Abstract
Interleukin-18 (IL-18) has been demonstrated to synergize with BCG for induction of a T-helper-type 1 (Th1) immune response. Since successful treatment of superficial bladder cancer with BCG requires proper induction of Th1 immunity, we have developed a recombinant (r) BCG strain that functionally secretes mouse (m) IL-18. This rBCG-mIL-18 strain significantly increased production of the major Th1 cytokine IFN-γ in splenocyte cultures, at levels comparable to that elicited by control BCG plus exogenous rIL-18. IFN-γ production by splenocytes was eliminated by addition of neutralizing anti-IL-18 antibody. Endogenous IL-12 played a favourable role whereas IL-10 played an adverse role in rBCG-mIL-18-induced IFN-γ production. Enhanced host antimycobacterial immunity was observed in mice infected with rBCG-mIL-18 which showed less splenic enlargement and reduced bacterial load compared to control mice infected with BCG. Further, splenocytes from rBCG-mIL-18-infected mice, in response to BCG antigen, displayed increased production of IFN-γ and GMCSF, decreased production of IL-10, elevated cellular proliferation and higher differentiation of IFN-γ-secreting cells. rBCG-mIL-18 also enhanced BCG-induced macrophage cytotoxicity against bladder cancer MBT-2 cells in a dose-dependent manner. Neutralizing all endogenous macrophage-derived cytokines tested (IL-12, IL-18 and TNF-α) as well as IFN-γ severely diminished the rBCG-mIL-18-induced macrophage cytolytic activity, indicating a critical role for these cytokines in this process. Cytokine analysis for supernatants of macrophage-BCG mixture cultures manifested higher levels of IFN-γ and TNF-α in rBCG-mIL-18 cultures than in control BCG cultures. Taken together, this rBCG-mIL-18 strain augments BCG's immunostimulatory property and may serve as a better agent for bladder cancer immunotherapy and antimycobacterial immunization.
Keywords: BCG, IL-18, IFN-γ, bladder cancer, cytotoxicity
INTRODUCTION
In addition to being one of the world's most widely used tuberculosis vaccines, BCG has also been successfully applied to the treatment of superficial bladder cancer for over two decades through intravesical instillation [1–3]. Although the exact mechanism by which BCG mediates antitumour immunity remains unclear, a local nonspecific immunological reaction reflecting various activities of immune cells has been suggested [2–8]. One of the remarkable phenomena after BCG bladder instillation is a transient secretion of several cytokines in patients’ voided urine [9–16]. Among them, a massive burst of urinary IFN-γ, along with other Th1 cytokines (IL-12 and IL-2), has been observed to be a common feature in BCG responders [11,16–18]. In contrast, higher levels of Th2 cytokines IL-10 and/or IL-6 appear to be associated with BCG failure [12,16,18]. Correlatively, in mouse models IFN-γ and IL-12 but not IL-10 and IL-4 are suggested to be required for immunotherapeutic control of orthotopic bladder cancer [16,19,20]. These observations suggest that effective BCG therapy of bladder cancer requires proper activation of the Th1 immune pathway [16,21,22].
Despite the success of current BCG therapy in superficial bladder cancer, 30–50% of patients do not respond to BCG therapy and long-term remission (>5 years) is only achieved in about 50% of responding patients [23–25]. Clearly, such conventional BCG therapy needs to be improved for its therapeutic efficacy. IL-18, a cytokine mainly produced by activated macrophages, possesses pleiotropic immunological activities, such as enhancement of IFN-γ production from T and NK cells and up-regulation of Fas ligand expression on these cells [26–29]. IL-18 has also been found to directly costimulate mycobacterium for induction of Th1 immune responses. IL-18 synergizes BCG for IFN-γ production from splenocytes [30], up-regulates secretion of matrix metalloproteinases (MMPs) from macrophages upon BCG infection [31], enhances host protective immunity against mycobacterial infection [32–38], and shows predictive value for a favourable bladder response to intravesical BCG therapy [10]. These immune properties of IL-18 make this cytokine ideal to supplement BCG for a better treatment of bladder cancer or even develop a better tuberculosis vaccine.
Our laboratory has pioneered methods to genetically engineer BCG to express various biologically active molecules of interest [39–43]. Based on the current understanding that IL-18 synergizes BCG for induction of Th1 immune responses, we created a new strain of BCG that constitutively secretes rmIL-18. We demonstrated that this newly constructed rBCG-mIL-18 strain possesses substantially enhanced immunogenicity compared to control BCG, showing elevated splenocyte IFN-γ production, host antimycobacterial immunity, and macrophage cytotoxicity against bladder cancer line MBT-2 cells.
MATERIALS AND METHODS
Reagents
The medium used for culturing splenocytes and peritoneal exudate cells (PECs) was RPMI 1640 (Gibco BRL) containing 10% fetal calf serum (FCS) and 30 µg/ml kanamycin. BCG was cultured in Middlebrook 7H9 Bacto broth (Difco, Detroit, MI, USA) supplemented with 10% ADC (5% bovine serum albumin fraction V, 2% dextrose and 0·85% NaCl), 0·05% Tween 80, and 30 µg/ml kanamycin. Antibodies used for enzyme-linked immunosorbent assay (ELISA) included paired capture and detecting antibodies for mouse (m) IL-18 (Hayashibara, Hyogo, Japan), mIFN-γ (Endogen, Woburn, MA, USA), mTNF-α (Genzyme, Cambridge, MA, USA), mIL-10 (PharMingen, San Diego, CA, USA), mIL-6 (PharMingen), and mGMCSF (PharMingen). Cytokine-neutralizing antibodies were obtained as follows: mIL-18 (rabbit polyclone) from Hayashibara, mIL-12 (clone C15·6, rat IgG1) from PharMingen, mIFN-γ (clone R4·6A2, rat IgG1) from Endogen, and mTNF-α (clone MP6-XT3, rat IgG1) from PharMingen. Species and isotype matched control antibodies were obtained from Sigma for rabbit IgG and from PharMingen for rat IgG1. Recombinant mIL-18 was obtained from Hayashibara. The high protein binding immobilon-P membrane wells of 96-well plates (MultiScreen-IP) for ELISPOT assay were purchased from Millipore (Bedford, MA, USA).
Mice
C57BL/6 and C3H/HeN mice were purchased from the National Cancer Institute. C57BL/6 IL-10–/– mice were kindly provided by Dr Donna Rennick and kept under the specific pathogen-free conditions. Mice were allowed free access to food and water. All mice were female and used for experiments at age of 6–8 weeks old.
