of individual monoclonal polyreactive antibodies, prepared by hybridoma technology, revealed that the affinity for different antigens varied by as much as 1000 fold [1,48]. Depending on the antigen, the dissociation constants (Kd) ranged from 10−3 to 10−7 (mol/l). In contrast, the Kd of high affinity monoclonal monoreactive antibodies ranged from 10−7 to 10−11 (mol/l) and these antibodies failed to bind any of the antigens in the panel used to detect polyreactive antibodies. Similarly, the B cell Ig receptors on monoclonal PAB+ cells bind a broad range of antigens, whereas the B cell Ig receptors on monoclonal PAB– cells generally bind only a single antigen [25]. Analysis of PAB+ cells, in the B cell population from different organs, however, presents a unique problem in that overall antigen binding represents an average from a mixture of the thousands of different PAB+ cells each with a different B cell Ig receptor and each with its own affinity for specific antigens.
In the studies described here, we chose several different representative self (e.g. Tg, Ins, Actin) and nonself antigens (e.g. β-gal, TT, LPS). FACS analysis of cells from different organs showed that certain antigens (e.g. Tg and β-gal) consistently resulted in higher binding than other antigens (e.g. Ins or TT). Thus, the fluorescence intensity profile and percentage of B cells binding antigens provides a range rather than the absolute number of PAB+ cells in a population. In principle, measuring the binding of antigens to polyreactive B cells by FACS is not different than measuring the expression of cell surface markers by the binding of specific antibodies. Appropriate controls, however, are extremely important since an antibody to a specific cell surface marker on a PAB+ cell might bind not only to that marker but also be bound by the polyreactive Ig receptor. This could give false positive results and might represent a general problem for evaluating the intensity of expression of certain B cell surface markers.
The relationship of PAB+ cells to B-1 cells was studied by measuring the binding of several different antigens to cells that expressed B-1 cell surface markers. These studies showed that a high percentage of PAB+ cells expressed B-1 cell surface markers, but that many PAB+ cells did not express B-1 cell surface markers. Conversely, a high percentage of cells expressing B-1 cell surface markers were PAB+, but many B-1 cells did not bind antigens and were scored as PAB–. Thus, there are four populations of cells based on antigen-binding and expression of B-1 cell surface markers: PAB+/B-1+; PAB+/B-1–; PAB–/B-1+; PAB–/B-1–. In some cases the PAB+ and B-1+ phenotypes are present on the same cell, whereas in other cases the PAB+ and B-1+ phenotypes are present on different cells and represent independent populations. Still not totally resolved is what percentage of B-1+ cells in different organs are PAB+ and PAB–. The problem is complicated by the fact that there is no unanimity as to how to identify B-1+ cells because the expression of B-1 cell surface markers is highly variable. Moreover, estimates of the percentage of PAB+ cells in a particular organ varies depending on the antigen(s) used. Nonetheless, estimates of the relative number of PAB+ and B-1+ can be obtained. For example, in the PerC, we estimate that between 50 and 75% of cells with B-1+ surface markers are PAB+ cells. Viewed in the reverse, 25–50% of the B-1+ cells in the PerC are not PAB+ and may represent an important source of natural antibody that is not polyreactive. These nonpolyreactive antibodies from the PAB–/B-1+ population, in fact, may represent monoreactive high affinity antibodies and be the source of previously reported monoclonal antibodies (e.g. phosphatidyl choline) isolated from B-1 cells of the PerC [40]. In the future it should be possible to see if there are any additional biological differences in B-1+ cells that are PAB+ as compared to B-1+ cells that are PAB– and to study in greater depth the antibodies that they actually make. This will be of particular interest since both PAB+ and B-1+ cells show phenotypic differences, in terms of expression and intensity of cell surface markers and antigen-binding, depending on the organs from which they are isolated. PAB+ cells are formed as a result of V, D, J rearrangement, but the question of whether the B-1+ or B-1– phenotype associated with PAB+ cells is the result of a separate lineage or differentiation from a common cell precursor has not been addressed. The recent findings with B-1+ cells which tend to favour some form or variation of the differentiation hypothesis [30] would be consistent with the B-1+ phenotype being superimposed on some PAB+ cells.
