Abstract
Dendritic cells (DC) genetically engineered to express Fas (CD95) ligand (FasL-DC) have been proposed as immunotherapeutic tools to induce tolerance to allografts. However, we and others recently showed that FasL-DC elicit a vigorous inflammatory response involving granulocytes and can promote Th1-type CD4+ and cytotoxic CD8+ T lymphocytes. This prompted us to evaluate the pathology induced by intravenous injection of FasL-DC in mice. We observed that FasL-DC obtained after retroviral gene transfer of bone marrow precursors derived from Fas-deficient C57Bl/6 mice induce massive pulmonary inflammation and pleuritis one day after a single intravenous injection in C57Bl/6 mice. Two months later, all mice presented granulomatous vasculitis of small to medium sized vessels, alveolar haemorrhage and pleuritis. In these lesions, apoptotic bodies were found in large number. Anti-neutrophilic cytoplasmic and anti-myeloperoxidase autoantibodies were not detected. This study documents that intravenous injection of FasL-DC causes severe lung granulomatous vasculitis. This new animal model for vasculitis is inducible, highly reproducible and shares many features with human Wegener granulomatosis. This model may be an appropriate tool to further investigate the pathogenesis of vasculitis and test new therapeutic strategies. Moreover, our findings highlight the potential severe complications of FasL-DC-based immunotherapy.
Keywords: vasculitis, animal model, Fas ligand, apoptosis, dendritic cell
INTRODUCTION
Fas (CD95) is a death receptor involved in apoptosis induction on engagement by Fas ligand (FasL, CD95L). In T cells the Fas/FasL pathway is involved in the homeostasis of the T-cell pool and thus essential for prevention of lymphoproliferative disorders and for autoimmunity [1]. However, apoptosis induced by inappropriate or excessive expression of FasL has been proposed as a pathogenic mechanism in several diseases including acute hepatitis [2,3], acute graft-versus-host disease [4], multiple sclerosis [5], autoimmune diabetes [6], dermatitis [7], lung silicosis [8], autoimmune myocarditis [9]. Perturbations in the Fas/FasL system may also play a role in vasculitis. Indeed, anti-neutrophilic cytoplasmic autoantibodies (ANCA)-positive vasculitis patients have high soluble Fas receptor levels [10] and soluble Fas receptor has also been suggested to be involved in the pathogenesis of Churg–Strauss syndrome [11].
Systemic Fas engagement in vivo by agonist Fas-specific antibody or soluble multimeric FasL causes rapid, extensive, and disseminated endothelial cell apoptosis and is rapidly lethal [12]. In addition, we and others have shown that subcutaneous injection of dendritic cells (DC) overexpressing FasL (FasL-DC) exerts a powerful chemoattractant effect on neutrophils leading to a massive inflammatory reaction which is dependent on Interleukin (IL)-1 [13]. This neutrophil recruitment is associated with the development of vigorous T-cell responses involving Th1-type CD4+ and cytotoxic CD8+ T lymphocytes. These observations prompted us to evaluate the pathology induced by intravenous (IV) injection of DC overexpressing FasL (FasL-DC) in mice. In the present study, we reported that a single IV injection of FasL-DC induces lung vasculitis in mice.
MATERIALS AND METHODS
Mice
C57Bl/6 were purchased from Harlan Nederland (Horst, the Netherlands) and C57Bl/6-lpr/lpr Fas-deficient (lpr/lpr) mice from Jackson Laboratories (Bar Harbor, ME, USA). Mice were bred in our animal facilities (Hôpital Erasme, Brussels) and treated according to institutional guidelines.
Reagents and cell lines
The rmGM-CSF used for the DC generation was produced as previously described [14]. The PhoenixECO ecotropic packaging cell line was provided by Dr G. P. Nolan (Stanford, CA, USA) and was grown in the same medium.
Cloning of retroviral vector constructs
For retrovirus production the retroviral vector MFG, derived from Moloney murine leukaemia virus, was used. This vector does not contain a drug resistance marker, nor does it express any potential antigenic protein other than the inserted cDNA [15]. The P1A and CD95L cDNAs were obtained by PCR. The amplification products were sequenced before insertion into the MFG vector. P1A gene was amplified from P1HTR cells (DBA/2 syngeneic tumour) and cloned in pMFG/Nco1-BamH1. The P1A recombinant retrovirus was used to generate the ‘control’ transduced-DC. PIA is a tumour-associated antigen expressed by the P815 mastocytoma cell line isolated from DBA/2 mice.