Construction of mouse IL-18 expression plasmid and the recombinant BCG strain
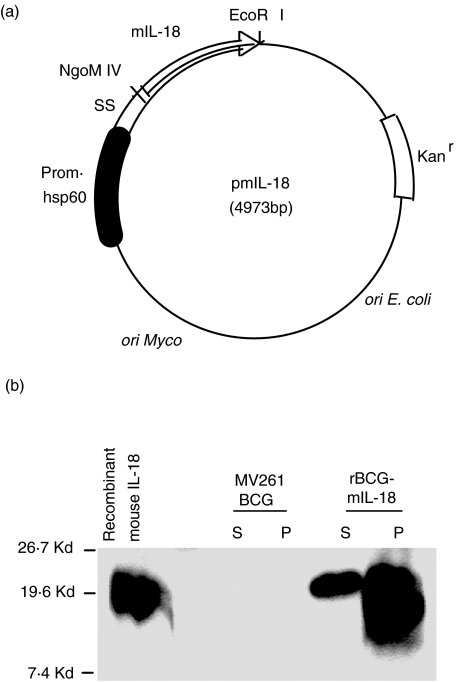
The previously described mouse IL-2 expression vector pRBD3 [39] was engineered to express mouse IL-18 by replacing the IL-2 coding sequence with mouse IL-18 coding sequence at the NgoM IV-EcoRI sites. The NgoM IV cutting site is located within the BCG α-antigen signal sequence that is linked to the N-terminal of IL-18 coding sequence. The insert IL-18 sequence was derived from PCR amplification of mouse IL-18 containing plasmid (a gift from Dr Andrew M. Jackson) using a pair of primers for the mature form of the cytokine. The sequence of the sense primer was 5′-CAAGgccggcGGAGCGGCAACCGCGGGCGC GAACTTTGGCCGACTTCACTGTA-3′ (NgoM IV site in lowercase) and that of the antisense primer was 5′-GCCGgaattc CTAACTTTGATGTAAGTTAGTGAG-3′ (EcoRI site in lowercase). The newly constructed plasmid pmIL-18 is illustrated in Fig. 1a. E. coli competent cells (XL1-Blue MR) obtained from Stratagene were transformed with the E. coli-BCG shuttle plasmid according to the manufacturer's instructions and selected on kanamycin (30 µg/ml) Luria-Bertani agar (Difco) plates. The E. coli-derived plasmid with correct structure verified by restriction analysis and sequencing was transformed into BCG Pasteur strain (ATCC) by electroporation as described previously [44]. The transformed BCG cells were plated on Middlebrook 7H10 Bacto agar (Difco) supplemented with 10% ADC and 30 µg of kanamycin per ml. Individual colonies containing pmIL-18 verified by PCR were picked and grown in Middlebrook 7H9 medium as described in Reagents. Construction of BCG Pasteur containing control vector (MV261 BCG) was described previously [39].
Fig. 1.
(a) Schematic illustration of the mouse IL-18-containing E. coli-BCG shuttle plasmid (pmIL-18). The IL-18 coding sequence was placed downstream of BCG α-antigen signal sequence (SS). Expression of IL-18 is driven by the BCG hsp60 promoter (black box). The arrow indicates the direction of transcription. The kanamycin resistance cassette (open box) and a mycobacterial as well as an E. coli origin of replication are indicated. The illustration is not to scale. (b) Expression of IL-18 in Western blot of BCG culture supernatant (S) and pellet lysate (P) from rBCG-mIL-18. For controls, culture supernatant and pellet lysate were prepared in parallel from MV261 BCG. Purified recombinant mouse IL-18 was used as a positive control shown on the left. The blot was probed with a specific rat anti-mouse IL-18 monoclonal antibody. Standard molecular weights are indicated.
Western blot analysis
BCG cellular lysates were prepared from log-phase growing cultures at 1 OD600 density (one unit of optical density at 600 nm was calculated as 2·5 × 107 CFUs) by heating the BCG pellet in water at 100°C for 10 min. Culture supernatants were concentrated using a protein G immunoprecipitation kit (Roche Molecular Biochemicals) as described previously [42]. Briefly, 1·5 ml of culture supernatants collected from 1 OD600 density of log-phase BCG were precleared by incubation with 50 µl of protein G agarose at 4°C for 3 h on a rocking platform. After centrifugation, supernatants were collected and incubated with 20 µg of rat anti-mIL-18 monoclonal antibody (clone ♯39–3F, MBL International Corporation) at 4°C for 1 h followed by further incubation with 100 µl of protein G agarose at 4°C for overnight. The IL-18-removed supernatants were collected and used in bioassays for comparisons. The bound IL-18 was released from protein G agarose after 3 cycle washes. Following electrophoresis in a 15% SDS-polyacrylamide gel (Bio-Rad) and transfer to nitrocellulose, the filters were blocked in 5% nonfat powdered milk and then probed with rat anti-mIL-18 monoclonal antibody (clone ♯74, MBL International Corporation). The blots were subsequently probed with a horseradish peroxidase-labelled rabbit anti-rat immunoglobulin G antibody (Sigma) and a chemiluminescent substrate (Santa Cruz Biotechnology) to detect membrane-bound IL-18 protein.
In vitro splenocyte cytokine production
Spleen cells were lysed using ACK lysis buffer (0·15 m NH4Cl, 1 mm KHCO3, 0·1 mm EDTA; pH 7·4) to remove erythrocytes, washed with RPMI 1640 medium, and then plated in triplicate in flat-bottomed 96-well (2·5 × 105 cells per well) or 24-well (2 × 106 cells per well) tissue culture plates (Nunc) in RPMI complete medium as described in Reagents. Cells were cultured in a humidified 5% CO2 incubator at 37°C for 3 days. To evaluate the in vitro ability of rBCG-mIL-18 to regulate splenocyte IFN-γ production, spleen cells were incubated with indicated doses of either control MV261 BCG or rBCG-mIL-18 or with BCG culture supernatants collected from either control MV261 BCG or rBCG-mIL-18. To confirm the specific role of IL-18 secreted from rBCG-mIL-18 in splenocyte IFN-γ production, neutralizing IL-18 antibody was added. To investigate the role of endogenous IL-12 in splenocyte IFN-γ production induced by rBCG-mIL-18, neutralizing IL-12 antibody was added. To evaluate the development of host BCG-specific immune responses, splenocytes were collected 4 or 5 weeks after infection of mice with either control MV261 BCG or rBCG-mIL-18 and stimulated in vitro with MV261 BCG or Mycobacterium tuberculosis culture filtrate protein (CFP). As previously reported [41,45], ELISPOT assays were performed after 16- h stimulation for enumerating the number of IFN-γ secreting cells and ELISAs were performed after 3-day stimulation for evaluating the expression of cytokines (IFN-γ, TNF-α, IL-10, IL-6 and GMCSF).
BCG infection of mice
As previously reported [41], C57BL/6 mice (8 mice per group) were infected intravenously through the lateral tail vein with 5 × 105 CFU of either rBCG-mIL-18 or control MV261 BCG suspended in 0·1 ml of phosphate-buffered saline (PBS). No apparent signs of illness were observed with the experimental infection. Mice (eight mice per group) were sacrificed at 4 weeks after infection. Spleens were removed aseptically, weighed, and then filtered through a fine nylon mesh to generate single-cell suspensions. The cell suspension were appropriately diluted and plated on Middlebrook 7H10 agar plates. The BCG colonies were counted after incubation at 37°C for 3 weeks. The remaining spleen cell suspensions of the same group were pooled together and used for in vitro cytokine production and proliferation assays.
C57BL/6 mice (5 mice per group) were also subcutaneously infected at the base of the tail with 5 × 105 CFU of either rBCG-mIL-18 or control MV261 BCG suspended in 0·2 ml PBS for five weeks. Mice were then sacrificed and spleens from each group of mice were aseptically harvested for in vitro analysis of their IFN-γ responses.