Natural antibodies are thought to play an important role in immunologic defense [33–36], but since sera contains both polyreactive and monoreactive antibodies, the respective role of each is not known. The fact that polyreactive antibodies bind to bacteria argues that they may prevent the dissemination of pathogens and/or improve immunogenicity by trapping antigens in lymphoid organs. The presence of polyreactive IgA in PP and LP also argues that polyreactive antibodies may be involved in mucosal immunity. Yet solid evidence is lacking and the low affinity of these antibodies would seem to make them poor candidates for effective defense against foreign invaders. Moreover, because polyreactive antibodies bind to endogenous antigens they are rapidly cleared from the circulation, leaving very little in the free unbound form [49]. Also, since much of the polyreactive antibody in serum is bound to endogenous antigens they only can be detected by dissociating the polyreactive antibody-antigen complex [50]. Initially, because they were found to bind to self-antigens, polyreactive antibodies were thought to be autoantibodies, but their low affinity argues against an important pathogenic role. Thus, the actually function of polyreactive antibodies still remains unclear.
In addition to secreting polyreactive antibodies, PAB+ cells themselves may have a function independent of the secreted antibodies. Because PAB+ cells are widely distributed and present in high numbers in the adult and are the predominant B cell type in the newborn [51], they are ideally suited to bind the vast array of endogenous antigens to which the host might make an autoimmune response. As recently demonstrated, PAB+ cells can process and present antigens to T cells [25] and at least with antigens that bind with low affinity, this occurs without activating the costimulatory molecules B7-1 and B7-2. Thus, PAB+ cells have all the properties known to be required to induce and/or maintain peripheral immunological tolerance. The demonstration in the present paper of PAB+ cells in the thymus raises the possibility that PAB+ cells might carry endogenous antigens from peripheral tissues back to the thymus. Here they could contribute to central tolerance, particularly for host antigens not ordinarily displayed by the stromal cells of the thymus [52,53]. Thus, PAB+ cells may have a dual role: one in defense against foreign invaders and the other in defense against autoimmunity by contributing to the development and/or maintenance of immunological tolerance.
The immune universe consists of the innate, natural and adaptive immune systems. The studies described here show that PAB+/B-1+, PAB+/B-1– and PAB–/B-1+ cells are major components of the natural immune system. The relationship of the innate immune system to the natural immune system has received little attention. Interaction of pathogens with toll receptors results in the release of a variety of cytokines that can activate cells of the adaptive immune system [54]. Although still not specifically studied, it is not unreasonable to think that the same cytokines will activate cells of the natural immune system. The relationship of the natural immune system to the adaptive immune system also remains unresolved. Although there is some evidence that B-1 cells that make low affinity polyreactive antibody can differentiate into cells that make high affinity antibody [31,55,56], the extent to which this actually occurs in nature is not known. Conversely, there is evidence that B-2 cells can differentiate into B-1 cells [57], but again the extent to which this occurs in nature is not clear. The ability to identify and isolate PAB+/B-1+, PAB+/B-1–, PAB–/B-1+ and PAB–/B-1– cells by antigen-binding and B-1 cell surface markers should make it easier, in the future, to answer some of these questions about the relationship between the natural immune system and the innate and adaptive immune systems.
Acknowledgments
We thank Neil Hardegen for excellent assistance in FACS analysis.
