Abstract
We established a mouse model in which fatal pneumonia was induced by pneumococcal superinfection following influenza virus infection in chronic Pseudomonas aeruginosa infected mice. In this mouse model, influenza virus infection caused a significant increase in inflammatory cells, cytokines and severe tissue damage in the lungs of these P. aeruginosa infected mice, before pneumococcal infection. Intrapulmonary virus titres were significantly increased in mice with chronic P. aeruginosa infection, compared with control mice. Neutrophil function analysis showed significant reduction of myeloperoxidase (MPO) activity and lysozyme secretion by influenza virus infection in these mice. Our results suggest that influenza virus infection may play an important role in inducing pneumococcal pneumonia in chronic P. aeruginosa infected mice. Our results suggested that our mouse model is useful for investigating the pathogenesis of influenza virus infection in patients with chronic lung infection.
Keywords: influenza virus, superinfection, Pseudomonas aeruginosa, Streptococcus pneumoniae, neutrophil function
INTRODUCTION
Influenza virus infection is one of the most pandemic infectious diseases in the world [1,2]. From 1972 through 1992, influenza epidemics accounted for a total of 426 000 deaths in the United States, even if there was no pandemic of influenza in this period [3]. In general, influenza virus infection can induce bronchitis and pneumonia, but severe lethal pneumonia is usually seen when complications involve bacterial infections. Respiratory virus infections, including influenza virus, could also trigger serious acute respiratory illness that result in hospitalization of patients with chronic underlying conditions, including chronic pulmonary conditions. The Center for Disease Control (CDC) recommends influenza vaccination for adults and children who have chronic disorders of the pulmonary to avoid influenza-related complications, including bacterial pneumonia [4].
Chronic respiratory tract infections caused by Pseudomonas aeruginosa, such as the infection in bronchiectasis, cystic fibrosis and diffuse panbronchiolitis (DPB), are one of most difficult infections to control. It is well known that the patients with such infections often develop acute exacerbations with viral and/or bacterial superinfection [5]. These serious complications require hospitalization or can sometimes be fatal. In patients with cystic fibrosis, although Streptococcus pneumonia is reported to be the fourth most common bacteria isolated from the sputum, the majority of these patients has high levels of pneumococcal antibody before immunization with pneumococcal vaccine [6], suggesting that they had frequent or persistent infection with S. pneumoniae.
Bacterial adherence to the surface of cells and respiratory tract is enhanced by influenza virus infection and is reported as a contributing factor to the increased secondary bacterial infection in influenza virus infectious diseases in vitro and in vivo[7,8]. However, the involvement of previous influenza virus infection in bacterial superinfection has not been reported in patients with chronic pulmonary disorders, including bronchiectasis, cystic fibrosis or DPB. We previously reported the murine model of chronic P. aeruginosa infection, in which P. aeruginosa infection of the bronchus persists for lifetime.
In this study, we found that previous influenza virus infection enhanced the development of fatal pneumococcal pneumonia in chronic P. aeruginosa infection mouse model, and induced neutrophil dysfunction, which may play an important role in establishing pneumococcal pneumonia. These results suggested that vaccination or chemoprophylaxis against influenza virus may be important to prevent secondary pneumonia in patients with cystic fibrosis or DPB as much as the importance of antibacterial treatment.
MATERIALS AND METHOD
Laboratory animals
Male, ddY, 7-week old, 30–35 g body weight, specific pathogen-free mice were purchased from Shizuoka Agricultural Cooperative Association Laboratory Animals (Shizuoka, Japan). All animals were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Center for Biomedical Science at Nagasaki University. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of our institution.
Bacterial strains
We used P. aeruginosa S10 strain isolated at Nagasaki University and S. pneumoniae (penicillin-resistant S. pneumoniae-187; PRSP-187, serotype; 19, isolated at Nagasaki University, Japan). The bacteria were stored at −70°C in brain heart infusion broth (BBL Microbiology System, USA) supplemented with 10% (v/v) glycerol and 5% (w/v) skimmed milk until use.
Viral preparation and viral titres
We used mouse-adapted influenza virus A strain (A/PR8/34 strain, H1N1) in our experiments. Madin-Darby canine kidney (MDCK) cells were used for maintenance of influenza virus and plaque formation assays to analyse viral titres in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Gaithersburg, MD, USA). Influenza virus were cultured in these cells for a few days and the supernatants were collected and stored at −80°C as viral stocks until used in experiments.
