Abstract
Alterations in the expression of CXCR4 and CCR5, the co-receptors for HIV entry, may be associated with susceptibility of monocytic cells to HIV infection. Interferon (IFN)-γ has been shown to inhibit HIV replication in monocytic cells, but the molecular mechanism involved is not well understood. To determine if IFN-γ regulates HIV replication by altering CXCR-4/CCR-5 expression and hence virus entry into monocytic cells, we investigated the effects of IFN-γ on CXCR-4 and CCR-5 expression and its biological implications with respect to HIV entry, replication and chemotaxis towards the CXCR-4 and CCR-5 ligands SDF-1 and MIP-1α, respectively. IFN-γ decreased CXCR-4 and CCR-5 expression on monocytes derived from HIV-negative adults, HIV-positive adults and HIV-negative cord blood. This down-regulation of chemokine receptor expression did not result in a corresponding change in mRNA expression but was associated with elevated levels of the endogenously produced chemokines SDF-1 and RANTES. Furthermore, IFN-γ inhibited chemotaxis in response to SDF-1 and MIP-1α, inhibited HIV replication, but failed to inhibit virus entry in monocytic cells. These results suggest that although IFN-γ-induced down-regulation of CXCR-4 and CCR-5 expression is associated with an inhibition of SDF-1-/MIP-1α-mediated chemotaxis, IFN-γ-induced inhibition of HIV replication may be mediated at levels subsequent to the virus entry.
Keywords: CXCR4, CCR5, chemotaxis, HIV, IFN-γ, monocytes
INTRODUCTION
Chemokines play a critical role in immune and inflammatory reactions as well as in infectious diseases, including acquired immunodeficiency syndrome (AIDS) [1,2]. The two main classes of chemokines, C-X-C (α) and C-C (β), act on several leucocyte populations including lymphocytes, monocytes, neutrophils, eosinophils and natural killer (NK) cells. Chemokines bind to and activate G-protein-coupled, seven transmembrane domain-spanning chemokine receptors [3]. The chemokine receptors CXCR-4, whose natural ligand is SDF-1, and CCR-5, whose natural ligands are MIP-1α, MIP-1β and RANTES, are considered to be the major co-receptors responsible for infection by T-tropic and M-tropic HIV-1 strains, respectively [4–6]. Monocytic cells are the primary targets of M-tropic CCR-5 HIV strains that dominate the early stages of infection [6–8]. Although monocytic cells express both CCR-5 and CXCR-4 receptors, infection by HIV in this cell type is mediated by interaction of viral gp120 with CD4 and CCR5 [6,9,10]. Primary HIV-1 infection typically involves a CCR5-using (R5) variant, and the switch to a CXCR-4-using (X4) strain is associated with a rapid decline in CD4+ T cell numbers and with disease progression [6,8,11,12].
In light of the observation that the level of expression of specific chemokine receptors on the cell surface correlates with susceptibility to infection with HIV [13–16], strategies aimed at down-regulating chemokine receptors may be potentially significant in preventing infection. It has been suggested that chemokine receptors expression on T cells and monocytic cells may be modulated by T helper (Th) cytokines [2,5,10,14,17–22], and consequently may alter the susceptibility of these cells to T-tropic HIV infection.
Interferon (IFN)-γ, a Th1-type cytokine produced by T cells and NK cells, is a potent activator of monocytic cells and modulates a variety of immune and inflammatory responses, including HIV infection [23]. IFN-γ has been shown to have suppressive as well as enhancing effects on HIV replication depending on the cell type and state of cell activation and differentiation [17,18,24,25]. For example, exposure of HIV-infected macrophages to IFN-γ has been shown to reduce viral replication [17,26]. In contrast, exposure of macrophages or promonocytic cells to IFN-γ prior to HIV infection has been reported to enhance virus production [24]. In addition, elevated levels of plasma IFN-γ have been detected in HIV patients in the absence of concurrent opportunistic infections [27,28]. These observations suggest that the presence of IFN-γ in the microenvironment of HIV-infected individuals may influence the ability of HIV to enter into, or replicate within, monocytic cells [29]. IFN-γ may regulate HIV infection by modulating the expression of chemokine receptors, resulting in alterations in virus entry into monocytic cells. In one study, IFN-γ was shown to enhance CCR-5 expression and chemotaxis in monocytes and macrophages [26]. Interestingly, IFN-γ inhibited HIV replication in these cells; however, the rationale for these contrasting observations was not clear [26]. In this study, we wished to determine if IFN-γ regulates HIV replication by altering chemokine receptor expression by, and hence virus entry into, resting monocytic cells. We thus investigated the effects of IFN-γ on the expression of the chemokine receptors CXCR-4 and CCR-5 in resting monocytic cells obtained from three different sources: HIV-negative adults, HIV-infected adults and HIV-negative umbilical cord blood. In addition, we determined the physiological significance of IFN-γ-induced alterations of chemokine receptor expression with respect to monocyte chemotaxis, HIV entry and HIV replication.
SUBJECTS AND METHODS
Monocyte isolation from PBMCs and cell culture
This study was approved by the Research Ethics Committee of the Children's Hospital of Eastern Ontario and by the Ethics Review Board of the Ottawa Hospital, General Campus. Blood was obtained from HIV-negative healthy adults and from HIV-infected adults with CD4+ T cell counts ranging from 100 to 400 cells/ml. All HIV-positive patients were receiving antiretroviral therapy and none had evidence of acute infection at the time of specimen collection. Cord blood was drawn from the umbilical cords of full-term, healthy, newborns. PBMCs were isolated by density-gradient centrifugation using Ficoll-Hypaque (Pharmacia, Baie D'Urfe, QC, Canada) as described earlier [28,30]. The PBMCs were cultured in Iscove's modified Dulbecco's medium (IMDM; Sigma Chemical Company, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco Laboratories, Grand Island, NY, USA), 100 U/ml penicillin and 100 µg/ml gentamicin (Sigma, Oakville, ON, Canada). Peripheral blood mononuclear cells (PBMCs) (1 × 106 cells/ml) were cultured in 24-well plates for 24–48 h (Falcon, Becton Dickinson, Lincoln Park, NJ, USA) in the presence or absence of recombinant human (rh) IFN-γ or rhIL-2 (as a control; Research Diagnostics, Flanders, NJ, USA).