The cDNA encoding mFasL.2, the murine CD95L gene, was obtained by RT/PCR on RNA from activated T cells of BALB/c origin. After cloning in pCR2, the mCD95L gene was excised from the plasmid as a BspH 1-BamH1 fragment and cloned in pMFG/Nco1-BamH1. The eGFP (enhanced green fluorescent protein) gene was obtained as a Nco1-Bcl1 fragment by digestion of peGFP-C1 (Clonetech Westburg, Leusden, the Netherlands), and ligated in pMFG/Nco1-BamH1.
Retrovirus production and DC transduction
Ten millions PhoenixECO producer cells were transfected with 40 µg of retroviral vector DNA by calcium phosphate precipitation method [16]. Cells were incubated in complete DMEM medium supplemented with 25 µm chloroquine (Sigma-Aldrich) at 37°C for 10 h. The medium was renewed with Opti-MEM (Gibco BRL, UK) after 14 h, and the retrovirus-containing medium was harvested 48 h after transfection. The retroviral supernatants were filtered (0·22 µm pore size), snap-frozen and stored at −80°C.
To generate DC from bone marrow cultures, we used a modified protocol described by Lutz et al. [17]. Briefly, bone marrow was flushed from the femurs and tibiae of mice, disintegrated by vigorous pipetting, filtered through a nylon mesh and depleted of red blood cells with ammonium chloride. At day 0, bone marrow progenitors were seeded in a 6 well plate at the rate of 1 × 106 per well in 4 ml of RPMI 1640 (BioWhittaker) medium containing 10% heat-inactivated FBS (SB0012 BioWhittaker) 20 mm HEPES, 2 mm glutamine, 1 mm non essential amino acids (BioWhittaker), sodium pyruvate (BioWhittaker), 2-mercaptoethanol and 20 ng/ml of rmGM-CSF. At day 3, another 4 ml of complete medium containing 20 ng/ml rmGM-CSF was added to each well. At day 6 and 8, half of the culture supernatant was collected, centrifuged, the cell pellet was resuspended in 4 ml fresh medium supplemented with 20 ng/ml rmGM-CSF and given back into the original well.
The transduction of DCs was carried out at day 1, 2, and 3 after the start of the bone marrow cell culture (3 retroviruses infections), the medium was removed and replaced with 2 ml of each viral supernatant containing 8 µg/ml polybrene (Sigma-Aldrich). In a first set of experiments, DC were generated from wild type C57BL/6 BM-progenitors and were submitted to mFasL.2MFG retroviral transduction. This resulted in massive cell death as more than 90% of BM-cells were annexin V and propidium iodide positive 48 h after the first CD95L transduction compared with 5% after the control transduction. Suicidal or fratricidal death was most likely involved since more than 85% viability of CD95L-transduced DC was obtained at the end of the culture when CD95-deficient lpr/lpr mice were used as bone marrow donors [13]. To generate eGFP FasL-DC and eGFP control P1A DC, bone marrow cells were cotransduced with 2 ml of eGFP and 2 ml of FasL or P1A retroviruses.
The cells were transduced by centrifugation of the 6-well plates for 2 h at 130 g at room temperature. The retroviral supernatant was removed, and the cells were resuspended in cytokine-containing medium. To evaluate our retroviral transduction efficiency and to determine the homing of the DCs, we used eGFP as reporter system. Transduction efficiency was monitored by flow cytometry on the 10th day of the DC culture (78% ± 10, mean ± SEM).
The DC culture purity was evaluated with a FITC-conjugated anti-CD11c mAb (HL3) (Becton Dickinson Pharmingen, Mountain View, CA, USA) in the presence of 2·4G2 supernatant and analysed on a FACScalibur flow cytometer (Becton Dickinson).
Experimental protocol
Six to 8 week-old C57Bl/6 mice received a single injection of 1 × 106 C57Bl/6-lpr/lpr FasL-DC (FasL-DC), C57Bl/6-lpr/lpr PIA control DC (control DC), or saline alone in the tail vein. Groups of mice were sacrificed 1 day (n = 6) and 2 months (n = 8) after injection by exsanguination under general anaesthesia. Blood was collected for indirect immunofluorescence and ELISA. Mice autopsy was performed by a pathologist and representative samples of lungs, kidneys, liver, abdominal lymph nodes were taken. One piece was snap frozen in liquid nitrogen and stored at − 80°C for immunofluorescence staining and one piece was fixed in 10% neutral-buffered formalin and embedded in paraffin and stained with haematoxylin and eosin (HE) for histological analysis.