Experimental model of chronic respiratory infection
Disposable sterile plastic cut-down intravenous catheters with 3 Fr. (1 mm) outer diameter (Atom Co., Tokyo) were used for intubation. The tube was 3·0 mm long with a few slits made at the proximal end to prevent blockage by oral secretions. To prepare the inoculum, P. aeruginosa was cultured on trypticase soy agar plates for 24 h. The bacteria were suspended in saline, harvested by centrifugation (3000× g, 4°C, 10 min), resuspended in sterile saline and adjusted to 1–2 × 108 cfu/ml as estimated by turbidimetry. The intubation tube was then immersed in the bacterial saline suspension for 3 days at 37°C. On day 3 postinoculation, just prior to intubation, bacterial counts in these tubes were found to be 6·0 ± 0·6 (log10cfu/ml, mean ± SD, n = 10). The method used for inducing infection has been described in detail previously [9]. Briefly, the intubation tube harbouring the bacteria was attached to the blunted tip of the needle of an intravenous catheter (Angiocath; Becton Dickinson vascular access products, USA). The needle-tube was inserted through the oral cavity, and then advanced through the vocal cords. When the tip of the tube was in the trachea, the needle/catheter was pulled out and the outer sheath was pushed gently to place the precoated tube into the main bronchus.
Influenza virus inoculation and sampling
Influenza virus A (A/PR8/34, H1N1) was inoculated intranasally in a 50-µl suspension containing 5 × 104 pfu/ml to wild type animals or the murine model of chronic P. aeruginosa respiratory infection described above under anaesthesia. The virus was inoculated at 80 days after P. aeruginosa intubation, when the bacterial pneumonia was suppressed after P. aeruginosa intubation. Control mice received an equal volume of diluted DMEM. Mice were sacrificed 2 days after influenza virus infection by cervical dislocation.
S. pneumoniae inoculation
Bacteria were incubated overnight in brain-heart infusion broth at 37°C, and harvested by centrifugation at 10 000 × g for 10 min. The harvested bacteria were suspended in phosphate buffered saline (PBS) to a density of 108 CFU/ml, determined by OD660 and confirmed by colony forming assay on blood agar plates. Two days after influenza virus- or mock-infection of chronic P. aeruginosa infected mice, the mice were re-anaesthetized with 50 mg/kg pentobarbital and then inoculated intranasally with S. pneumoniae at 50 µl of 1 × 108 CFU/ml. The mice were observed over 10 days for survival studies. As controls, we also determined the survival rates of chronic P. aeruginosa infected mice after influenza virus alone infection (without S. pneumoniae infection), and both influenza virus and S. pneumoniae infected, but not P. aeruginosa infected mice.
Bronchoalveolar lavage (BAL) and histopathological analysis
Bronchoalveolar lavage (BAL), staining of the cells in lung lavage, and histopathological analysis were performed at 2 days after influenza virus infection, just before pneumococcal superinfection, as described previously [10]. Briefly, mice were sacrificed at 2 days after influenza virus infection, just before pneumococcal superinfection. After the chest was opened to expose the lungs, a disposable sterile plastic cutdown intravenous catheter was inserted into the trachea. BAL was performed in situ four times sequentially using 1 ml saline each time and the recovered fluid fractions were pooled for each animal. Leucocytes in BALF samples obtained from each mouse were washed and counted with a haemocytometer. For differential cell counts, cells were centrifuged onto a slide in a tabletop centrifuge at 1000× g for 1 min and the slides were stained with May–Giemsa stain, and differential cell counts were performed by counting 100 cells. To prepare lungs for tissue sectioning, the lungs were excised, and immersed in 10% phosphate-buffered formalin. Paraffin embedding and tissue staining with haematoxylin and eosin (HE) staining were performed using standard methods.
Determination of viral and bacterial titres
The numbers of P. aeruginosa and titres of influenza virus in the lung homogenates were determined by colony formation assay [9] and plaque formation assay [11], respectively.
Cytokine analysis
The concentrations of tumour necrosis factor-alpha (TNF)-α, interleukin (IL)-6, macrophage inflammatory protein-2 (MIP-2) in aqueous lung extracts from lung homogenates [12] were assayed by mouse Quantikine Kit (R & D Systems, Minneapolis, MN, USA).
Analysis of neutrophil function
Myeloperoxidase (MPO) activity in the lung homogenates was measured by the method reported previously by LeVine et al. [13]. Antigenic lysozyme in the lung homogenates was detected by Western blotting using rabbit antilysozyme antibody (dilution 1 : 10 000, Rockland-Inc., Gilbertsville, PA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (diluted 1 : 20 000, Santa Cruz Technology, CA, USA) was used as secondary antibody, and the signals were developed with ECL-plus (Amersham, Arlington Heights, IL, USA).