Purified, non-activated monocytes were isolated from PBMCs by negative-selection by depleting T cells and B cells using polystyrene M-450 Dynabeads (Dynal, Oslo, Norway) coated with antibodies specific for CD2 (T cells) and CD19 (B cells), as described earlier [28,30]. CD2-negative and CD19-negative cells were incubated at 37°C for 2 h following which non-adherent cells were removed. The adherent mononuclear cells thus obtained contained fewer than 1% CD2+ T cells and CD19+ B cells as determined by flow cytometric analysis.
Flow cytometric analysis of monocyte CXCR4 and CCR5 expression
CXCR-4 and CCR-5 expression by CD14+ monocytes was determined by flow cytometry, as described earlier [31]. Briefly, cells were double-stained with FITC-conjugated anti-CD14 MoAb (Becton Dickinson Biosciences, San Jose, CA, USA) and either PE-conjugated anti-CXCR4 MoAb (12G5; Pharmingen, San Diego, CA, USA) or Cy-Chrome-conjugated anti-CCR5 MoAb (2D7; Pharmingen). Autofluorescent controls and isotype (IgG2b)-matched control MoAb (Becton Dickinson) were also included. Stained monocytic cells were gated based on forward scatter/side scatter characteristics and CD14 labelling. Data was acquired on a Becton Dickinson FACScan Flow Cytometer and analysed using the WinMDI software package (J. Trotter, Scripps Institute, San Diego, CA, USA). The validity of comparisons of CXCR-4 and CCR-5 expression between different patients and populations was ensured with the use of Calbrite™ Beads (Becton Dickinson, Franklin Lakes, NJ, USA).
Measurement of CXCR4 and CCR5 mRNA expression by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis
Purified CD14+ monocytes were cultured in the absence of exogenous cytokines or in the presence of either IFN-γ or interleukin (IL)-2 for various periods of time ranging from 0 to 12 h. Total RNA was extracted using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) and reverse-transcribed, as described earlier [31]. The resulting cDNA was subjected to PCR analysis using 1·25 units of Amplitaq DNA polymerase, 1 µm of each of the appropriate sense and antisense primers (University of Ottawa Biotechnology Research Institute), 0·5 mm of each dNTP, and 2 mm MgCl2 (Roche Diagnostic Systems, Laval, PQ, Canada). The primer sequences used were as follows: β-actin sense 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′; β-actin antisense 5′-CTA GAA GCA TTG CGG TGG CAG ATG GAG GG-3′[31,32]; CXCR-4 sense 5′-AGC TGT TGG CTG AAA AGC TGG TCT ATG-3′; CXCR-4 antisense 5′-GCG CTT CTG GTG GCC CTT GGA GTG TG-3′[33]; CCR-5 sense 5′-GCT CTC TCC CAG GAA TCA TCT TTA C-3′; CCR-5 antisense 5′-TTG GTC CAA CCT GTT AGA GCT ACT G-3′[34]. The amplification conditions for CXCR-4 were 35 cycles of denaturing at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min. The amplification conditions for CCR-5 were 35 cycles of denaturing at 94°C for 2 min, annealing at 56·C for 2 min and extension at 72·C for 2 min. The PCR products for β-actin [610 base pairs (bp)], CXCR-4 (250 bp) and CCR-5 (320 bp) were resolved by electrophoresis on 1·2% agarose gels and were visualized by ethidium bromide staining. Densitometry was performed to normalize the expression of CXCR-4 and CCR-5 to β-actin using the GS-670 densitometer and the Molecular Analyst Image Analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Enzyme-linked immunosorbent assay (ELISA) measurement of RANTES, MIP-1α and SDF-1
RANTES, MIP-1α and SDF-1 were measured by ELISA as described earlier [35]. Briefly, plates (Nunc Immunomodules, Roskilde, Denmark) were coated overnight with primary murine MoAb diluted in coating buffer (0·04 m Na2CO3, 0·06 m NaHCO3, pH 9·6). The chemokine-specific MoAbs used were: mouse antihuman RANTES (cat. no. MAB-678; 10 µg/ml), mouse antihuman SDF-1 (cat. no. MAB-310; 25 µg/ml) and mouse antihuman MIP-1α (cat. no. MAB-670; 10 µg/ml) (R&D Systems, Minneapolis, MN, USA). The detection step was performed using biotinylated goat polyclonal antibody: goat antihuman SDF-1 (cat. no. BAF-310; 25 ng/ml), goat-antihuman MIP-1α (cat. no. BAF-270; 25 ng/ml) and goat-antihuman RANTES (cat. no. BAF-278; 5 ng/ml) (R&D Systems). Streptavidin–peroxidase was used at a final concentration of 1 : 1000 (Jackson Immuno-Research, West Grove, PA, USA). The colour reaction was developed by OPD (Sigma) and hydrogen peroxide, and read at 450 nm. Recombinant SDF-1, MIP-1α and RANTES (R&D Systems) were used as standards. The sensitivity of these assays was 16 pg/ml.