Macrophage cytotoxicity assay
Macrophage cytolytic activity was evaluated using PECs as previously reported but with slight modifications [46,47]. PECs were prepared in C3H/HeN mice by a single intraperitoneal injection with 2 ml of 3% thioglycollate (Sigma). After 3 days, PECs were collected by washing out the peritoneal cavity with PBS for several times and then suspended in culture medium containing 30 µg/ml kanamycin. PECs prepared were composed of >90% macrophages and <10% lymphocytes and NK cells by flow cytometric analysis. 1 × 105 PECs in 100 µl culture medium was seeded in 96-well flat-bottomed plates (Nunc). After 2- h culture, BCG (0·005 OD/well for most assays otherwise as indicated) alone or together with various doses of rmIL-18 or neutralizing antibodies (anti-IL-18, anti-IL-12, anti-IFN-γ, and anti-TNF-α) was added and the final volume in each well adjusted to 200 µl. The plates were incubated at 37°C in 5% CO2. For evaluation of PEC cytokine production, culture supernatants were harvested after 12, 24 and 48 h and analysed by ELISA. For evaluation of PEC cytolytic activity, 51Cr-labelled MBT-2 cells were added after 24 h. MBT-2 cells were radiolabelled with 200 µCi[51Cr] sodium chromate (Amersham) for 2 h at 37°C in 5% CO2, washed 3 times with RPMI 1640 medium, and suspended at a concentration of 2 × 105/ml in culture medium containing 30 µg/ml kanamycin. For most cytotoxicity assays, effector:target (E : T) ratio of 10 : 1 was performed (otherwise as indicated) by adding 50 µl of 51Cr-labelled MBT-2 suspension (1 × 104 cells/well) to each well that contained 200 µl of 1 × 105 PECs. After a further 20-h incubation, the supernatants were harvested and released 51Cr counted with a γ-counter. All assays were performed in triplicate. Spontaneous 51Cr release was determined by incubating radiolabelled target cells in the absence of PECs. Maximal 51Cr release was determined by incubating the same amount of target cells in 2% Triton X-100 (Sigma). The percentage of specific cytotoxicity was calculated as follows:
% Cytotoxicity = [(Experimental release − Spontaneous release)/(Maximal release − Spontaneous release)] × 100.
RESULTS
Expression of mouse IL-18 in mycobacteria
The PCR-derived mouse IL-18 coding sequence was placed into the immediate downstream site of the BCG alpha-antigen signal sequence (SS) under control of the constitutively active hsp60 promoter (Fig. 1a). From BCG transformed with this construct, mIL-18 was readily detectable in both BCG lysates and culture supernatants on Western blot (Fig. 1b). MV261 BCG, a control BCG strain carrying the empty vector but retaining the similar immunostimulatory properties of wild-type BCG [39,48], showed no specific band on the same blots. The concentration of mIL-18 in culture was quantified by ELISA and calculated to be approximately 3000 pg/ml (data not shown).
Augmentation of splenocyte IFN-γ production by rBCG-mIL-18
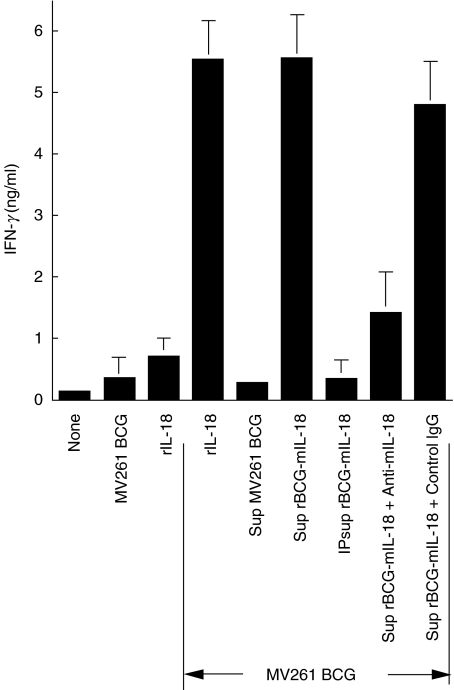
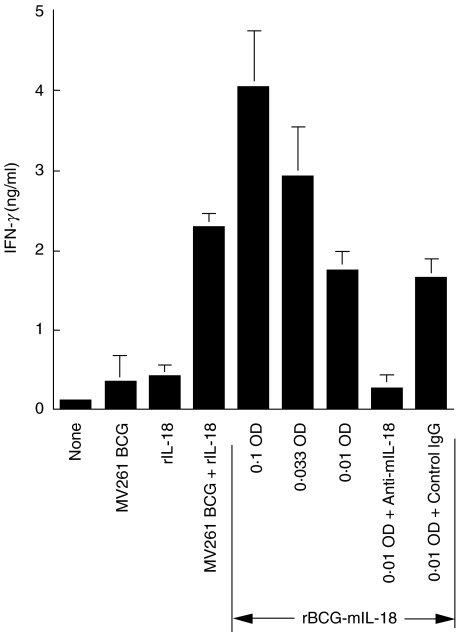
Enhanced production of IFN-γ in mouse splenocyte cultures by rBCG-mIL-18 was readily demonstrable. When combined with cell free-culture supernatant of BCG-mIL-18, MV261 BCG augmented IFN-γ production 20-fold compared to control MV261 BCG (Fig. 2). Supernatant alone showed a marginal effect on IFN-γ production (data not shown). This increased level of IFN-γ expression was comparable with that of exogenous rIL-18 (1000 pg/ml) combined with MV261 BCG, a synergistic effect as observed previously [30], and could be completely eliminated by the removal of IL-18 from the supernatant through immunoprecipitation and severely reduced by adding neutralizing anti-mIL-18 antibody. Figure 3 demonstrated that rBCG-mIL-18 was superior to control MV261 BCG for splenocyte IFN-γ production (5·6-fold increase at 0·01 OD/ml). This increased level of IFN-γ production was also comparable with that of synergistic effect between MV261 BCG and exogenous rIL-18 and could also be removed by adding neutralizing anti-mIL-18 antibody. These data demonstrated that rBCG-mIL-18 secreted functional IL-18 and was more potent than wild-type BCG for IFN-γ induction.
Fig. 2.
Biological activity of IL-18 secreted from rBCG-mIL-18. IFN-γ production in culture supernatants was measured by ELISA after 3-day incubation of splenocytes from naïve C57BL/6 mice with medium, control MV261 BCG (0·01 OD/ml) alone, rIL-18 (1000 pg/ml) alone, or combinations of control MV261 BCG (0·01 OD/ml) with rIL-18 (1000 pg/ml), culture supernatant (Sup) of MV261 BCG, Sup of rBCG-mIL-18, Sup of rBCG-mIL-18 after the removal of IL-18 by immunoprecipitation (IP sup rBCG-mIL-18), or Sup of rBCG-mIL-18 plus either rabbit neutralizing IL-18 antibody (3 µg/ml) or control IgG (3 µg/ml). All BCG supernatants used were collected from 1 OD density of BCG cultures. Values were expressed as means ± SD from triplicate-well incubations.
Fig. 3.
Augmentation of splenocyte IFN-γ production by rBCG-mIL-18. IFN-γ production in culture supernatants was measured by ELISA after 3-day incubation of splenocytes from naïve C57BL/6 mice with medium, control MV261 BCG (0·01 OD/ml), rIL-18 (250 pg/ml), MV261 BCG (0·01 OD/ml) plus rIL-18 (250 pg/ml), different doses of rBCG-mIL-18 (0·1 OD/ml, 0·033 OD/ml and 0·01 OD/ml), or rBCG-mIL-18 (0·01 OD/ml) plus either rabbit neutralizing IL-18 antibody (3 µg/ml) or control IgG (3 µg/ml). Values were expressed as means ± SD from triplicate-well incubations.