Immunohistological stainings
To detect apoptotic cells, deparaffinized sections were boiled 2 × 5 min in citrate buffer (pH 6·0). Endogenous peroxydase activity was quenched by a solution of methanol/0·03% H2O2 (Merck, Darmstadt, Germany) and nonspecific binding was blocked by 10% normal goat serum (Dako, Glostrup, Denmark). The sections were incubated with a rabbit anti-human active caspase 3 polyclonal antibody (Cell Signalling, Beverly MA, USA) followed by a further incubation with poly horse radish peroxydase goat anti-rabbit IgG (Powervision, Immunovision Technologies, Duiven, the Netherlands) and developed using a solution of 1% H2O2 and 3·3′-diaminobenzidin-tetra-hydrochloride (Sigma) in Tris-HCl. The sections were mounted in glycerin gelatin after rapid counter staining with methyl green. To detect granulocytes and GFP-positive cells, the same procedure was followed. Slides were incubated with Ly6-G (Pharmingen, Erembodegem, Belgium) and rabbit anti-aequoreo victoria GFP (Molecular Probes, Leiden, the Netherlands), respectively.
To detect immunoglobulin and complement deposits in the lungs, direct immunofluorescence was performed on 4 µm frozen sections. Slides were air-dried, fixed in an acetone-ethanol solution, and incubated with fluoroscein isothiocyanate (FITC)-conjugated antisera against mouse IgM, IgG, or C3 (all from Nordic Immunology, Tilburg, the Netherlands).
Scoring of the histopathology
Five sections obtained at different levels in an all lung were analysed for each mouse. Twenty vessels were randomly analysed on each slide and the number of affected vessels counted. Results are expressed as percentage. The number of granulocytes, active caspase 3 positive cells and eosinophils was quantified in 10 nonoverlapping high power field (×400) and results are expressed as average of number of cells/mm2. The thickness of the pleura was measured at 10 different randomly points for each slide using a digital image analysis program (Image pro-plus®, Mediacybernetics, Germany).
ANCA and anti-MPO detection
Detection of ANCA in sera was performed by indirect immunofluorescence as previously described 18. Briefly, mouse neutrophils were harvested from the peritoneum of C57Bl/6 mice 1 day after intraperitoneal injection of 3% sterilized Proteose peptone (Difco Laboratories, Detroit, MI, USA). Isolated cells were washed in PBS with 0·05 mm EDTA and adjusted to 1 × 106 cells per millilitre. More than 50% of isolated cells were polymorphonuclear neutrophils, and the remainder were mononuclear leucocytes. Cells were cytocentrifuged onto glass slides, air-dried, and fixed with 100% ethanol for 5 min. Serum diluted 1 : 20 was incubated with the substrate neutrophils for 45 min at room temperature, then washed with PBS. Bound antibody was detected with a 1 : 50 dilution of FITC-conjugated rabbit anti-mouse IgG (Dako).
Detection of anti-myeloperoxidase (MPO) antibodies was performed by ELISA as previously described [18]. Microtitre plates were coated with 0·5 µg per well murine MPO, incubated with 50 fold dilutions of mouse sera, developed with alkaline phosphatase-conjugated goat antibodies specific for mouse IgG, and analysed spectrophotometrically at OD 405 nm. Results were expressed as percentage of a positive control serum pool. This positive control consisted of pool serum obtained from MPO knock-out mice immunized with MPO. This positive pool serum diluted at 1 : 50·000 gave an absorption of 50% at OD 405 nm.
RESULTS
DC overexpressing FasL induce granulocytic inflammatory infiltrates in lungs after one day
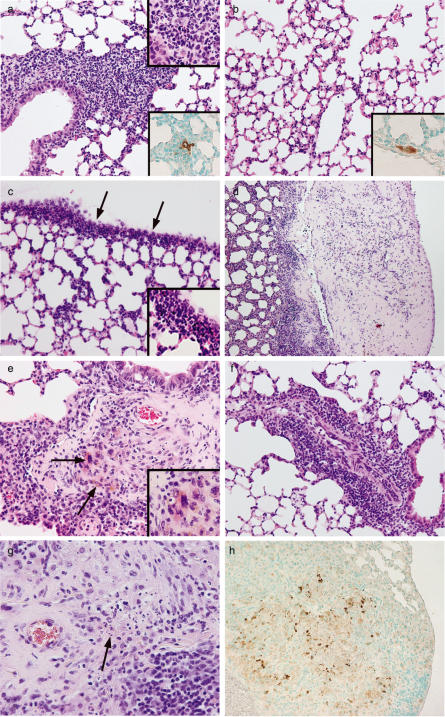
One day after a single IV injection of 1 × 106 C57Bl/6-lpr/lpr FasL-DC all mice developed a massive granulocytic inflammation of the lungs predominantly localized around vessels and bronchi (Fig. 1a). Moreover, a severe granulocytic pleuritis was observed (Fig. 1c). No significant pathological findings could be observed in other organs in these mice. This observation can be explained by the preferential trapping of dendritic cells in the lungs since lungs are the first microvascular bed encountered after IV injection. Accordingly, one day after IV injection of 1 × 106 GFP positive C57Bl/6-lpr/lpr FasL-DC (insert Fig. 1a) or 1 × 106 GFP positive C57Bl/6-lpr/lpr control DC (insert Fig. 1b), solitary GFP positive cells could be identified under the endothelium of small vessels in the lungs. No GFP-positive cells could be detected in other organs such as liver, kidney or lymph nodes. The homing pattern of C57Bl/6-lpr/lpr Fas-L DC and C57Bl/6-lpr/lpr control DC was similar (0·8 ± 0·3 and 0·9 ± 0·4, mean ± SD of positive GFP cells/10 mm2, GFP positive FasL-DC and GFP positive control DC, respectively). Despite similar DC homing, mice injected with 1 × 106 control DC presented discrete interstitial inflammatory infiltrates predominantly composed of monocytes (Fig. 1b) and no signs of pleuritis. No pathological findings were observed in saline injected mice.