Statistical analysis
Data were expressed as mean ± SEM. Differences between groups were examined for statistical significance using the unpaired t-test. Kaplan–Meier analysis was used for analysis of survival rates. Geometric analysis was used for analysis of the number of bacteria and viral titres. A P-value less than 0·05 denoted the presence of a statistically significant difference. Statistical analysis was performed using StatView software (Abacus Concept, Inc., Berkeley, CA, USA).
RESULTS
Effects of influenza virus and S. pneumoniae infections on mortality of mice with chronic P. aeruginosa infection
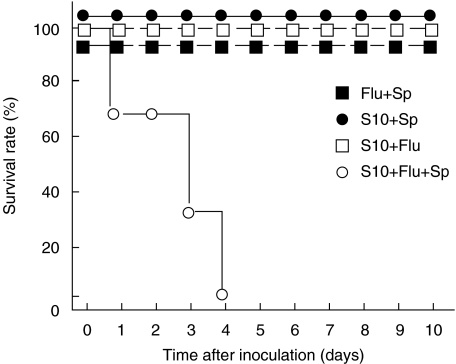
To investigate the effects of influenza virus infection on bacterial superinfection in chronic P. aeruginosa infection in vivo, we inoculated chronic P. aeruginosa infected mice with live S. pneumoniae, with or without influenza virus. As shown Fig. 1, mice with chronic P. aeruginosa infection died only when superinfected with both S. pneumoniae and influenza virus. These mice showed massive pneumonia at macroscopic and microscopic examinations at 2 days after S. pneumonia infection (data not shown). S. pneumoniae infection did not affect the mortality rate of these mice with chronic P. aeruginosa infection in the absence of influenza virus infection. Likewise, influenza virus infection alone did not cause death of these mice. Furthermore, none of the control mice (without P. aeruginosa infection) died even when coinfected with S. pneumoniae and influenza virus (Fig. 1).
Fig. 1.
Survival rates of mice superinfected with S. pneumoniae. Day 0 = the day of S. pneumoniae inoculation. Mice colonized with P. aeruginosa (S10) were infected with influenza virus (Flu) 2 days before S. pneumoniae (Sp) instillation. ○influenza virus and S. pneumoniae-infected mice (S10 + Flu + Sp); • S. pneumoniae-infected mice (S10 + Sp); □influenza virus-infected mice (S10 + Flu). ▪influenza virus and S. pneumoniae-infected mice without P. aeruginosa (Flu + Sp). Each group comprised 6 mice. Significantly shorter survival was observed in mice infected with both influenza virus and S. pneumoniae (P < 0·01). No mice died in the other groups during the observation period (10 days).
Histopathological evidence of acute on chronic bronchopulmonary infection
To further study the role of influenza virus on the lungs of mice with chronic P. aeruginosa infection, we examined the lungs histopathologically at 2 days after influenza virus infection (Fig. 2), just before pneumococcal superinfection. In control mice, we found almost no inflammatory cells or tissue damage (Fig. 2a). Mice infected with P. aeruginosa for 80 days showed accumulation of lymphocytes in the peribronchial walls of the distal bronchi and hyperplasia of the bronchial epithelium (Fig. 2b), as reported previously [9]. In mice infected with influenza virus alone, without P. aeruginosa infection, neutrophils and macrophages appeared in the alveolar walls as well as the alveolar space, consistent with acute interstitial pneumonia (Fig. 2c). In mice colonized with P. aeruginosa and subsequently infected with influenza virus, accumulation of acute inflammatory cells, mainly neutrophils and macrophages, was noted throughout the whole lung, in addition to accumulation of lymphocytes in the peribronchial walls (Fig. 2d). Moreover, massive accumulation of neutrophils was noted inside the bronchial lumen, representing bronchopneumonia, together with damage of bronchial epithelial cells. BALF analysis at 2 days after influenza virus infection, just before pneumococcal superinfection, showed significant accumulation of inflammatory cells, compared with mice infected with P. aeruginosa alone (Table 1). Mice infected with the influenza virus and P. aeruginosa showed the highest accumulation of inflammatory cells (Table 1).
Fig. 2.