Role of IL-12 and IL-10 in splenocyte IFN-γ production induced by rBCG-mIL-18
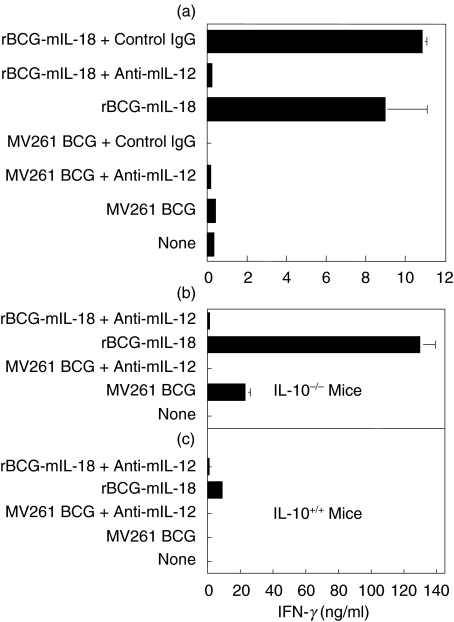
Both IL-12 and IL-10 had been known to affect the overall output of IFN-γ production from immune cells. Thus, the role of endogenous IL-12 and IL-10 in rBCG-mIL-18 induced IFN-γ production was investigated. Neutralizing endogenous IL-12 completely eliminated the effect of rBCG-mIL-18 on induction of IFN-γ for both C57BL/6 (Fig. 4a,c) and Balb/c mice (data not shown). Abolition of endogenous IL-10 dramatically up-regulated the expression of IFN-γ induced by rBCG-mIL-18, although the expression of IFN-γ was also increased by control MV261 BCG (Fig. 4b). This up-regulated IFN-γ expression was most likely due to the absence of the Th1 antagonistic cytokine IL-10 in IL-10–/– mice. Again, neutralizing endogenous IL-12 showed a potent inhibitory effect on IFN-γ production in IL-10–/– mice. These data demonstrated that IL-12 played a positive role whereas IL-10 played a negative role in splenocyte IFN-γ production in response to BCG as well as BCG expressing IL-18.
Fig. 4.
Role of IL-12 and IL-10 in splenocyte IFN-γ production induced by rBCG-mIL-18. (a) IFN-γ production in culture supernatants was measured by ELISA after 3-day incubation of splenocytes from naïve C57BL/6 mice with medium, control MV261 BCG (0·1 OD/ml), rBCG-mIL-18 (0·1 OD/ml), rBCG-mIL-18 (0·1 OD/ml) plus neutralizing IL-12 antibody (100 µg/ml) or control IgG1 (100 µg/ml), or MV261 BCG (0·1 OD/ml) plus neutralizing IL-12 antibody (100 µg/ml) or control IgG (100 µg/ml). (b,c) Splenocytes from naïve C57BL/6 IL-10–/– mice (b) and naïve C57BL/6 mice (c) were incubated with medium, control MV261 BCG (0·1 OD/ml) alone or plus neutralizing IL-12 antibody (100 µg/ml), or rBCG-mIL-18 (0·1 OD/ml) alone or plus neutralizing IL-12 antibody (100 µg/ml). IFN-γ production in culture supernatants was measured by ELISA following 3-day incubation. Values were expressed as means ± SD from triplicate-well incubations.
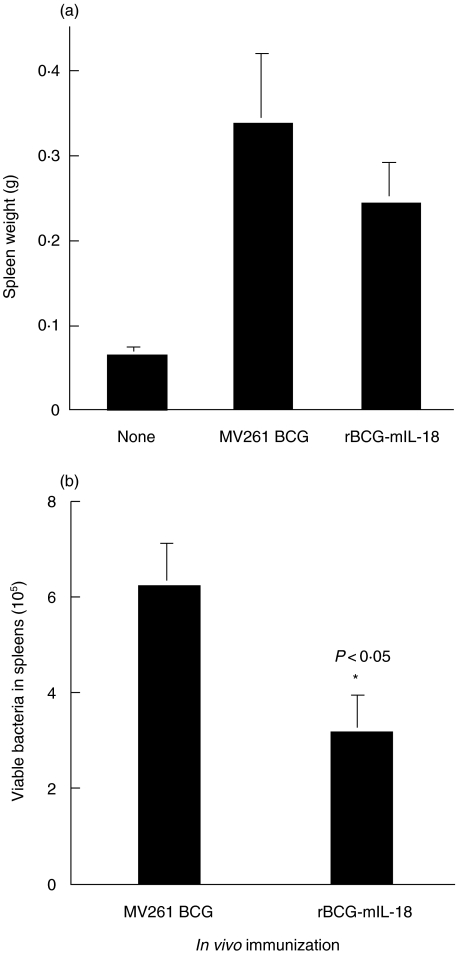
Reduced growth of rBCG-mIL-18 in mouse spleens after infection
To test if IL-18 expressed by rBCG-mIL-18 could enhance host antimycobacterial immunity, C57BL/6 mice were intravenously infected with this BCG strain as well as control MV261 BCG strain. C57BL/6 mice have previously been observed to readily develop a Th1 type protective immunity upon BCG infection [41,49]. Bacterial growth in mice was monitored at week 4 after BCG infection by measuring splenic enlargement (Fig. 5a) as well as bacterial counts in spleens (Fig. 5b). As observed previously [41], spleen weights in BCG infected mice were much heavier than those in uninfected mice. However, spleen weights of rBCG-mIL-18 infected mice were 28% less than those of the control MV261 BCG infected mice (Fig. 5a). Accordingly, bacterial counts in spleens of mice infected with rBCG-mIL-18 were 2-fold less than those in spleens of mice infected with control MV261 BCG strain (Fig. 5b). These data suggested that the early expression of IL-18 by rBCG-mIL-18 enhanced host antimycobacterial immunity with resultant accelerated mycobacterial clearance.
Fig. 5.
Reduced in vivo growth rate of rBCG-mIL-18. (a) Spleen weights at week 4 after intravenous infection of C57BL/6 mice with rBCG-mIL-18 (5 × 105 CFU) or controlMV261 BCG (5 × 105 CFU). (b) Total viable BCG counts in spleens of the same mice at week 4 after BCG infection. Values were expressed as means ± SD from 8 mice per group.