Fig. 1.
(a) Heavy granulocytic inflammation (insert, ×60) predominantly localized around vessels and bronchi in the lung of C57Bl/6 mouse one day after IV injection of 1 × 106 FasL-DC, H&E staining ×10. Insert: immunostaining showing a single GFP-positive FasL DC surrounded by an inflammatory infiltrate, GFP-immunostaining, ×40. (b) Slight interstitial inflammation in the lung of C57Bl/6 mouse one day after IV injection of 1 × 106 control DC, H&E staining ×10. Insert: immunostaining showing a single GFP-positive control DC under the endothelium of a small vessel, GFP-immunostaining, ×40. (c) Severe pleuritis (arrow) with dense granulocyte infiltration (insert, ×60), one day after IV injection of 1 × 106 FasL-DC, H&E staining ×10. (d) Extensive fibrotic pleuritis 2 months after IV injection of 1 × 106 FasL-DC, H&E staining ×4. (e) Small vessel vasculitis with excentric granulomatous inflammatory reaction 2 months after IV injection of 1 × 106 FasL-DC, H&E staining ×10. Arrows and insert (×60): multinucleated cells. (f) Perivascular inflammatory infiltrate predominantly composed of lymphocytes and monocytes 2 months after IV injection of 1 × 106 control DC, H&E staining ×10. (g) Close-up on the inflammatory infiltrate surrounding a vessel showing destruction of the vessel wall, extensive fibrosis, numerous apoptotic bodies, and a small focus of fibrinoid necrosis (arrow), H&E staining ×40. (h) Anti-active caspase 3 immunostaining demonstrating the presence of large numbers of apoptotic cells, ×20.
DC overexpressing FasL elicit small vessel lung vasculitis
After 2 months all mice injected with FasL-DC developed granulomatous vasculitis of small and medium-sized arteries and veins in the lungs. The lesions were numerous involving 67 ± 12% of the vessels (Table 1). Involved vessels show transmural infiltration by neutrophils, eosinophils, lymphocytes, plasma cells and giant cells in varying proportions (Fig. 1e,g). Fibrinoid necrosis was focally present. Prominent and eccentric intimal fibrosis was observed surrounded by inflammation (Fig. 1g). Extensive alveolar haemorrhage developed resulting in accumulation of haemosiderin-laden macrophages. A lot of apoptotic bodies were present resulting in the accumulation of fine haematoxyphilic dust (Fig. 1g). This was confirmed by immunostaining for anti-active caspase 3 (Table 1, Fig. 1h). Moreover, extensive fibrotic pleuritis characterized by thick fibrotic pleura and dense inflammatory infiltrate in the underlying lung parenchyma was also observed in all mice (Table 1, Fig. 1d). Three out of 8 mice that received FasL-DC developed small granulomas in the subcapsular sinus of lymph nodes (data not shown). No other significant pathological finding was found in other organs including kidney and liver. Mice that received control DC developed only monocytic inflammatory infiltrates around vessels and bronchi without signs of vasculitis (Fig. 1f). Saline injected mice did not develop any histological abnormalities.
Table 1.
Quantitative analysis of lung lesions
| Mice injected with† | Pleura thickness‡ | Vasculitis§ | Granulocytes¶ | Eosinophils¶ | Apoptotic cells¶ |
|---|---|---|---|---|---|
| FasL DC | 117 ± 88* | 67 ± 12* | 227 ± 30* | 17·0 ± 13* | 95 ± 17* |
| CTRL-DC | 11 ± 2 | 0 | 125 ± 32 | 0·5 ± 0·8 | 21 ± 5 |
| Saline | 6 ± 2 | 0 | 97 ± 23 | 0·3 ± 0·5 | 19 ± 3 |
Mice received an IV injection of either 1 × 106 C57Bl/6-lpr/lpr FasL-DC (FasLDC), 1 × 106 C57Bl/6-lpr/lpr PIA (CTRL-DC) or saline, n = 8 in each group.