REFERENCES
- 1.Casali P, Notkins AL. Probing the human B-cell repertoire with EBV. polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–35. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 2.Satoh J, Prabhakar BS, Haspel MV, Ginsberg-Fellner F, Notkins AL. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983;309:217–20. doi: 10.1056/NEJM198307283090405. [DOI] [PubMed] [Google Scholar]
- 3.Dighiero G, Lymberi P, Mazie JC, et al. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983;131:2267–72. [PubMed] [Google Scholar]
- 4.Prabhakar BS, Saegusa J, Onodera T, Notkins AL. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984;133:2815–7. [PubMed] [Google Scholar]
- 5.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 6.Burastero SE, Casali P, Wilder RL, Notkins AL. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J Exp Med. 1988;168:1979–92. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harindranath N, Ikematsu H, Notkins AL, Casali P. Structure of the VH and VL segments of polyreactive and monoreactive human natural antibodies to HIV-1 and Escherichia coli beta-galactosidase. Int Immunol. 1993;5:1523–33. doi: 10.1093/intimm/5.12.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain V segments. J Exp Med. 1994;180:885–95. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung SC, Takeda S, Notkins AL. Both VH and VL chains of polyreactive IgM antibody are required for polyreactivity: expression of Fab in Escherichia coli. Clin Exp Immunol. 1995;101:383–6. doi: 10.1111/j.1365-2249.1995.tb08368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adib-Conquy M, Gilbert M, Avrameas S. Effect of amino acid substitutions in the heavy chain CDR3 of an autoantibody on its reactivity. Int Immunol. 1998;10:341–6. doi: 10.1093/intimm/10.3.341. [DOI] [PubMed] [Google Scholar]
- 11.Rini JM, Schulze-Gahmen U, Wilson IA. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992;255:959–65. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- 12.Stanfield RL, Wilson IA. Antigen-induced conformational changes in antibodies: a problem for structural prediction and design. Trends Biotechnol. 1994;12:275–9. doi: 10.1016/0167-7799(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 13.Wedemayer GJ, Patten PA, Wang LH, Schultz PG, Stevens RC. Structural insights into the evolution of an antibody combining site. Science. 1997;276:1665–9. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A, Keitel T, Winkler K, Stocklein W, Hohne W, Schneider-Mergener J. Molecular basis for the binding promiscuity of an anti-p24 (HIV-1) monoclonal antibody. Cell. 1997;91:799–809. doi: 10.1016/s0092-8674(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 15.Keitel T, Kramer A, Wessner H, Scholz C, Schneider-Mergener J, Hohne W. Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity. Cell. 1997;91:811–20. doi: 10.1016/s0092-8674(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 16.Ramsland PA, Guddat LW, Edmundson AB, Raison RL. Diverse binding site structures revealed in homology models of polyreactive immunoglobulins. J Comput Aided Mol Des. 1997;11:453–61. doi: 10.1023/a:1007932211514. [DOI] [PubMed] [Google Scholar]
- 17.Schultz PG, Lerner RA. Completing the circle. Nature. 2002;418:485. doi: 10.1038/418485a. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–53. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 19.Notkins AL. Polyreactive antibodies and polyreactive antigen-binding B (PAB) Cells. Curr Top Microbiol Immunol. 2000;252:241–9. doi: 10.1007/978-3-642-57284-5_25. [DOI] [PubMed] [Google Scholar]
- 20.Potter M, Melchers F. Opinions on the nature of B-1 cells and their relationship to B cell neoplasia. Curr Top Microbiol Immunol. 2000;252:307–24. doi: 10.1007/978-3-642-57284-5_32. [DOI] [PubMed] [Google Scholar]
- 21.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–30. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 22.Scicluna LA, Bruckner L, McCullough KC. Qualitative assessment of the humoral immune status against FMDV in post-vaccination cattle. Vaccine. 2001;19:2975–86. doi: 10.1016/s0264-410x(00)00538-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZJ, Wheeler J, Notkins AL. Antigen-binding B cells and polyreactive antibodies. Eur J Immunol. 1995;25:579–86. doi: 10.1002/eji.1830250241. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZJ, Shimizu F, Wheeler J, Notkins AL. Polyreactive antigen-binding B cells in the peripheral circulation are IgD+ and B7. Eur J Immunol. 1996;26:2916–23. doi: 10.1002/eji.1830261217. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Chen ZJ, Wheeler J, Shen S, Notkins AL. Characterization of murine polyreactive antigen-binding B cells: presentation of antigens to T cells. Eur J Immunol. 2001;31:1106–14. doi: 10.1002/1521-4141(200104)31:4<1106::aid-immu1106>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 27.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J Immunol. 2002;168:4257–61. doi: 10.4049/jimmunol.168.9.4257. [DOI] [PubMed] [Google Scholar]