Analysis of monocyte chemotaxis
Chemotaxis was performed in 96-well ChemoTx plates (NeuroProbe Inc., Gaithersburg, MD, USA). The lower chambers of the plates were filled with 30 µl of either phosphate buffered saline (PBS), SDF-1 (1 µg/ml; BD Pharmingen, Mississauga, ON, Canada) or MIP-1α (100 ng/ml; BD Pharmingen). A polycarbonate filter with 5 µm pores was placed on top of the lower wells to separate them from the upper wells. PBMCs (1 × 105/ml) cultured in the presence or absence of IFN-γ for 24 or 48 h were added to the upper chamber of each well and incubated for 1·5 h at 37°C. After incubation, cells from the lower wells were removed and CD14+ cells were enumerated by flow cytometry using anti-CD14 MoAb (Leu-M3; Becton Dickinson). The number of cells that had migrated in response to a chemokine was calculated as the difference between the number of cells that migrated in response to a chemokine solution and those that migrated towards medium alone. Percentage chemotaxis of cytokine-stimulated monocytes was calculated as follows:
 |
HIV p24 ELISA for the determination of HIV replication in monocytes
Monocytes (1 × 103/well) from HIV-negative individuals were cultured in the presence or absence of either IFN-γ or IL-2 (negative control). After 48 h, monocytes were infected for 12 h at an MOI of 0·1 with dual-tropic HIV clinical isolate no. 204, washed three times, resuspended in media and cultured with the same cytokine used originally to stimulate the cells. Supernatants were collected on days 0, 5, 10 and 15 post-infection and were analysed using a HIV p24 ELISA kit (PerkinElmer NEN Life Sciences Products, Woodbridge, Ontario, Canada) as per the manufacturer's instructions. The dual-tropic HIV-1 clinical isolate, no. 204, isolated from a HIV-infected individual, was a gift from Dr F. Diaz-Mitoma (Children's Hospital of Eastern Ontario, Ottawa, Canada) and has been described previously [36]. This HIV isolate was propagated in PBMCs stimulated with PHA and IL-2. The virus stock was titrated against both THP-1 cells and adherence-derived monocytes to determine TCID50 values. The p24 standards and sample supernatants were added to Triton X-100-containing antip24 antibody-coated plates and incubated for 2 h at 37°C. Plates were washed six times with p24 wash buffer and incubated for 1 h at 37°C with biotin-labelled detector antibody. Plates were washed again, followed by incubation at room temperature for 30 min with streptavidin-conjugated horseradish peroxidase. Subsequently, the plates were incubated for 30 min at room temperature with the OPD substrate. The colour reaction was read at 450 nm p24 concentrations were calculated using Microplate Manager 4·0 software (Bio-Rad).
PCR analysis of HIV entry into monocytes
HIV entry into the target cells was measured by a PCR reaction specific for HIV proviral gag DNA as described previously [37,38]. Purified CD14+ monocytes were cultured for 48 h in the presence or absence of IFN-γ, following which they were exposed to HIV clinical isolate no. 204 for 8 h at an MOI of 0·1. After infection, DNA was isolated from the monocytes using TRI reagent, as described previously [31,32]. One µg DNA was used as the template for all PCR reactions. PCR reaction mixtures contained 1·25 units of Amplitaq DNA polymerase, 0·5 mm of each dNTP, 2 mm MgCl2 (all from Roche Diagnostic Systems) and 1 µm of each of the sense and antisense primers for β-actin, or 250 µm of each of the sense and antisense primers for HIV gag (University of Ottawa Biotechnology Research Institute). The primer sequences for β-actin were described above. The primer sequences for HIV gag were as follows: gag sense 5′-ATA ATC CAC CTA TCC CAG TAG GAG AAA T-3′; gag antisense 5′-TTT GGT CCT TGT CTT ATG TCC AGA ATG C-3′[37]. The amplification conditions for gag were 35 cycles of denaturing at 94·C for 1 min, annealing at 56·C for 2 min and extension at 72·C for 2 min. The amplification conditions for β-actin were 30 cycles of denaturing at 94·C for 1 min, annealing at 56·C for 2 min and extension at 72·C for 2 min. The amplified PCR products for HIV gag (114 bp) and β-actin (610 bp) were resolved by electrophoresis on 2% agarose gels and were visualized by ethidium bromide staining.
Statistical analysis
Means were compared by the two-tailed Student's t-test. The results are expressed as mean ± standard error (s.e.m.).
RESULTS
IFN-γ down-regulates CXCR-4 and CCR-5 expression on human monocytes
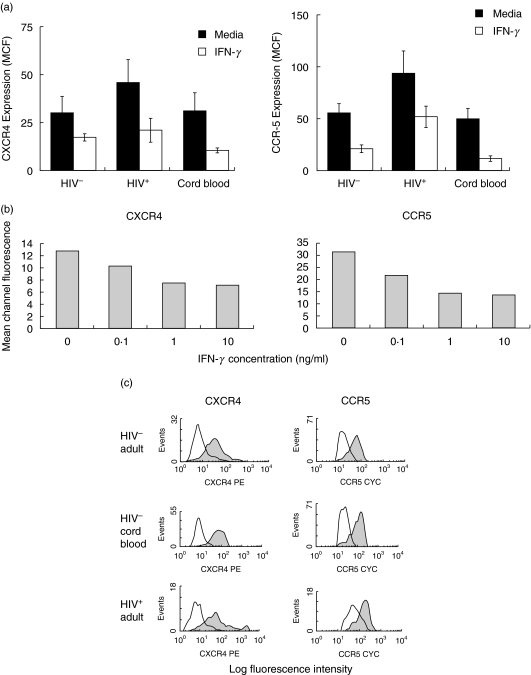
PBMCs obtained from 10 HIV-negative adults, six HIV-positive adults and 10 HIV-negative cord blood donors were stimulated with IFN-γ for 24 and 48 h, following which CD14+ monocytes were analysed by flow cytometric analysis. IFN-γ significantly reduced the expression of CXCR-4 and CCR-5 by monocytes isolated from HIV-negative adults (CXCR-4, P = 0·029; CCR-5, P = 0·003), HIV-infected individuals (CXCR-4, P = 0·078; CCR-5, P = 0·003) and HIV-negative cord blood (CXCR-4, P = 0·047; CCR-5, P = 0·034) following culture for 24 h (Fig. 1a) and 48 h (data not shown). It may be noted that the expression of both CXCR-4 and CCR-5 on monocytes derived from HIV-infected individuals was significantly higher than that observed on monocytes obtained from HIV-negative adult and cord blood (P < 0·05; Fig. 1a). To determine the optimal dose of IFN-γ, dose–response experiments were performed and the results revealed that 1 ng/ml of IFN-γ induced the maximum down-regulation of CXCR-4 and CCR-5 expression by CD14+ monocytes (Fig. 1b).
Fig. 1.