Pleura thickness is expressed in microns, mean ± SD.
Vasculitis is expressed as percentage of inflamed vessels, mean ± SD.
Number of granulocytes, eosinophils and apoptotic cells (antiactive caspase 3 positive cells) per mm2, mean ± SD.
P < 0·05 compared to CTRL-DC.
Lung vasculitis induced by FasL DC is not mediated by immune-complex formation and is not associated with ANCA formation
Since lung vasculitis can be elicited by the in situ formation or deposition of immune-complexes, we performed direct immunofluoresence using anti-IgG, anti-IgM and anti-C3 antibodies. No significant deposits of IgG or IgM could be observed in the lungs of mice injected with FasL-DC. C3 deposits were observed in the destroyed vessel walls (data not shown). As this pauci-immune vasculitis induced by FasL-DC shares common features with Wegener granulomatosis, we searched for ANCA using indirect immunofluorescence on human and mouse granulocytes and anti-MPO ELISA. Neither ANCA nor anti-MPO were detected by these assays in serum of FasL-DC injected mice (data not shown).
DISCUSSION
Our findings establish a new model of experimental vasculitis elicited by a single IV injection of DC overexpressing FasL. This new animal model for vasculitis is inducible, highly reproducible and characterized by a granulomatous inflammatory reaction destroying the wall of small to medium sized vessels mimicking Wegener granulomatosis. We found that after IV injection DC are trapped into the lungs and home under the endothelium of small vessels. This is in agreement with previous studies using radioactive labelled DC showing a concentration of DC in lungs and liver after IV injection [19–21]. Already one day after a single intravenous injection of FasL DC, a massive infiltration of neutrophils was observed around small vessels in lungs and pleura. This is reminiscent of previous reports in which FasL overexpression in different tissues induced neutrophil infiltration leading to tissue destruction [13,22–24]. We previously showed that IL-1 was instrumental for the recruitment of granulocytes following FasL DC injection [13] and interestingly, Nicklin et al. [25] described that mice lacking the IL-1 receptor antagonist gene developed arterial inflammation characterized by massive transmural infiltration of neutrophils, macrophages, and T cells. This suggests that granulocyte infiltration mediated by IL-1 in the early phase of the pathological process may be instrumental in the later development of vasculitis.
The common denominator of small vessel vasculitis is necrotizing inflammation of the vessel walls affecting a variety of organ systems. Systemic vasculitides are a heterogenous group of syndromes characterized by relatively similar clinical and pathological findings [26]. Current classification scheme of vasculitides is primarily based on the type of vessel affected and the pattern of injury [27]. Based on this scheme, our model of lung vasculitis induced by FasL-DC shares the classical pathological features encountered in Wegener granulomatosis namely granulomatous inflammation and necrotizing vasculitis affecting small to medium-sized vessels in the respiratory tract. The granulomatous character of the inflammatory infiltrates observed in our model fits better with Wegener granulomatosis than with microscopic polyangiitis. The absence of immune deposits rules out immune complex-mediated and anti-basement membrane antibody mediated pulmonary vasculitic syndromes. Patients suffering from Wegener granulomatosis usually also have a pauci-immune necrotizing and crescentic glomerulonephritis. However, Wegener granulomatosis limited to the lungs as in our model is not rare and this was also observed in the vasculitis model induced by infusion of neutrophil lysosomal enzyme extract where a segmental small vessel vasculitis develops in the lungs and the gut in the absence of glomerulonephritis [28].
The severe fibrotic pleuritis observed in our model is not a characteristic feature of Wegener granulomatosis and may be the sequelae of Fas-mediated apoptosis and/or reflects intrinsic properties of DC. Indeed, in vitro and in vivo studies have shown that Fas-mediated apoptosis induces the release of TGF-β1, one of the most powerful profibrotic cytokine [29–31]. Intimal fibrosis is reminiscent of the healing stage of Langerhans cell histiocytosis and Wegener granulomatosis which is characterized by fibrous scars with pigment-laden macrophages, lymphocytes, plasma cells and fibroblasts. The fibrosis may therefore also be caused by fibroblast-stimulating cytokines such as PDGF, TGF-β, TNF-α and IL-1 which can be produced by activated Langerhans cells and DC [32–34]. Even though eosinophils were not predominant in the inflammatory infiltrate, they were clearly present as in Wegener granulomatosis [35] and Langerhans cell histiocytosis [33]. Eosinophils could also have contributed to the development of fibrosis through the secretion of TGF-β1 and IL-13 [36]. The presence of eosinophils in our vasculitis model is not surprising since soluble FasL has been identified has a survival factor for eosinophils [11].