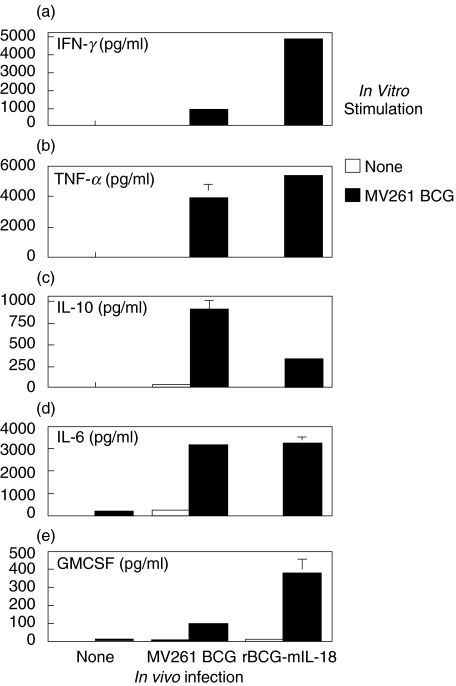
Enhanced BCG-specific cellular immune responses in mice infected with rBCG-mIL-18
Splenocytes were prepared from mice at 4 weeks after infection with either rBCG-mIL-18 or control MV261 BCG and placed in culture in the presence or absence of MV261 BCG. The cytokine profile in the splenocyte cultures was analysed with ELISA after 3-day incubation (Fig. 6). A marked increase in cytokine production in response to in vitro BCG stimulation was observed. Higher levels of Th1 cytokines IFN-γ and TNF-α were observed in rBCG-mIL-18 infected mice (5-fold higher for IFN-γ and 25% higher for TNF-α over the control MV261 BCG infected mice, respectively). Infection with rBCG-mIL-18 resulted in increased GMCSF production in response to BCG stimulation in vitro. The cells also produced Th2 cytokines IL-10 and IL-6 upon BCG stimulation. Although there was no obvious difference for IL-6 production between rBCG-mIL-18 infected and control MV261 BCG infected groups, levels of the major Th2 cytokine IL-10 were significantly reduced in the former group. These observations suggested that the IL-18 secreted from rBCG-mIL-18 strain selectively sensitized Th1 but not Th2 immune cells to response to mycobacterial infection in vivo.
Fig. 6.
Splenocyte cytokine production from mice infected with rBCG-mIL-18 or control MV261 BCG strains. Splenocytes were prepared at week 4 after intravenous infection of C57BL/6 mice with BCG (the same mice as in Fig. 5) and cultured in the absence or presence of MV261 BCG (0·01 OD/ml) for 3 days. Cytokine productions (IFN-γ, TNF-α, IL-10, IL-6 and GMCSF) were measured by ELISA. Values were expressed as means ± SD from triplicate-well incubations.
The results obtained from in vitro splenocyte proliferation assay further substantiated the role of IL-18 in enhancing the BCG-specific immune response (data not shown). An approximate two-fold increase of thymidine uptake by splenocytes from the mice infected with rBCG-mIL-18 was observed in response to MV261 BCG stimulation vs. to medium alone. The increased proliferation correlated with the elevated production of Th1 cytokines by splenocytes from the same group (Fig. 6).
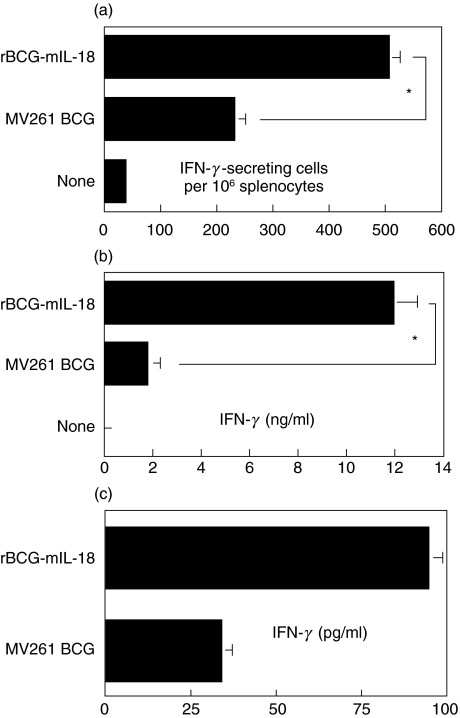
The effect of IL-18 secretion by BCG on the potential of splenocytes to produce IFN-γ was next investigated. After vaccination of mice, ELISPOT analysis revealed a two-fold increase in the number of IFN-γ-secreting splenocytes in mice vaccinated with BCG secreting IL-18 compared to control MV261 BCG (Fig. 7a). The level of total IFN-γ produced, as determined by ELISA, revealed a six-fold increase (P < 0·05) in cells from rBCG-mIL-18-infected mice compared to mice immunized with MV261 BCG (Fig. 7b). Therefore, each antigen-reactive cell from mice immunized with BCG secreting IL-18 is producing approximately three times as much IFN-γ than antigen-reactive cells from mice immunized with BCG not secreting the cytokine (Fig. 7c).
Fig. 7.
Increase of IFN-γ-secreting cells in rBCG-mIL-18 infected mice. Splenocytes were isolated from C57BL/6 mice (5 mice per group) 5 weeks after infection with rBCG-mIL-18 (5 × 105 CFU) or control MV261 BCG (5 × 105 CFU) and incubated with 10 µg/ml M. tuberculosis CFP for stimulation. (a) The number of IFN-γ secreting cells was enumerated by ELISPOT after 16-h incubation. (b) IFN-γ production in culture supernatants was measured by ELISA after 3-day incubation. (c) The amount of IFN-γ produced per cell was calculated by taking the ratio of IFN-γ produced per IFN-γ-secreting cell. Statistical analysis by anova showed significant differences between the mice infected with rBCG-mIL-18 and those infected with control MV261 BCG (*P < 0·05).
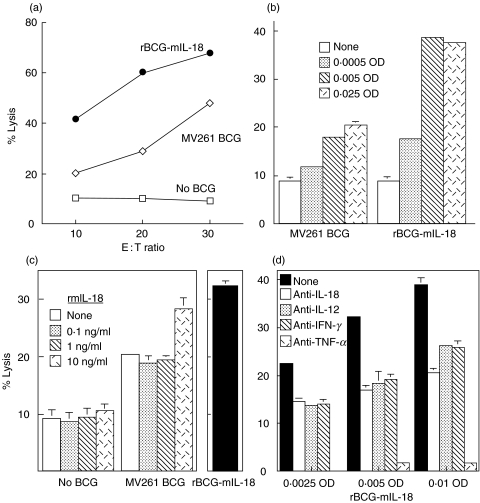
Augmentation of macrophage cytotoxicity against bladder cancer MBT-2 cells by rBCG-mIL-18
PECs were prepared by intraperitoneal injection of thioglycollate and served as a conventional source for macrophages (>90% by flow cytometry) [46, 47, 50]. PECs without BCG stimulation showed weak cytotoxicity (<10% lysis at E : T = 10 : 1) against MBT-2 cells, a bladder cancer line derived from C3H mice (Fig. 8a–c). Enhanced cytotoxicity (about 20% lysis at E : T = 10 : 1) in a dose-dependent manner was observed upon stimulation with control MV261 BCG (Fig. 8a–c). Stimulation with rBCG-mIL-18 further enhanced PEC cytolytic activity (about 40% lysis at E : T = 10 : 1) that was also BCG dose-dependent (Fig. 8a–). These increased levels of cytotoxicity were comparable with those induced by control MV261 BCG plus exogenous rmIL-18 (Fig. 8c). To investigate the role of endogenous macrophage-derived cytokines in enhancing PEC cytolytic activity, neutralizing antibodies to IL-18, IL-12, and TNF-α as well as IFN-γ were added during the incubation of PECs with rBCG-mIL-18. Although neutralizing all endogenous cytokines tested showed an inhibitory effect on rBCG-mIL-18-induced PEC cytotoxicity (Fig. 8d), neutralizing TNF-α inhibited nearly all MBT-2 killing (95–99%), indicating that TNF-α played a critical role in PEC cytotoxic activity. Species and isotype control antibodies did not reduce PEC cytotoxicity against MBT-2 (data not shown). These data indicated that rBCG-mIL-18 was more potent than control BCG for induction of PEC cytolytic activity against MBT-2 cells and that this enhanced cytotoxicity was mediated by a number of endogenous Th1-stimulating cytokines produced by PECs.