IFN-γ down-regulates CXCR-4 and CCR-5 expression by monocytic cells. (a) PBMCs (1 × 106/ml) from 10 HIV-negative, six HIV-infected and 10 HIV-negative cord blood donors were incubated with IFN-γ (1 ng/ml) for 24 h, and the effects on cell surface CXCR-4 and CCR-5 expression by CD14+ monocytes were determined by flow cytometry. (b) PBMCs (1 × 106/ml) from a representative HIV-negative individual were incubated with various concentrations of IFN-γ for 24 h followed by analysis of cell surface CXCR-4 and CCR-5 expression on CD14+ monocytes by flow cytometry. (c)Representative flow cytometry histograms of the effect of IFN-γ on the expression of CXCR-4 and CCR-5 by purified monocytes. CD14+ purified monocytes (2 × 105/ml) isolated from a HIV-negative individual, an HIV-negative cord blood donor and an HIV-positive adult were stimulated with IFN-γ (1 ng/ml) for 24 h followed by determination of cell surface CXCR-4 and CCR-5 expression by CD14+ monocytes by flow cytometry. Shaded and unshaded histogram represent chemokine receptor expression in unstimulated and IFN-γ-stimulated cells, respectively.
To determine that the effects of IFN-γ were not mediated by cytokines produced by other cell types present in PBMC cultures, purified monocytes obtained from the PBMCs of five HIV-negative individuals were stimulated with IFN-γ for 48 h. IFN-γ decreased significantly the expression of CXCR-4 (MCF of 8·2 ± 1·2 for untreated monocytes versus 3·9 ± 1·0 for IFN-γ-treated monocytes; P < 0·007) as well as of CCR-5 (MCF of 20·3 ± 2·4 for untreated monocytes versus 5·4 ± 0·4 for IFN-γ-treated monocytes; P < 0·001) on the surface of purified monocytes. Similar results were obtained when purified monocytes obtained from HIV-negative cord blood and from HIV-infected individuals were stimulated with IFN-γ (Fig. 1c). Because the effects of IFN-γ on chemokine receptor expression in cultures of purified monocytes were identical to those observed for monocytes present in PBMCs, PBMC cultures were used in subsequent experiments. Representative histograms showing the effects of IFN-γ on CXCR-4 and CCR-5 expression by purified monocytes isolated from a HIV-negative adult, a HIV-infected individual and a HIV-negative cord blood donor are depicted in Fig. 1c.
IFN-γ-induced down-regulation of CXCR4 and CCR5 expression in monocytic cells is not mediated at the transcriptional level
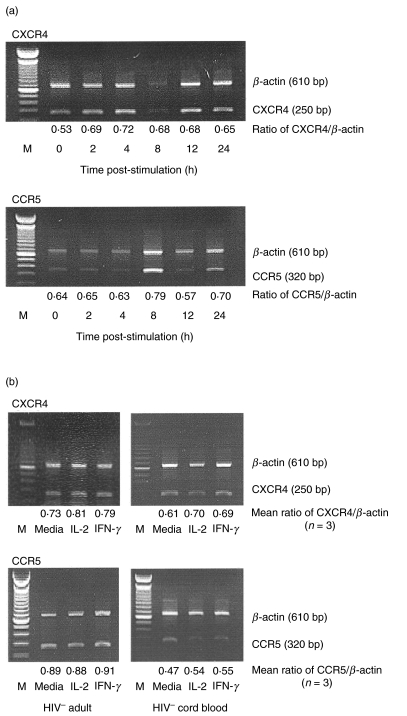
To determine the effect of IFN-γ on the transcription of CXCR-4 and CCR-5, the expression of mRNA encoding these chemokine receptors was examined by semiquantitative RT-PCR analysis. Purified monocytes from HIV-negative adults were stimulated with IFN-γ for various periods of time (0–24 h) and the mRNA isolated from these cells was subjected to semiquantitative RT-PCR analysis. IFN-γ did not induce statistically significant changes in CXCR-4 and CCR-5 mRNA levels in monocytes from HIV-negative adults at any time following culture. The results from a representative experiment are shown in Fig. 2a. In additional experiments, monocytes isolated from HIV-negative adults (n = 3) and HIV-negative cord blood donors (n = 3) were subjected to RT-PCR analysis after culture for 8 h in the presence or absence of IFN-γ or IL-2 (negative control). Neither IFN-γ nor IL-2 influenced the expression of CXCR4 or CCR5 mRNA by HIV-negative adult or cord blood monocytes (Fig. 2b). Similar results were obtained when monocytes from HIV-infected individuals were stimulated with IFN-γ (data not shown).
Fig. 2.
The effect of IFN-γ on the transcription of mRNA encoding CXCR-4 and CCR-5. (a) Purified, CD14+ non-activated monocytes (5 × 105/ml) isolated from HIV-negative adults were incubated with IFN-γ (1 ng/ml) for the indicated lengths of time. At various times following stimulation, RNA was isolated from these cells and subjected to RT-PCR analysis using primers specific for RNA encoding β-actin and either CXCR4 or CCR5. Amplified products were electrophoresed and analysed by densitometry using β-actin as an internal control. (b) Purified monocytes from three HIV-negative individuals and three cord blood donors were stimulated with either IL-2 or IFN-γ for 8 h. RNA isolated from these cells was subjected to semiquantitative RT-PCR analysis for CXCR-4 and CCR-5 expression. The results of one representative individual are shown (M = MW markers). The mean ratios of CXCR-4/β-actin and CCR-5/β-actin as determined by densitometric analysis from three individuals are shown.