The aetiology of systemic vasculitis syndromes and the exact mechanisms causing their characteristic combination of lesions remain poorly understood. Our current understanding of the pathogenesis of pauci-immune vasculitis is largely derived from clinical phenomenological studies [37] and from a few animal models of vasculitis [38]. In the present model, overwhelming apoptosis and secondary necrosis induced by FasL DC are likely to play a central role in the development of the vasculitic lesions. Although numerous studies have shown that the clearance of apoptotic cells by phagocytosis can result in powerful anti-inflammatory and immunosuppressive effects [39,40], defective clearance of apoptotic cells and secondary necrosis have been linked closely to autoimmune and persistent inflammatory diseases [41,42]. Moreover, the phagocytic clearance of apoptotic cells can, under certain circumstances, induce immune responses and inflammation [43–45]. Defective clearance or increased exposure to apoptotic neutrophils may be the initiation mechanism for the development of systemic vasculitis [46]. Apoptotic bodies are commonly observed in injured vessels [47] and the antigens recognized by ANCA are expressed at the surface of apoptotic neutrophils [48,49].
Recent reviews have discussed in details the advantages and disadvantages of different animal models of vasculitides [38]. Briefly, we wanted to pinpoint the most striking features of our model compared to the established ones. Vasculitides observed in spontaneous models of systemic lupus erythematosus such as MRL/lpr or (NZBxBXSB)F1 are immune-complex mediated diseases in which lungs are generally not involved [50]. The occurrence of small vessel vasculitis in the lungs has been described in association with polyclonal B cell activation induced by heavy metals by some authors but not all suggesting the contribution of unknown environmental factors [51,52]. The pathogenic role of MPO-ANCA has been demonstrated in different models [53,54]. Brouwer et al. [55] demonstrated that Brown Norway rats immunized with human MPO developed a necrotizing crescentic glomerulonephritis after renal perfusion with MPO. Using a similar approach, Heeringa et al. [56] demonstrated that the site of the lesion is determined by the localization of the interaction of MPO-ANCA with its target antigen. Despite these experimental data showing that ANCA and anti-MPO autoantibodies are pathogenic [18,54], there is a loose correlation between ANCA titre and disease activity [26]. ANCA are identified in the circulation of approximately 80% of patients with pauci-immune small vessel vasculitis and MPO is the most common target for ANCA [57,58]. Since we were unable to detect circulating ANCA or anti-MPO autoantibodies and involvement of the kidney, the present model of small vessel lung vasculitis elicited by FasL-DC mimics the limited form of Wegener's granulomatosis and may provide pathogenic clues in the understanding of ANCA-negative small vessel vasculitis. Our model suggests an additional role for Fas–FasL interaction and dendritic cells in the pathogenesis of small vessel vasculitis. Along this line, activation of adventitial dendritic cells has been recently shown to initiate and maintain T cell response in giant cell arteritis and break tissue tolerance in the perivascular space [59].
Beside its interest for the pathogenesis and perhaps the treatment of small vessel vasculitis, this new model also draws attention to a possible complication of FasL-DC-based immunotherapy, a therapeutic approach which has been proposed to induce tolerance to transplanted organs [60,61].
References
- 1.Krammer PH. CD95′s deadly mission in the immune system. Nature. 2000;407:789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 2.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–13. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 4.Via CS, Nguyen P, Shustov A, Drappa J, Elkon KB. A major role for the Fas pathway in acute graft-versus-host disease. J Immunol. 1996;157:5387–93. [PubMed] [Google Scholar]
- 5.D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–70. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su X, Hu Q, Kristan JM, Costa C, Shen Y, Gero D, Matis LA, Wang Y. Significant role for Fas in the pathogenesis of autoimmune diabetes. J Immunol. 2000;164:2523–32. doi: 10.4049/jimmunol.164.5.2523. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Akdis M, Trautmann A, Blaser K. Immune regulation in atopic dermatitis. Curr Opin Immunol. 2000;12:641–6. doi: 10.1016/s0952-7915(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 8.Borges VM, Falcao H, Leite-Junior JH, et al. Fas ligand triggers pulmonary silicosis. J Exp Med. 2001;194:155–64. doi: 10.1084/jem.194.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiyama S, Hiroe M, Nishikawa T, et al. The Fas/Fas ligand system is involved in the pathogenesis of autoimmune myocarditis in rats. J Immunol. 1998;161:4695–701. [PubMed] [Google Scholar]