Histopathological analysis of acute exacerbations caused by influenza virus in the lungs of mice with chronic respiratory P. aeruginosa infection, just before S. pneumonia infection. The lungs were collected at day 2 after instillation of influenza virus. (a) Mock (control) mouse (b) P. aeruginosa colonized mouse (c) influenza virus infected mouse (d) influenza virus infection of P. aeruginosa colonized mouse. Sections were stained with H&E. Original magnification, ×40.
Table 1. Inflammatory cells in the bronchoalveolar lavage fluid (BALF) of mice infected with Flu and/or S10.
| Cell numbers (×104, mean ± SD) (n = 5) | ||||
|---|---|---|---|---|
| Total cells | Neutrophil | Macrophages | Lymphocytes | |
| Mock | 13·3 ± 5·6 | 0 | 13·0 ± 5·5 | 0·3 ± 0·1 |
| Flu | 78·2 ± 9·0* | 38·1 ± 1·2* | 39·4 ± 7·7* | 0·6 ± 0·1* |
| S10 | 119·0 ± 14·5*† | 27·1 ± 4·3* | 77·0 ± 9·1* | 15·6 ± 1·1*† |
| S10 + Flu | 226·2 ± 28·5*†‡ | 100·8 ± 12·2*†‡ | 103·4 ± 14·4*† | 23·0 ± 1·9*†‡ |
Mock; control. Flu.; influenza virus, S10; P. aeruginosa, S10 + Flu; Superinfection.
P < 0·05, compared with Mock-, Flu, or S10 infected mice, respectively.
Elevated lung cytokine levels in mice infected with influenza virus
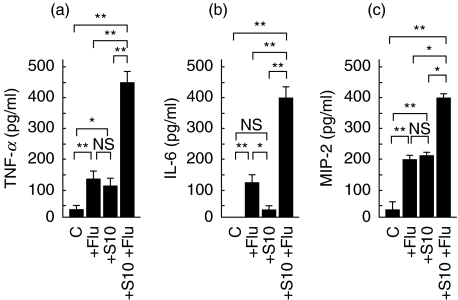
Cytokines are suggested to play important roles in protection against infection within the airways, and activation of inflammatory cells, including neutrophils, macrophages, and T cells. Therefore, we measured the concentration of secreted inflammatory cytokines, such as TNF-α, IL-6, and MIP-2 at 2 days after influenza virus infection, just before pneumococcal superinfection. As shown Fig. 3, intrapulmonary levels of TNF-α, IL-6, and MIP-2 increased significantly in the lungs of mice infected with P. aeruginosa and influenza virus, compared with those in mice with either P. aeruginosa or influenza virus alone infection.
Fig. 3.
Concentrations of inflammatory cytokines in the lungs of mice with chronic respiratory P. aeruginosa infection and influenza viral infection (n = 5), just before S. pneumonia infection. (a) TNF-α(b) IL-6 (c) MIP-2. C: control (mock), +Flu: acute influenza virus infection, +S10; chronic P. aeruginosa infection, +S10 +Flu; chronic P. aeruginosa+influenza virus. Data are mean ± SEM. *P < 0·05, **P < 0·01, NS; not significant.
Viral titres and bacterial numbers in lungs of mice with acute on chronic bronchopulmonary infection
We next analysed changes in P. aeruginosa numbers and influenza virus titres in the lungs of mice at 2 days after influenza virus infection. Results of the plaque formation assay showed almost 100-fold increase of influenza virus titres in the lungs of mice infected with both P. aeruginosa and influenza virus, compared with those of mice infected with influenza virus alone (Fig. 4a). In contrast, results of the colony forming assay showed that the number of P. aeruginosa in the lungs of mice infected with both P. aeruginosa and influenza virus was not significantly different from that of mice infected with P. aeruginosa alone (Fig. 4b).
Fig. 4.
Comparison of viral titres (a) and bacterial numbers (b) in the lungs of mice with single infection (either +Flu or +S10 alone) and those with chronic respiratory P. aeruginosa infection and acute influenza viral infection (+S10 +Flu), just before S. pneumonia infection. Data are mean ± SEM. **P < 0·01, NS; not significant (n = 5).