IFN-γ-induced down-regulation of CXCR-4 and CCR-5 is associated with chemokine secretion
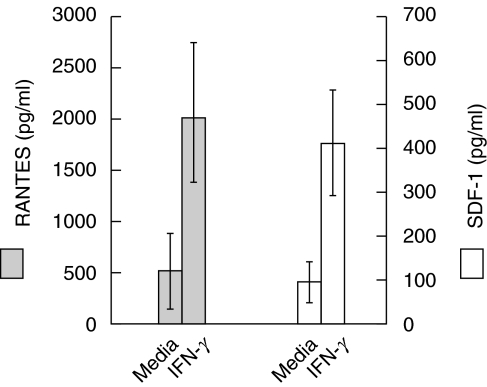
The absence of transcriptional regulation of IFN-γ-mediated CXCR-4 and CCR-5 receptor down-regulation suggested that IFN-γ might mediate this effect by inducing the secretion of the chemokines, SDF-1 (the CXCR-4 ligand) and MIP-1γ, MIP-1β and RANTES (CCR-5 ligands). To investigate this possibility, PBMCs from five HIV-negative adults were cultured with IFN-γ and the culture supernatants were analysed for the production of SDF-1, MIP-1α and RANTES. Stimulation of monocytes with IFN-γ enhanced the production of SDF-1 from an average of 90 ± 30 pg/ml to 392 ± 112 pg/ml (P = 0·023) after 24 h culture, and RANTES from an average of 483 ± 348 pg/ml to 1925 ± 680 pg/ml (P < 0·017) after 48 h of culture (Fig. 3). However, MIP-1α production was not detectable in the supernatants of cells cultured in the presence or absence of IFN-γ (data not shown). It is likely that increases in chemokine secretion in response to IFN-γ may down-regulate CXCR-4 and CCR-5 by ligand-induced receptor internalization. To determine the effect of endogenous SDF-1 and RANTES on down-regulation of CXCR-4 or CCR-5 receptors, culture supernatants obtained following stimulation with IFN-γ were treated with either neutralizing anti-SDF-1 or anti-RANTES antibodies followed by incubation with PBMC for a period ranging from 30 min to 24 h. Addition of anti-SDF-1 or anti-RANTES antibodies failed to cause down-regulation of CXCR-4 and CCR-5 receptors at any point (data not shown). However, cells incubated with recombinant SDF-1 as well as with RANTES did cause down-regulation of CXCR-4 and CCR-5 receptors, respectively (data not shown). These results suggest that down-regulation of CXCR-4 and CCR-5 receptors by IFN-γ may be due, at least in part, to the chemokine-mediated internalization of their corresponding receptors as well as in part to other unknown effects of IFN-γ.
Fig. 3.
IFN-γ induces the synthesis of RANTES and SDF-1 by monocytic cells. Purified monocytes (5 × 105/ml) were stimulated with IFN-γ (1 ng/ml) for 24 and 48 h followed by ELISA analysis of the culture supernatants collected at 24 and 48 h for the measurement of SDF-1 and RANTES, respectively. The results shown are the mean ± s.d.
IFN-γ-induced down-regulation of CXCR-4 and CCR-5 is associated with decreased chemotaxis
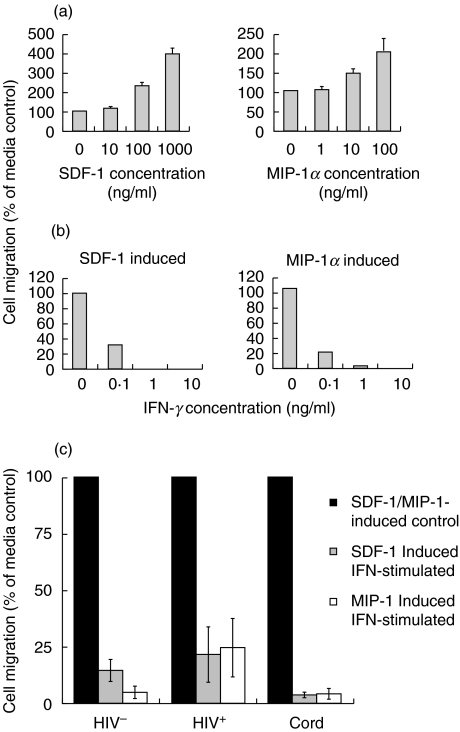
The down-regulation of monocyte CXCR-4 and CCR-5 receptors by IFN-γ may inhibit the chemotaxis mediated by their respective chemokine ligands. To determine the physiological significance of IFN-γ-induced down-regulation of CXCR-4 and CCR-5 receptors by monocytes, chemotactic migration of CD14+ monocytes in response to the CXCR-4 ligand, SDF-1 and to the CCR-5 ligand, MIP-1α was examined. The optimal dose of each chemokine (100 ng/ml of MIP-1α and 1000 ng/ml of SDF-1) required to induce maximal monocyte chemotaxis was determined in a dose–response experiment (Fig. 4a). Furthermore, we determined that 1 ng/ml of IFN-γ was required to inhibit the chemotaxis of CD14+ monocytic cells in response to SDF-1 and MIP-1α (Fig. 4b). PBMCs from six HIV-negative adults, six HIV-positive individuals and six HIV-negative cord blood donors were cultured for 24 h in the presence or absence of IFN-γ. Following culture, chemotaxis of CD14+ monocytic cells towards either SDF-1 or MIP-1α was measured. IFN-γ-stimulated PBMCs from both HIV-negative and cord blood HIV-positive individuals, exhibited significantly reduced chemotaxis towards both SDF-1 and MIP-1α compared to unstimulated PBMCs (Fig. 4c). Similar inhibitory effects of IFN-γ on chemotaxis were observed when PBMCs were stimulated for 48 h (data not shown). These results suggest that the down-regulation of monocyte CXCR-4 and CCR-5 receptor expression by IFN-γ may be associated with the decreased ability of monocytes to migrate towards their respective chemokine ligands.
Fig. 4.
IFN-γ down-regulates SDF-1- and MIP-1α-mediated chemotaxis of CD14+ monocytic cells. (a) PBMCs (1 × 105/ml) from HIV-negative adult blood were subjected to chemotaxis using increasing doses of SDF-1 or MIP-1α in order to determine the optimal dose for use in subsequent experiments. Migrated CD14+ monocytes were enumerated by flow cytometry and are expressed as a percentage of the control (cells incubated in the absence of chemokines). The results shown are a mean ± s.d. of three experiments. (b) PBMCs (1 × 105/ml) from HIV-negative adult blood were analysed for chemotaxis towards SDF-1 or MIP-1α after culture with increasing doses of IFN-γ. Migrated CD14+ monocytes were enumerated by flow cytometry and are expressed as a percentage of the unstimulated control. The results shown are a mean of two experiments. (c) PBMCs (1 × 105/ml) from six HIV-negative adults, six cord blood donors and six HIV-infected individuals were cultured for 24 h in the presence and absence of 1 ng/ml of IFN-γ followed by analysis for chemotaxis towards SDF-1 or MIP-1α. Migrated CD14+ monocytes were enumerated by flow cytometry and expressed as a percentage of the unstimulated control.