Fig. 8.
Augmentation of cytotoxic activity of PECs against mouse bladder cancer cells by rBCG-mIL-18. Thioglycollate-elicited PECs from C3H/HeN mice were stimulated with rBCG-mIL-18 or control MV261 BCG for 24 h. Cytotoxicity of the BCG-stimulated PECs was evaluated by coculturing the PECs with 51Cr-labelled MBT-2 cells for 20 h as follows: (a) at 3 different E:T ratios after PEC stimulation by BCG (0·005 OD); (b) at the indicated BCG doses for PEC stimulation (E : T ratio = 10 : 1); (c) under the supplementation of exogenous rmIL-18 at the indicated doses to MV261 BCG (0·005 OD) for PEC stimulation (E : T ratio = 10 : 1); (d) in the presence of various neutralizing antibodies (3 µg/ml for each) during PEC stimulation with the indicated doses of rBCG-mIL-18 (E : T ratio = 10 : 1).
Up-regulation of PEC IFN-γ and TNF-α production by rBCG-mIL-18
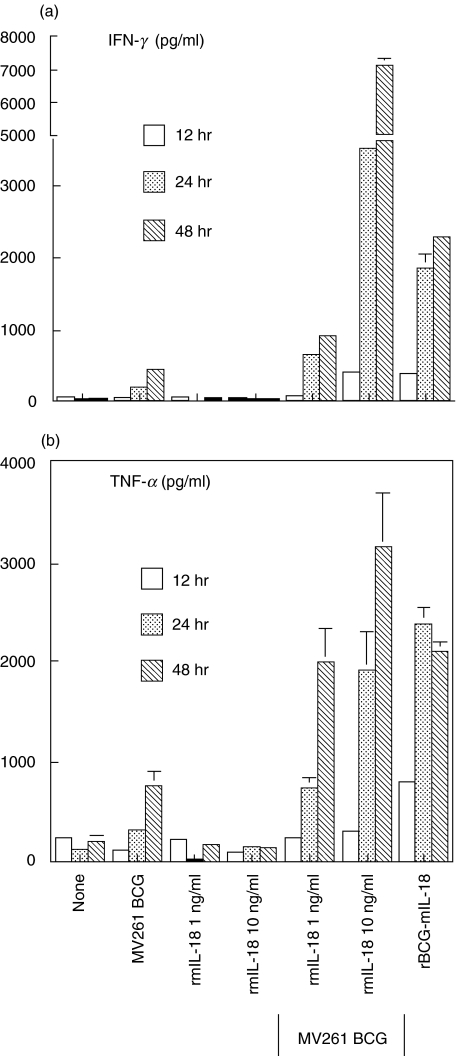
Since both IFN-γ and TNF-α had been known to play critical roles in BCG-induced PEC cytotoxicity against MBT-2 cells [46,47], expression of these cytokines by PECs in response to rBCG-mIL-18 was evaluated. Significantly higher levels of IFN-γ and TNF-α were observed in PEC cultures stimulated with rBCG-mIL-18 than with control MV261 BCG (Fig. 9). This increased expression of cytokines was comparable to PEC cultures stimulated with control MV261 BCG plus exogenous rmIL-18. rBCG-mIL-18 not only enhanced but also accelerated the production of these cytokines. Assays using trans-well apparatus to separate effectors from targets demonstrated that soluble factors, including IFN-γ and TNF-α, contributed to about 50% of total MBT-2 cell killing by PECs (data not shown), indicating that both direct cell to cell contact and soluble factors were required for the maximal PEC cytotoxicity.
Fig. 9.
Up-regulation of PEC IFN-γ and TNF-α production by rBCG-mIL-18. Thioglycollate-elicited PECs from C3H/HeN mice were incubated with medium, control 261 BCG (0·005 OD), rmIL-18 (1 ng/ml and 10 ng/ml), MV261 BCG (0·005 OD) plus the same doses of rmIL-18, or rBCG-mIL-18 (0·005 OD). Culture supernatants were harvested after incubation for 12, 24 and 48 h. Production of IFN-γ and TNF-α in culture supernatants was measured by ELISA. Values were expressed as means ± SD from triplicate-well incubations.
DISCUSSION
To improve immunogenicity of BCG, an immunotherapeutic agent commonly used for bladder cancer treatment, we have created a novel mIL-18-secreting rBCG strain using DNA cloning techniques and evaluated its immunostimulatory properties compared with control BCG. Consistent with our previous observations that combination of BCG with exogenous rmIL-18 synergized the induction of Th1 immune responses [30], this newly developed rBCG-mIL-18 strain significantly enhances splenocyte IFN-γ production, host antimycobacterial immunity, and macrophage cytotoxicity against MBT-2 bladder cancer cells.
Like other rBCG strains expressing Th1-stimulating cytokines generated in our laboratory [39,41,42], this rBCG-mIL-18 functionally expressed bioactive IL-18 that enhanced BCG-induced Th1 immune responses. The synergistic effect of rBCG-mIL-18 on splenocyte IFN-γ production was observed in both BCG-naïve and -primed mice (data not shown). This effect was IL-18 specific since neutralizing IL-18 in splenocyte-rBCG cultures or the removal of IL-18 in rBCG-mIL-18 culture supernatants could compromise its augmentation for IFN-γ production. Strikingly, neutralizing endogenous IL-12 completely inhibited the effect of rBCG-mIL-18 on enhancing IFN-γ production. It has been reported that IL-12 induces IL-18 receptor expression on T cells and thus deprivation of IL-12 could significantly inhibit IFN-γ production [26,28]. As previously observed [16,30,51,52], endogenous IL-10 antagonized IFN-γ production induced by BCG or rBCG-mIL-18 as IL-10 knockout mice showed substantially elevated IFN-γ production, especially for splenocytes stimulated with rBCG-mIL-18.
Protective immunity against mycobacterial infection is primarily mediated by a cellular immune response of the Th1 type [41,49,53–55]. IL-18, produced mainly by macrophages, plays a critical role in induction of cellular immunity. IL-18 has been reported to enhance host defense against several mycobacterial infections including BCG in humans and mice showing significant production of IFN-γ and reduction of bacterial counts [32–38]. Using IL-18-deficient (knockout) mice, reduced bacterial clearance, lower IFN-γ production, and marked development of granulomatous lesions were observed [56–58]. Based on our previous observations that splenic enlargement and bacterial counts in spleen peaked at 2–4 weeks post intravenous BCG infection of C57BL/6 mice [41], we performed the similar BCG infection procedure for rBCG-mIL-18 in this study. At week 4, less spleen enlargement and shorter BCG persistence in spleens were observed in mice infected with rBCG-mIL-18 than those infected with control BCG. This difference was not likely due to the difference in the growth rates of these two strains, as they both showed the same growth rate in vitro (data not shown). Correlatively, enhanced BCG-specific responses were also observed in splenocytes from mice infected with rBCG-mIL-18, showing increased production of IFN-γ (a major Th1 cytokine) and GMCSF and decreased production of IL-10 (a major Th2 cytokine), pronounced cellular proliferation, and elevated differentiation of IFN-γ-secreting cells. These data indicated that the in vivo expression of IL-18 by rBCG greatly potentiated the host specific cellular immunity against BCG infection in C57BL/6 mice. These observations were discrepant from those in our previous study using BALB/c mice as an infection model where rBCG-mIL-18 did not provide a better protection against mycobacterial challenge compared to control BCG [59]. Thus, induction of antimycobacterial immunity by rBCG-mIL-18 is likely strain dependent. In support of this, it was previously observed that administration of IL-18 to BALB/c mice exacerbated infections by Leishmania major in this strain of animals and that IL-18 augmented expression of Th2 cytokines (IL-4 and IL-10) in BALB/c mice but Th1 cytokine (IFN-γ) in C57BL/6 and CBA mice [60]. Thus, it is quite possible that IL-18 secreted by rBCG may also up-regulate Th2 responses in BALB/c but Th1 responses in C57BL/6 mice in our rBCG-mIL-18 infection models. In addition to C57BL/6 mice, augmentation of IFN-γ production by IL-18 secreting rBCG has also been observed in C3H mice [61].