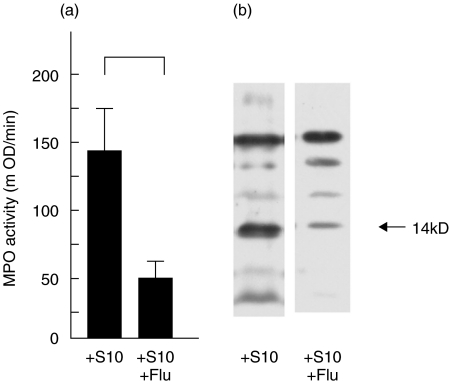
MPO activity and lysozyme secretions in lung homogenates
In chronic P. aeruginosa infected mice, significant decreases of MPO production in whole lungs occurred at 2 days after influenza virus infection (Fig. 5a). Furthermore, secretion of lysozymes was significantly reduced by influenza virus infection in the whole lungs of chronic P. aeruginosa infected mice (Fig. 5b). We also observed decrease of MPO and lysozyme secretion by influenza virus infection alone (mice without P. aeruginosa infection), but the decrease was less than P. aeruginosa colonized mice (data not shown).
Fig. 5.
(a) MPO activity and (b) lysozyme secretion in the whole lungs of the mice chronic P. aeruginosa infected mice at 2 days after influenza virus infection (just before S. pneumonia infection). The data were compared with or without acute influenza virus infection in P. aeruginosa colonized mice. (a) Data are mean ± SEM MPO activity in 5 mice in each group, NS; not significant. (b) Lysozyme secretions were analysed by Western blotting and representative data from 4 mice are presented. C: control (mock), +Flu: acute influenza virus infection, +S10; chronic P. aeruginosa infection, +S10 +Flu; chronic P. aeruginosa+ influenza virus.
DISCUSSION
The synergic effects between influenza virus and S. pneumoniae has been investigated and observed to induce death of mice in vivo[8,13]. The doses of influenza virus and S. pneumoniae used in our study were probably insufficient to induce death of ddY mice, although when used at the same doses, both organisms caused death of other murine strains, such as Balb/c or CBA/J mice, respectively (data not shown).
Viral infections do not only worsen the histopathological findings, but also increase the risk of further (secondary) bacterial infections such as by S. pneumoniae and H. influenzae[1,2,14,15]. We found histopathological worsening of the lung and lower airway inflammation, and increase number of inflammatory cells and cytokine concentrations in the lungs following influenza virus infection, compared with mice infected with either P. aeruginosa alone or influenza virus alone, before pneumococcal superinfection. These results may explain the destruction of lung tissue. Furthermore overproduction of cytokines may make mice with chronic P. aeruginosa infection more susceptible to severe pneumonia induced by pneumococcal superinfection.
Surprisingly, we found that increased influenza virus titres did not influence the bacterial numbers even after influenza viral infection. These results suggest that viral infection do not seem to be associated with a significant growth of colonized bacteria. Destruction of lung tissue or/and overproduction of cytokines may be largely induced by the viral infection, rather than the colonized bacterial infection. In other words, the results suggest that treatment of influenza virus infection might be more important than that of colonized bacterial infection to prevent secondary bacterial superinfection, which could potentially cause severe pneumonia.
Neutrophils are considered as key immune cells against infection, and their functions, reflected by MPO and lysozyme levels, are thought to modulate the risk of subsequent bacterial pneumonia [16]. Neutrophils are central to control of infection within the bronchial mucosa, and the link between bacteria and influenza viral infection in the respiratory tract can be partly explained by acute reduction of neutrophil function by influenza virus infection [16]. In the present study, we found influenza virus infection caused reduction of secreted lysozyme in chronic P. aeruginosa infected mice. Neutrophil–derived lysozymes play a major role in intracellular destruction of ingested bacteria, through the formation of phagolysosomes with primary (or azurophilic) and secondary granules [17,18]. Reduced MPO activity in influenza virus infection was also observed in the whole lungs of mice with chronic P. aeruginosa infection. Influenza virus has been demonstrated to have direct effects on neutrophil functions in vitro, including inhibition of chemotaxis, oxidative function and lysosome-phagosome fusion [19,20]. Pang et al. [16] reported that sputum neutrophils obtained from patients with bronchiectasis showed a significantly reduced capacity to secrete lysozyme, but not MPO by influenza virus infection. They explained those relative differences in lysozyme and MPO by different transport characteristics. MPO is largely intracellular and its activity is difficult to detect in supernatants of cell cultures, whereas lysozymes are released extracellularly. Because we used sonicated lung samples, we were able to detect the decrease of intracellular MPO activity in this study. The down-regulation of neutrophil functions may result in reduced bactericidal activity against bacteria superinfection, and consequently contribute to reduction in the capacity to control bacterial infection in respiratory tract following influenza virus infection. These data suggest that influenza virus infection may reduce the bactericidal activity partly through down-regulation of MPO activity and lysozyme secretion. Furthermore, chronic P. aeruginosa infection of bronchial tree may further enhance the decrease of host defense against future infections. In this regard, it has been reported that some proteases secreted from bacteria could activate haemagglutinin of influenza virus by proteolytic cleavage, and that these strains promote the replication of influenza virus in the respiratory tract and the subsequent development of pneumonia [21,22].