IFN-γ-induced down-regulation of monocyte CXCR-4 and CCR-5 does not impair HIV entry
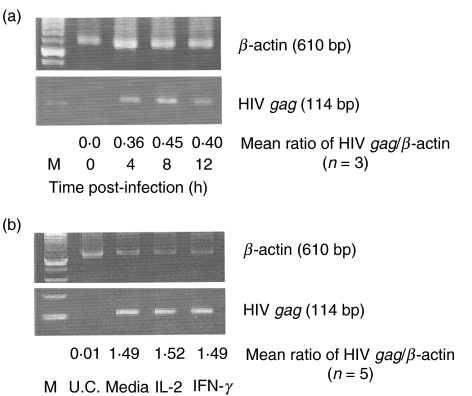
Because chemokine receptors act as co-receptors for HIV infection, we hypothesized that IFN-γ-induced down-regulation of CXCR-4 and CCR-5 receptors on monocytic cells may result in decreased HIV entry. To determine the ability of HIV to enter monocytes, PCR was used to detect the presence of HIV gag DNA as unintegrated viral DNA and as integrated proviral DNA [37,38]. Analysis of HIV gag DNA by PCR is a suitable measure of viral entry because gag DNA forms are present at early stages of virus replication [37,38].
To determine the optimal time for HIV gag expression, monocytes from an HIV-negative adult were infected with the dual-tropic HIV clinical isolate no. 204 over a period of time ranging from 0 to 12 h, followed by PCR analysis of HIV gag DNA expression. Maximal HIV gag DNA was detected 8 h post-infection (Fig. 5A). To determine whether the IFN-γ-induced down-regulation of CXCR-4 and CCR-5 receptors results in decreased or delayed entry of HIV, monocytes isolated from the PBMC of five HIV-negative adults were cultured with either IFN-γ or IL-2 for 48 h prior to HIV infection. Prior treatment of monocytic cells with IFN-γ did not influence the level of HIV gag DNA expression compared to the untreated or IL-2-treated cells (Fig. 5b). These results suggest that the IFN-γ-induced down-regulation of monocyte CXCR-4 and CCR-5 does not influence the ability of HIV to enter these cells.
Fig. 5.
IFN-γ does not prevent HIV entry into monocytic cells. (a) Purified monocytes (1 × 104/ml) obtained from HIV-negative individuals were incubated with a dual-tropic HIV clinical isolate no. 204 with a MOI of 0·1 for the indicated periods of time. DNA extracted at these times was subjected to PCR analysis for HIV gag; β-actin was used as an internal control. Amplified products were electrophoresed and analysed by densitometry. (b) Purified monocytes (1 × 104/ml) from five HIV-negative individuals were cultured for 48 h with 1 ng/ml of IFN-γ or IL-2 followed by infection with a dual tropic HIV clinical isolate no. 204 at a MOI of 0·1 for 8 h. The DNA was extracted and subjected to PCR analysis for gag amplification as described above. One representative sample is shown. The average ratio of HIV-gag to β-actin calculated from the results obtained from five different individuals is also shown. UC, uninfected controls.
IFN-γ inhibits HIV replication in monocytic cells
Because IFN-γ inhibits monocyte chemokine receptor expression and chemokine-induced chemotaxis, but does not affect HIV entry, we wished to determine whether IFN-γ inhibits HIV replication. Monocytes (1 × 104/ml) from five HIV-negative adults were cultured with either IFN-γ or IL-2 for 48 h, followed by infection with dual-tropic HIV clinical isolate no. 204. Virus replication was determined by assaying p24 concentrations in the culture supernatants at various times post-infection. In unstimulated monocytes, p24 production was undetectable 5 days after infection, but increased to an average of 15·0 ± 2·2 pg/ml by day 10 and reached a maximal level of 17·0 ± 2·3 pg/ml by day 15. In contrast, stimulation of monocytes with IFN-γ significantly reduced the production of p24 at days 10 (less than 2·5 pg/ml) and 15 (less than 5 pg/ml) post-infection compared to either untreated or IL-2-treated monocytes. It may be pointed out that the low levels of p24 detected in these experiments were due to the low numbers of monocytic cells used for infection with HIV. These results suggest that although IFN-γ inhibits monocyte expression of the chemokine receptors CXCR-4 and CCR-5, chemokine-induced chemotaxis and HIV replication, it fails to inhibit HIV entry. These results further suggest that IFN-γ may inhibit HIV replication by targeting pathways subsequent to virus entry.
DISCUSSION
In this study, we investigated the role of IFN-γ in regulating the expression of chemokine receptors by monocytic cells, as well as the physiological impact of IFN-γ on cell migration and HIV infection and replication. Our results show that IFN-γ inhibited the expression of the chemokine receptors CXCR-4 and CCR-5 on freshly isolated monocytic cells derived from three different sources, namely, HIV-negative adults, HIV-infected individuals and HIV-negative umbilical cord blood. IFN-γ-mediated down-regulation of CXCR-4 and CCR-5 expression was correlated with reduced chemokine-induced chemotaxis. Although IFN-γ inhibited viral replication in monocytic cells, IFN-γ-mediated down-regulation of CXCR-4 and CCR-5 was not sufficient to prevent the entry of HIV into monocytic cells.