We previously demonstrated that macrophages exhibited cytotoxicity against MBT-2 cells (a mouse bladder cancer line of C3H origin) in vitro upon BCG activation [46,47]. In these studies, PECs were used as a conventional source for macrophages. Although the PEC preparations contained a small population of T (<5%) as well as NK cells (<5%), macrophages served as primary effectors in lysis of MBT-2 targets since depletion of T or NK cells showed only marginal reduction of MBT-2 killing [46,47]. These studies also demonstrated that endogenous IFN-γ and TNF-α produced by BCG-stimulated PECs contributed to MBT-2 killing since simultaneously neutralizing these two cytokines significantly reduced MBT-2 killing [46,47]. To investigate if rBCG-mIL-18 works better than control BCG to induce macrophage cytotoxic activity, PECs were stimulated with the rBCG or MV261 BCG for 24 h followed by incubation with 51Cr-labelled MBT-2 cells. About two-fold higher killing of MBT-2 cells was observed in PECs stimulated with rBCG-mIL-18. This augmented cytolytic activity was BCG-dose dependent, comparable with combination of control BCG plus exogenous rmIL-18 (10 ng/ml), and diminishable by neutralizing anti-IL-18 antibody. This rBCG-mIL-18 was also observed to significantly enhance and accelerate PECs to produce both IFN-γ and TNF-α compared with control BCG. Although neutralizing endogenous IL-12 and IFN-γ reduced MBT-2 killing by 30–40%, neutralizing endogenous TNF-α abolished >95% killing, indicating that TNF-α played an indispensable role in rBCG-mIL-18-induced PEC cytotoxicity against MBT-2 cells. These observations suggested that direct physical contact between BCG-activated PECs and MBT-2 targets as well as soluble cytokines (such as IFN-γ, TNF-α, IL-12 and IL-18) produced by BCG-activated PECs were required for the maximal cytotoxicity against MBT-2 cells. Indeed, using trans-well apparatus to separate PECs from MBT-2 cells, we observed that soluble factors secreted by BCG-activated PECs contributed to about 50% of MBT-2 killing by PECs (data not shown). Combination of rIFN-γ and rTNF-α has also been shown to have direct cytotoxicity against MBT-2 cells in a dose-dependent manner [46].
The present study demonstrates that the newly developed rBCG-mIL-18 strain is superior to control BCG for induction of host Th1 immune responses and macrophage cytotoxicity. This rBCG strain may be particularly useful for treating diseases that require a desirable Th1 response such as superficial bladder cancer, intracellular pathogenic infection, allergies, and certain autoimmune diseases.
Acknowledgments
We are grateful to Dr Andrew M. Jackson (Imperial Research Fund, Cancer Medicine Research Unit, St James University Hospital, Leeds, United Kingdom) for supplying the mouse IL-18 containing plasmid (pIGIF-MODgfp) and Dr Donna Rennick (DNAX Research Institute, Palo Alto, CA, USA) for providing C57BL/6 IL-10–/– mice.
REFERENCES
- 1.Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guérin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 2.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell MA, DeWolf DC. Bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. New prospects for an old warhorse. Surg Oncol Clin N Am. 1995;4:189–202. [PubMed] [Google Scholar]
- 4.Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-gamma-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette Guérin. Eur J Immunol. 1999;29:650–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<650::AID-IMMU650>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Invest. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez-Samperio P, Trejo-Echeverria A, Ayala-Verdin H. Regulation of interleukin-12 production in human cells stimulated with Mycobacterium bovis BCG. Cell Immunol. 1998;189:25–30. doi: 10.1006/cimm.1998.1361. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto H, Suzuki K, Tsuyuguchi K, et al. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG. cytokine regulation and effect of NK cells. Infect Immun. 1997;65:4405–10. doi: 10.1128/iai.65.11.4405-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohle A, Gerdes J, Ulmer AJ, Hofstetter AG, Flad HD. Effects of local bacillus Calmette-Guérin therapy in patients with bladder carcinoma on immunocompetent cells of the bladder wall. J Urol. 1990;144:53–8. doi: 10.1016/s0022-5347(17)39365-5. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, Nowc C, Ulmer AJ, Musehold J, Gerdes J, Hofstetter AG, Flad HD. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette-Guérin immunotherapy. J Urol. 1990;144:59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- 10.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guérin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 11.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guérin therapy for superficial bladder cancer: processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 12.Taniguchi K, Koga S, Nishikido M, Yamashita S, Sakuragi T, Kanetake H, Saito Y. Systemic immune response after intravesical instillation of bacille Calmette-Guérin (BCG) for superficial bladder cancer. Clin Exp Immunol. 1999;115:131–5. doi: 10.1046/j.1365-2249.1999.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol. 1990;144:1248–51. doi: 10.1016/s0022-5347(17)39713-6. [DOI] [PubMed] [Google Scholar]
- 14.Saint F, Patard JJ, Maille P, Soyeux P, Hoznek A, Salomon L, Abbou CC, Chopin DK. Prognostic value of a T helper 1 urinary cytokine response after intravesical bacillus Calmette-Guérin treatment for superficial bladder cancer. J Urol. 2002;167:364–7. [PubMed] [Google Scholar]
- 15.Elsasser-Beile U, Gutzeit O, Bauer S, Katzenwadel A, Schultze-Seemann W, Wetterauer U. Systemic and local immunomodulatory effects of intravesical BCG therapy in patients with superficial urinary bladder carcinomas. J Urol. 2000;163:296–9. [PubMed] [Google Scholar]
- 16.Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, O'Donnell MA, Ratliff TL. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guérin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003;131:206–16. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Reijke TM, De Boer EC, Kurth KH, Schamhart DH. Urinary interleukin-2 monitoring during prolonged bacillus Calmette-Guérin treatment. can it predict the optimal number of instillations? J Urol. 1999;161:67–71. doi: 10.1097/00005392-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell MA, Chen X, DeWolf WC. Maturation of the cytokine immune response to BCG in the bladder: implications for treatment schedules. J Urol. 1996;155:1030A. [Google Scholar]
- 19.Riemensberger J, Bohle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. 2002;127:20–6. doi: 10.1046/j.1365-2249.2002.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAveney KM, Gomella LG, Lattime EC. Induction of TH1- and TH2-associated cytokine mRNA in mouse bladder following intravesical growth of the murine bladder tumor MB49 and BCG immunotherapy. Cancer Immunol Immunother. 1994;39:401–6. doi: 10.1007/BF01534428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–23. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 22.Ratliff TL. Role of the immune response in BCG for bladder cancer. Eur Urol. 1992;21(Suppl. 2):17–21. doi: 10.1159/000474916. [DOI] [PubMed] [Google Scholar]
- 23.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573–80. [PubMed] [Google Scholar]
- 24.De Jager R, Guinan P, Lamm D. Long-term complete remission in bladder carcinoma in situ with intravesical Tice bacillus Calmette-Guérin. Urology. 1991;38:507–13. doi: 10.1016/0090-4295(91)80166-5. [DOI] [PubMed] [Google Scholar]