- 10.Christensson M, Pettersson E, Eneslatt K, Christensson B, Bratt J, Rantapaa-Dahlqvist S, Sundqvist KG. Serum sFAS levels are elevated in ANCA-positive vasculitis compared with other autoimmune diseases. Jclinimmunol. 2002;22:220–7. doi: 10.1023/a:1016040925295. [DOI] [PubMed] [Google Scholar]
- 11.Muschen M, Warskulat U, Perniok A, et al. Involvement of soluble CD95 in Churg–Strauss syndrome. Am J Pathol. 1999;155:915–25. doi: 10.1016/S0002-9440(10)65191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janin A, Deschaumes C, Daneshpouy M, et al. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99:2940–7. doi: 10.1182/blood.v99.8.2940. [DOI] [PubMed] [Google Scholar]
- 13.Buonocore S, Paulart F, Le Moine A, et al. Dendritic cells overexpressing CD95 (Fas) ligand elicit vigorous allospecific T-cell responses in vivo. Blood. 2003;101:1469–76. doi: 10.1182/blood-2002-07-2042. [DOI] [PubMed] [Google Scholar]
- 14.Olivares Fontt E, Heirman C, Thielemans K, Vray B. Granulocyte-macrophage colony-stimulating factor. involvement in control of Trypanosoma cruzi infection in mice. Infect Immun. 1996;64:3429–34. doi: 10.1128/iai.64.8.3429-3434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–7. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pear WS, Scott ML, Nolan GP. Generation of high-titer, helper-free retroviruses by transient infection. In: Walker JM, editor. Methods in Molecular Medicine, Gene Therapy Protocols. New Jersey: Humana Press; 1997. pp. 41–57. [DOI] [PubMed] [Google Scholar]
- 17.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 18.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barratt-Boyes SM, Watkins SC, Finn OJ. In vivo migration of dendritic cells differentiated in vitro: a chimpanzee model. J Immunol. 1997;158:4543–7. [PubMed] [Google Scholar]
- 20.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–9. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Callahan RJ, Wilkenson RA, van Rooijen N, Schneeberger EE. Interferon-gamma reduces Ia+ dendritic cell traffic to the lung. J Leukoc Biol. 1996;60:519–27. doi: 10.1002/jlb.60.4.519. [DOI] [PubMed] [Google Scholar]
- 22.Kang SM, Hoffmann A, Le D, Springer ML, Stock PG, Blau HM. Immune response and myoblasts that express Fas ligand. Science. 1997;278:1322–4. doi: 10.1126/science.278.5341.1322. [DOI] [PubMed] [Google Scholar]
- 23.Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–43. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi T, Ueki T, Nishimatsu H, et al. Accelerated rejection of Fas ligand-expressing heart grafts. J Immunol. 1999;162:518–22. [PubMed] [Google Scholar]
- 25.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–23. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 27.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 28.Heeringa P, Foucher P, Klok PA, Huitema MG, Tervaert JW, Weening JJ, Kallenberg CG. Systemic injection of products of activated neutrophils and H2O2 in myeloperoxidase-immunized rats leads to necrotizing vasculitis in the lungs and gut. Am J Pathol. 1997;151:131–40. [PMC free article] [PubMed] [Google Scholar]
- 29.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freire -d, e-Lima CG, Nascimento DO, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 32.Corrin B, Butcher D, McAnulty BJ, Dubois RM, Black CM, Laurent GJ, Harrison NK. Immunohistochemical localization of transforming growth factor-beta 1 in the lungs of patients with systemic sclerosis, cryptogenic fibrosing alveolitis and other lung disorders. Histopathol. 1994;24:145–50. doi: 10.1111/j.1365-2559.1994.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 33.de Graaf JH, Tamminga RY, Dam-Meiring A, Kamps WA, Timens W. The presence of cytokines in Langerhans’ cell histiocytosis. J Pathol. 1996;180:400–6. doi: 10.1002/(SICI)1096-9896(199612)180:4<400::AID-PATH701>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 34.Foss HD, Herbst H, Araujo I, Hummel M, Berg E, Schmitt-Graff A, Stein H. Monokine expression in Langerhans’ cell histiocytosis and sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) J Pathol. 1996;179:60–5. doi: 10.1002/(SICI)1096-9896(199605)179:1<60::AID-PATH533>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel A, Csernok E, Braun J, Gross WL. Activation of neutrophils, eosinophils, and lymphocytes in the lower respiratory tract in Wegener's Granulomatosis. Am J Respir Crit Care Med. 2000;161:399–405. doi: 10.1164/ajrccm.161.2.9904076. [DOI] [PubMed] [Google Scholar]