Susceptibility to HIV infection may be regulated at the level of chemokine receptor expression by monocytic cells [13–16]. Differences in the levels of CCR-5 expression by monocytes from neonates and adults, as well as differences in the stages of monocyte differentiation may be associated with their susceptibility to HIV infection [14,29,39]. During the last few years, the role of cytokines as potent modulators of chemokine receptor expression by T cells and monocytic cells has been studied [10,14,17–21]. Our results show for the first time that IFN-γ consistently decreased the expression of both CXCR-4 and CCR-5 by CD14+ monocytes derived from HIV-negative adults, HIV-positive adults and HIV-negative cord blood. IFN-γ-induced down-regulation of monocyte CXCR-4 and CCR-5 expression was not associated with changes at the mRNA level. However, IFN-γ stimulation of monocytic cells enhanced significantly the production of the chemokines SDF-1 and RANTES. It is possible that the down-regulation of CXCR-4 and CCR-5 expression observed in our studies may thus be due to ligand-mediated receptor internalization in response to IFN-γ-induced secretion of RANTES and SDF-1. Anti-SDF-1 or anti-RANTES antibodies failed to cause down-regulation of CXCR-4 and CCR-5 receptors, respectively. However, as expected, cells incubated with SDF-1 as well as with RANTES induced down-regulation of CXCR-4 and CCR-5 receptors, respectively (data not shown). These results suggest that down-regulation of CXCR-4 and CCR-5 receptors by IFN-γ may be due at least in part to the chemokine-mediated internalization of their corresponding receptors. Alternatively, IFN-γ may induce the secretion of certain cytokines/factors with inhibitory effects on chemokine receptor expression. In that event, neutralizing anti-SDF-1 and anti-RANTES antibodies may not prevent down-regulation of chemokine receptor expression.
In this study, we have also shown that down-regulation of CXCR-4 and CCR-5 by IFN-γ was associated with a corresponding inhibition of viral replication and chemotaxis in response to SDF-1 and MIP-1α, ligands for CXCR-4 and CCR-5, respectively. These results are contrary to the studies reported by Hariharan et al. [26], who demonstrated that IFN-γ enhanced CCR5 expression on normal monocytes and differentiating MDMs with a corresponding increase in MIP-1α-induced chemotaxis. Interestingly, increases in chemokine receptor expression and chemotaxis were not associated with a corresponding increase in virus replication; rather, IFN-γ decreased HIV replication in these cells [26]. None the less, our results demonstrate clearly an association between chemokine receptor expression, chemotaxis and its sensitivity to HIV replication in monocytic cells. The reasons for the variable results in our studies and those of Hariharan et al. [26] are not clear. It is likely that different cell isolation techniques and culture conditions used to obtain monocytes may affect the level of cellular maturation, resulting in variable effects of IFN-γ with respect to chemokine receptor expression, chemotaxis and susceptibility to HIV infection.
Our results also show that although IFN-γ-induced significant down-regulation of CXCR-4 and CCR-5 expression is associated with an inhibition of SDF-1- and MIP-1α-mediated chemotaxis, IFN-γ failed to inhibit virus entry into the monocytic cells. These results suggest that viral entry requires a critical chemokine receptor expression threshold level, below which HIV entry may not occur. In addition, CD4 expression levels on T cells and monocytes may play a vital role in HIV entry. It has been shown that blocking of CD4 expression on MDMs with monoclonal antibodies abrogated M-tropic HIV entry, whereas blocking CCR-5 expression by similar methods was able to reduce entry of M-tropic HIV by only 60%[18]. This is supported by a recent study suggesting that when CD4 expression is high, maximal levels of viral entry can occur even in the presence of minimal levels of CCR-5 expression [16]. Furthermore, decreases in CCR-5 expression significantly affected HIV entry only when CD4 expression was very low [13–16]. Interestingly, IFN-γ did not inhibit CD4 expression on monocytic cells in this study (data not shown). The inability of IFN-γ to reduce CD4 expression on monocytes may be responsible for the failure to inhibit HIV entry despite the significant down-regulation of CXCR-4 and CCR-5 expression observed in this study. Additionally, the usage of other chemokine receptors such as CCR-2, etc. for HIV entry can not be ruled out in the present study. Because IFN-γ inhibited viral replication, our results suggest that IFN-γ may inhibit virus replication by targeting pathways subsequent to virus entry. The specific events in the viral life cycle subsequent to virus entry that are inhibited by IFN-γ remain to be determined.
IFN-γ appears to play a critical role in HIV infection and disease progression as high levels of IFN-γ are detected in the serum and cerebrospinal fluids of HIV-infected individuals [27,28,40–42]. IFN-γ has been shown to have biphasic effects on HIV infection and HIV replication in monocytic cells [17,18,24–26]. While treatment of HIV-infected macrophages with IFN-γ was shown to inhibit viral replication [17,26], IFN-γ was found to enhance HIV replication in vitro in terminally differentiated monocyte derived macrophages (MDMs) and in the promonocytic cell line U937 [24]. In another study, IFN-γ augmented susceptibility of MDMs to infection with T cell tropic CXCR-4-utilizing (X4) HIV-1 strains. However, in the same study IFN-γ decreased the susceptibility of MDMs to infection with M-tropic CCR-5-utilizing (X5) HIV-1 strains [12]. Our results show that IFN-γ inhibited HIV replication in monocytes obtained from HIV-negative individuals. The variable effects of IFN-γ on HIV replication in monocytes and highly differentiated MDM may be attributed to the state of cell activation and/or differentiation [14,29]. These observations suggest that IFN-γ may employ different signalling pathways, resulting in either inhibition or induction of viral replication in monocytic cells and MDMs, depending on their stage of maturation.
In summary, the results of this study demonstrate that IFN-γ, in addition to its role in the development of protective immune responses, may influence the migration of monocytic cells. The release of chemokines following stimulation of mononuclear phagocytes with IFN-γ, and the consequent inhibition of chemokine receptor expression, may limit the recruitment of monocytic cells to sites of inflammation and HIV infection, and hence may play a role in containing infection and inflammation. Given our observations regarding the IFN-γ-mediated inhibition of chemokine receptor expression and inhibition of HIV replication in monocytes, the enhanced levels of IFN-γ present in the serum and CSF of HIV-infected individuals may be beneficial. Therefore, strategies designed to drive the immune response towards a Th1-type response by the administration of either IFN-γ or agents that enhance IFN-γ production could be of clinical significance to HIV-infected individuals.