- 28.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5- B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690–702. [PMC free article] [PubMed] [Google Scholar]
- 29.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 30.Wortis HH, Berland R. Cutting edge commentary. origins of B-1 cells. J Immunol. 2001;166:2163–6. doi: 10.4049/jimmunol.166.4.2163. [DOI] [PubMed] [Google Scholar]
- 31.Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002;169:1735–43. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- 32.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 33.Kroese FG, de Waard R, Bos NA. B-1 cells and their reactivity with the murine intestinal microflora. Semin Immunol. 1996;8:11–8. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]
- 34.Ochsenbein AF, Fehr T, Lutz C, Suter M, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–9. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–80. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalinke U, Oxenius A, Lopez-Macias C, Zinkernagel RM, Hengartner H. Virus neutralization by germ-line vs. hypermutated antibodies. Proc Natl Acad Sci USA. 2000;97:10126–31. doi: 10.1073/pnas.97.18.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–8. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantor AB, Stall AM, Adams S, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–4. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson LD, Foy TM, Waldschmidt TJ. Murine B1 B cells require IL-5 for optimal T cell-dependent activation. J Immunol. 2001;166:1531–9. doi: 10.4049/jimmunol.166.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med. 1994;179:1585–95. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casali P, Burastero SE, Balow JE, Notkins AL. High-affinity antibodies to ssDNA are produced by CD-B cells in systemic lupus erythematosus patients. J Immunol. 1989;143:3476–83. [PubMed] [Google Scholar]
- 42.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 43.Heltemes LM, Manser T. Level of B cell antigen receptor surface expression influences both positive and negative selection of B cells during primary development. J Immunol. 2002;169:1283–92. doi: 10.4049/jimmunol.169.3.1283. [DOI] [PubMed] [Google Scholar]
- 44.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–12. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 45.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–207. [PubMed] [Google Scholar]
- 46.Tumas-Brundage KM, Notidis E, Heltemes L, Zhang X, Wysocki LJ, Manser T. Predominance of a novel splenic B cell population in mice expressing a transgene that encodes multireactive antibodies. support for additional heterogeneity of the B cell compartment. Int Immunol. 2001;13:475–84. doi: 10.1093/intimm/13.4.475. [DOI] [PubMed] [Google Scholar]
- 47.Ceredig R. The ontogeny of B cells in the thymus of normal, CD3 epsilon knockout (KO), RAG-2 KO and IL-7 transgenic mice. Int Immunol. 2002;14:87–99. doi: 10.1093/intimm/14.1.87. [DOI] [PubMed] [Google Scholar]
- 48.Ternynck T, Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and their affinities. Immunol Rev. 1986;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 49.Sigounas G, Harindranath N, Donadel G, Notkins AL. Half-life of polyreactive antibodies. J Clin Immunol. 1994;14:134–40. doi: 10.1007/BF01541346. [DOI] [PubMed] [Google Scholar]
- 50.Sigounas G, Kolaitis N, Monell-Torrens E, Notkins AL. Polyreactive IgM antibodies in the circulation are masked by antigen binding. J Clin Immunol. 1994;14:375–81. doi: 10.1007/BF01546322. [DOI] [PubMed] [Google Scholar]
- 51.Chen ZJ, Wheeler CJ, Shi W, Wu AJ, Yarboro CH, Gallagher M, et al. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol. 1998;28:989–94. doi: 10.1002/(SICI)1521-4141(199803)28:03<989::AID-IMMU989>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Ferrero I, Anjuere F, Martin P, Martinez del Hoyo G, Fraga ML, Wright N, et al. Functional and phenotypic analysis of thymic B cells: role in the induction of T cell negative selection. Eur J Immunol. 1999;29:1598–609. doi: 10.1002/(SICI)1521-4141(199905)29:05<1598::AID-IMMU1598>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 53.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 54.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 55.Ikematsu H, Kasaian MT, Schettino EW, Casali P. Structural analysis of the VH-D-JH segments of human polyreactive IgG mAb. Evidence for somatic selection. J Immunol. 1993;151:3604–16. [PMC free article] [PubMed] [Google Scholar]
- 56.Ichiyoshi Y, Zhou M, Casali P. A human anti-insulin IgG autoantibody apparently arises through clonal selection from an insulin-specific ‘germ-line’ natural antibody template. Analysis by V gene segment reassortment and site-directed mutagenesis. J Immunol. 1995;154:226–38. [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold LW, McCray SK, Tatu C, Clarke SH. Identification of a precursor to phosphatidyl choline-specific B-1 cells suggesting that B-1 cells differentiate from splenic conventional B cells in vivo: cyclosporin A blocks differentiation to B-1. J Immunol. 2000;164:2924–30. doi: 10.4049/jimmunol.164.6.2924. [DOI] [PubMed] [Google Scholar]