- 36.Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–12. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- 37.Kallenberg CG, Heeringa P. Pathogenesis of vasculitis. Lupus. 1998;7:280–4. doi: 10.1191/096120398678920109. [DOI] [PubMed] [Google Scholar]
- 38.Specks U. Are animal models of vasculitis suitable tools? Curr Opin Rheumatol. 2000;12:11–9. doi: 10.1097/00002281-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Botto M. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp Clin Immunogenet. 1998;15:231–4. doi: 10.1159/000019076. [DOI] [PubMed] [Google Scholar]
- 43.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 44.O'Connell J, Houston A, Bennett MW, O'Sullivan GC, Shanahan F. Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med. 2001;7:271–4. doi: 10.1038/85395. [DOI] [PubMed] [Google Scholar]
- 45.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 46.Esnault VL. Apoptosis: the central actor in the three hits that trigger anti-neutrophil cytoplasmic antibody-related systemic vasculitis. Nephrol Dial Transplant. 2002;17:1725–8. doi: 10.1093/ndt/17.10.1725. [DOI] [PubMed] [Google Scholar]
- 47.Rastaldi MP, Ferrario F, Crippa A, Dell’Antonio G, Casartelli D, Grillo C, D’Amico G. Glomerular monocyte-macrophage features in ANCA-positive renal vasculitis and cryoglobulinemic nephritis. J Am Soc Nephrol. 2000;11:2036–43. doi: 10.1681/ASN.V11112036. [DOI] [PubMed] [Google Scholar]
- 48.Gilligan HM, Bredy B, Brady HR, et al. Antineutrophil cytoplasmic autoantibodies interact with primary granule constituents on the surface of apoptotic neutrophils in the absence of neutrophil priming. J Exp Med. 1996;184:2231–41. doi: 10.1084/jem.184.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang JJ, Tuttle RH, Hogan SL, Taylor JG, Phillips BD, Falk RJ, Jennette JC. Target antigens for anti-neutrophil cytoplasmic autoantibodies (ANCA) are on the surface of primed and apoptotic but not unstimulated neutrophils. Clin Exp Immunol. 2000;121:165–72. doi: 10.1046/j.1365-2249.2000.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harper JM, Thiru S, Lockwood CM, Cooke A. Myeloperoxidase autoantibodies distinguish vasculitis mediated by anti-neutrophil cytoplasm antibodies from immune complex disease in MRL/Mp-lpr/lpr mice: a spontaneous model for human microscopic angiitis. Eur J Immunol. 1998;28:2217–26. doi: 10.1002/(SICI)1521-4141(199807)28:07<2217::AID-IMMU2217>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 51.Esnault VL, Mathieson PW, Thiru S, Oliveira DB, Martin-Lockwood C. Autoantibodies to myeloperoxidase in brown Norway rats treated with mercuric chloride. Laboratory Invest. 1992;67:114–20. [PubMed] [Google Scholar]
- 52.Mathieson PW, Thiru S, Oliveira DB. Mercuric chloride-treated brown Norway rats develop widespread tissue injury including necrotizing vasculitis. Laboratory Invest. 1992;67:121–9. [PubMed] [Google Scholar]
- 53.Heeringa P, Brouwer E, Cohen Tervaert JW, Weening JJ, Kallenberg CG. Animal models of anti-neutrophil cytoplasmic antibody associated vasculitis. Kidney Int. 1998;53:253–63. doi: 10.1046/j.1523-1755.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 54.Falk RJ, Jennette JC. ANCA are pathogenic – oh yes they are! J Am Soc Nephrol. 2002;13:1977–9. doi: 10.1681/ASN.V1371977. [DOI] [PubMed] [Google Scholar]
- 55.Brouwer E, Huitema M, Klok P, de Weerd H, Cohen Tervaert J, Weening J, Kallenberg C. Antimyeloperoxidase-associated proliferative glomerulonephritis: an animal model. J Exp Med. 1993;177:905–14. doi: 10.1084/jem.177.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heeringa P, Foucher P, Klok PA, Huitema MG, Cohen Tervaert JW, Weening JJ, Kallenberg CG. Systemic injection of products of activated neutrophils and H2O2 in myeloperoxidase-immunized rats leads to necrotizing vasculitis in the lungs and gut. Am J Pathol. 1997;151:131–40. [PMC free article] [PubMed] [Google Scholar]
- 57.Jennette JC, Hoidal JR, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–4. [PubMed] [Google Scholar]
- 58.Falk RJ, Jennette JC. ANCA small-vessel vasculitis. J Am Soc Nephrol. 1997;8:314–22. doi: 10.1681/ASN.V82314. [DOI] [PubMed] [Google Scholar]
- 59.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of Arterial Wall Dendritic Cells and Breakdown of Self-tolerance in Giant Cell Arteritis. J Exp Med. 2004;199:173–83. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–24. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 61.Sata M, Luo Z, Walsh K. Fas ligand overexpression on allograft endothelium inhibits inflammatory cell infiltration and transplant-associated intimal hyperplasia. J Immunol. 2001;166:6964–71. doi: 10.4049/jimmunol.166.11.6964. [DOI] [PubMed] [Google Scholar]