Acknowledgments
This work was supported by grants to A. K. from the Ontario Ministry of Health, the Canadian Foundation for AIDS Research and the Research Institute, Children's Hospital of Eastern Ontario. We thank Dr Gina Graziani-Bowering for critically reading the manuscript.
REFERENCES
- 1.Pelchen-Matthews A, Signoret N, Klasse PJ, Fraile-Ramos A, Marsh M. Chemokine receptor trafficking and viral replication. Immunol Rev. 1999;168:33–49. doi: 10.1111/j.1600-065x.1999.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 2.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med. 2003;13:39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 3.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 5.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 6.Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Juarez J, Alali M, et al. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73:9741–55. doi: 10.1128/jvi.73.12.9741-9755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaitseva M, Blauvelt A, Lee S, et al. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–75. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 10.Jourdan P, Vendrell JP, Huguet MF, et al. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165:716–24. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- 11.Wodarz D, Nowak MA. Evolutionary dynamics of HIV-induced subversion of the immune response. Immunol Rev. 1999;168:75–89. doi: 10.1111/j.1600-065x.1999.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 12.Zaitseva M, Lee S, Lapham C, et al. Interferon gamma and interleukin 6 modulate the susceptibility of macrophages to human immunodeficiency virus type 1 infection. Blood. 2000;96:3109–17. [PubMed] [Google Scholar]
- 13.Naif HM, Li S, Alali M, et al. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–6. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naif HM, Li S, Ho-Shon M, Mathijs JM, Williamson P, Cunningham AL. The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J Immunol. 1997;158:501–11. [PubMed] [Google Scholar]
- 15.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol. 1998;72:4962–9. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhawan S, Heredia A, Wahl LM, Epstein JS, Meltzer MS, Hewlett IK. Interferon-gamma-induced downregulation of CD4 inhibits the entry of human immunodeficiency virus type-1 in primary monocytes. Pathobiology. 1995;63:93–9. doi: 10.1159/000163939. [DOI] [PubMed] [Google Scholar]
- 18.Di Marzio P, Tse J, Landau NR. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–38. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Roderiquez G, Oravecz T, Norcross MA. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J Virol. 1998;72:7642–7. doi: 10.1128/jvi.72.9.7642-7647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailer RT, Lee B, Montaner LJ. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur J Immunol. 2000;30:1340–9. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Montaner LJ, Bailer RT, Gordon S. IL-13 acts on macrophages to block the completion of reverse transcription, inhibit virus production, and reduce virus infectivity. J Leukoc Biol. 1997;62:126–32. doi: 10.1002/jlb.62.1.126. [DOI] [PubMed] [Google Scholar]
- 22.Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–51. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–87. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 24.Biswas P, Poli G, Kinter AL, et al. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992;176:739–50. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyanagi Y, O'Brien WA, Zhao JQ, Golde DW, Gasson JC, Chen IS. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–5. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 26.Hariharan D, Douglas SD, Lee B, Lai JP, Campbell DE, Ho WZ. Interferon-gamma upregulates CCR5 expression in cord and adult blood mononuclear phagocytes. Blood. 1999;93:1137–44. [PubMed] [Google Scholar]
- 27.Vyakarnam A, Matear P, Meager A, et al. Altered production of tumour necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin Exp Immunol. 1991;84:109–15. doi: 10.1111/j.1365-2249.1991.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Mitoma F, Kumar A, Karimi S, et al. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–9. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fear WR, Kesson AM, Naif H, Lynch GW, Cunningham AL. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J Virol. 1998;72:1334–44. doi: 10.1128/jvi.72.2.1334-1344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 31.Lim W, Ma W, Gee K, et al. Distinct role of p38 and c-Jun N-terminal kinases in IL-10-dependent and IL-10-independent regulation of the costimulatory molecule B7·2 in lipopolysaccharide-stimulated human monocytic cells. J Immunol. 2002;168:1759–69. doi: 10.4049/jimmunol.168.4.1759. [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Lim W, Gee K, et al. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 33.Wang JF, Liu ZY, Groopman JE. The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–64. [PubMed] [Google Scholar]
- 34.Hermann E, Darcissac E, Idziorek T, Capron A, Bahr GM. Recombinant interleukin-16 selectively modulates surface receptor expression and cytokine release in macrophages and dendritic cells. Immunology. 1999;97:241–8. doi: 10.1046/j.1365-2567.1999.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar D, Parato K, Kumar A, Sun E, Cameron DW, Angel JB. Sustained suppression of plasma HIV RNA is associated with an increase in the production of mitogen-induced MIP-1alpha and MIP-1beta. AIDS Res Hum Retroviruses. 1999;15:1073–7. doi: 10.1089/088922299310368. [DOI] [PubMed] [Google Scholar]
- 36.Chambers KA, Parato KG, Angel JB. Active cellular infection of myeloid cells is required for HIV-1-mediated suppression of interleukin-12 p40 expression. Cell Immunol. 2002;215:120–32. doi: 10.1016/s0008-8749(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 37.Ou CY, Kwok S, Mitchell SW, et al. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;239:295–7. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- 38.Panther LA, Coombs RW, Aung SA, dela RC, Gretch D, Corey L. Unintegrated HIV-1 circular 2-LTR proviral DNA as a marker of recently infected cells: relative effect of recombinant CD4, zidovudine, and saquinavir in vitro. J Medicalvirol. 1999;58:165–73. doi: 10.1002/(sici)1096-9071(199906)58:2<165::aid-jmv11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Ho WZ, Lioy J, Song L, Cutilli JR, Polin RA, Douglas SD. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992;66:573–9. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyor WR, Glass JD, Griffin JW, et al. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 41.Boyle MJ, Berger MF, Tschuchnigg M, et al. Increased expression of interferon-gamma in hyperplastic lymph nodes from HIV-infected patients. Clin Exp Immunol. 1993;92:100–5. doi: 10.1111/j.1365-2249.1993.tb05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinicco A, Biglino A, Sciandra M, et al. Cytokine network and acute primary HIV-1 infection. AIDS. 1993;7:1167–72. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]