- 25.Herr HW, Pinsky CM, Whitmore WF. Long-term effect of intravesical bacillus Calmette-Guérin on flat carcinoma in situ of the bladder. J Urol. 1986;135:265–7. doi: 10.1016/s0022-5347(17)45604-7. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Chan WL, Leung BP, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–92. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–50. [PubMed] [Google Scholar]
- 28.Ahn HJ, Maruo S, Tomura M, et al. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–31. [PubMed] [Google Scholar]
- 29.Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell Immunol. 1996;173:230–5. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- 30.Luo Y, Chen X, O'Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine. 2003;21:17–26. doi: 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 31.Quiding-Jarbrink M, Smith DA, Bancroft GJ. Production of matrix metalloproteinases in response to mycobacterial infection. Infect Immun. 2001;69:5661–70. doi: 10.1128/IAI.69.9.5661-5670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi K, Kai M, Gidoh M, Nakata N, Endoh M, Singh RP, Kasama T, Saito H. The possible role of interleukin (IL)-12 and interferon-gamma-inducing factor/IL-18 in protection against experimental Mycobacterium leprae infection in mice. Clin Immunol Immunopathol. 1998;88:226–31. doi: 10.1006/clin.1998.4533. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Nakata N, Kai M, Kasama T, Hanyuda Y, Hatano Y. Decreased expression of cytokines that induce type 1 helper T cell/interferon-gamma responses in genetically susceptible mice infected with Mycobacterium avium. Clin Immunol Immunopathol. 1997;85:112–6. doi: 10.1006/clin.1997.4421. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Cho D, Kim TS. Induction of in vivo resistance to Mycobacterium avium infection by intramuscular injection with DNA encoding interleukin-18. Immunology. 2001;102:234–41. doi: 10.1046/j.1365-2567.2001.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugawara I. Interleukin-18 (IL-18) and infectious diseases, with special emphasis on diseases induced by intracellular pathogens. Microbes Infect. 2000;2:1257–63. doi: 10.1016/s1286-4579(00)01279-x. [DOI] [PubMed] [Google Scholar]
- 36.Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–21. [PubMed] [Google Scholar]
- 37.Song CH, Lee JS, Nam HH, et al. IL-18 production in human pulmonary and pleural tuberculosis. Scand J Immunol. 2002;56:611–8. doi: 10.1046/j.1365-3083.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- 38.Vankayalapati R, Wizel B, Weis SE, Samten B, Girard WM, Barnes PF. Production of interleukin-18 in human tuberculosis. J Infect Dis. 2000;182:234–9. doi: 10.1086/315656. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell MA, Aldovini A, Duda RB, Yang H, Szilvasi A, Young RA, DeWolf WC. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Szilvasi A, Chen X, DeWolf WC, O'Donnell MA. A novel method for monitoring Mycobacterium bovis BCG trafficking with recombinant BCG expressing green fluorescent protein. Clin Diagn Laboratory Immunol. 1996;3:761–8. doi: 10.1128/cdli.3.6.761-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Y, Chen X, Szilvasi A, O'Donnell MA. Co-expression of interleukin-2 and green fluorescent protein reporter in mycobacteria: in vivo application for monitoring antimycobacterial immunity. Mol Immunol. 2000;37:527–36. doi: 10.1016/s0161-5890(00)00077-8. [DOI] [PubMed] [Google Scholar]
- 42.Luo Y, Chen X, Han R, O'Donnell MA. Recombinant bacille Calmette-Guérin (BCG) expressing human interferon-alpha 2B demonstrates enhanced immunogenicity. Clin Exp Immunol. 2001;123:264–70. doi: 10.1046/j.1365-2249.2001.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung MA, Luo Y, O'Donnell M, Rodriguez C, Heber W, Sharma S, Chang HR. Development and preclinical evaluation of a Bacillus Calmette-Guérin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003;63:1280–7. [PubMed] [Google Scholar]
- 44.Snapper SB, Lugosi L, Jekkel A, Melton RE, Kieser T, Bloom BR, Jacobs Jr WR. Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci USA. 1988;85:6987–91. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palendira U, Kamath AT, Feng CG, Martin E, Chaplin PJ, Triccas JA, Britton WJ. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun. 2002;70:1949–56. doi: 10.1128/IAI.70.4.1949-1956.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada H, Kuroda E, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Prostaglandin E2 down-regulates viable Bacille Calmette-Guérin-induced macrophage cytotoxicity against murine bladder cancer cell MBT-2 in vitro. Clin Exp Immunol. 2002;128:52–8. doi: 10.1046/j.1365-2249.2002.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Enhancing effect of an inhibitor of nitric oxide synthesis on bacillus Calmette-Guérin-induced macrophage cytotoxicity against murine bladder cancer cell line MBT-2 in vitro. Jpn J Cancer Res. 2000;91:534–42. doi: 10.1111/j.1349-7006.2000.tb00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stover CK, de la Cruz VF, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–60. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 49.Huygen K, Abramowicz D, Vandenbussche P, et al. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–6. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang AS, Morales A. BCG induced murine peritoneal exudate cells: cytotoxic activity against a syngeneic bladder tumor cell line. J Urol. 1982;127:1225–9. doi: 10.1016/s0022-5347(17)54303-7. [DOI] [PubMed] [Google Scholar]
- 51.O'Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette-Guérin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 52.Luo Y, Chen X, Downs TM, DeWolf WC, O'Donnell MA. IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immunol. 1999;162:2399–405. [PubMed] [Google Scholar]
- 53.Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guérin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–9. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lagranderie MR, Balazuc AM, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64:1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutis T, Cornelisse YE, Ottenhoff TH. Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–95. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 56.Kinjo Y, Kawakami K, Uezu K, et al. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis: a comparative study with IL-12p40. J Immunol. 2002;169:323–9. doi: 10.4049/jimmunol.169.1.323. [DOI] [PubMed] [Google Scholar]
- 57.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–9. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–90. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 59.Young S, O'Donnell M, Lockhart E, Buddle B, Slobbe L, Luo Y, De Lisle G, Buchan G. Manipulation of immune response to Mycobacterium bovis by vaccination with IL-2 and IL-18-secreting recombinant bacillus Calmette Guerin. Immunol Cell Biol. 2002;80:209–15. doi: 10.1046/j.1440-1711.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- 60.Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, Mclnnes LB, Liew FY. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30:3147–56. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 61.Biet F, Kremer L, Wolowczuk I, Delacre M, Locht C. Mycobacterium bovis BCG producing interleukin-18 increases antigen-specific gamma interferon production in mice. Infect Immun. 2002;70:6549–57. doi: 10.1128/IAI.70.12.6549-6557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]