In conclusion, we established a mouse model, in which fatal pneumonia is induced by pneumococcal superinfection following influenza virus infection in chronic P. aeruginosa infected mice. Our results suggest that our mouse model might be useful for investigating the pathogenesis of influenza virus infection in patients with chronic lung infection.
Acknowledgments
The authors thank Masahiro Harada for the excellent technical support and Dr F. G. Issa (http://www.word-medex.com.au) for the careful reading and editing of the manuscript.
REFERENCES
- 1.Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 2.Schwarzmann SW, Adler JL, Sullivan RJ, Jr, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–69. Arch Intern Med. 1971;127:1037–41. [PubMed] [Google Scholar]
- 3.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2003;52:1–34. quiz CE1–4. [PubMed] [Google Scholar]
- 5.Wong K, Roberts MC, Owens L, Fife M, Smith AL. Selective media for the quantitation of bacteria in cystic fibrosis sputum. J Med Microbiol. 1984;17:113–9. doi: 10.1099/00222615-17-2-113. [DOI] [PubMed] [Google Scholar]
- 6.Lahiri T, Waltz DA. Preimmunization anti-pneumococcal antibody levels are protective in a majority of patients with cystic fibrosis. Pediatrics. 2001;108:E62. doi: 10.1542/peds.108.4.e62. [DOI] [PubMed] [Google Scholar]
- 7.Sanford BA, Davison VE, Ramsay MA. Staphylococcus aureus adherence to influenza A virus-infected and control cell cultures: evidence for multiple adhesins. Proc Soc Exp Biol Medical. 1986;181:104–11. doi: 10.3181/00379727-181-42230. [DOI] [PubMed] [Google Scholar]
- 8.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 9.Yanagihara K, Tomono K, Sawai T, Hirakata Y, Kadota J, Koga H, Tashiro T, Kohno S. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 1997;155:337–42. doi: 10.1164/ajrccm.155.1.9001333. [DOI] [PubMed] [Google Scholar]
- 10.Kadota J, Sakito O, Kohno S, et al. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–9. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 11.Matsukura S, Kokubu F, Noda H, Tokunaga H, Adachi M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J Allergy Clin Immunol. 1996;98:1080–7. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara K, Tomono K, Kuroki M, et al. Intrapulmonary concentrations of inflammatory cytokines in a mouse model of chronic respiratory infection caused by Pseudomonas aeruginosa. Clin Exp Immunol. 2000;122:67–71. doi: 10.1046/j.1365-2249.2000.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeVine AM, Koeningsknecht V, Stark JM. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J Virol Meth. 2001;94:173–86. doi: 10.1016/s0166-0934(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 14.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes ML, Kobzik L, Freihorst J. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J Immunol. 1995;154:268–80. [PubMed] [Google Scholar]
- 15.Raza MW, Blackwell CC, Elton RA, Weir DM. Bactericidal activity of a monocytic cell line (THP-1) against common respiratory tract bacterial pathogens is depressed after infection with respiratory syncytial virus. J Med Microbiol. 2000;49:227–33. doi: 10.1099/0022-1317-49-3-227. [DOI] [PubMed] [Google Scholar]
- 16.Pang G, Clancy R, Cong M, Ortega M, Zhigang R, Reeves G. Influenza virus inhibits lysozyme secretion by sputum neutrophils in subjects with chronic bronchial sepsis. Am J Respir Crit Care Med. 2000;161:718–22. doi: 10.1164/ajrccm.161.3.9812047. [DOI] [PubMed] [Google Scholar]
- 17.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 18.Buret A, Dunkley M, Clancy RL, Cripps AW. Effector mechanisms of intestinally induced immunity to Pseudomonas aeruginosa in the rat lung: role of neutrophils and leukotriene B4. Infect Immun. 1993;61:671–9. doi: 10.1128/iai.61.2.671-679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramson JS, Giebink GS, Mills EL, Quie PG. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981;143:836–45. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- 20.Abramson JS, Parce JW, Lewis JC, Lyles DS, Mills EL, Nelson RD, Bass DA. Characterization of the effect of influenza virus on polymorphonuclear leukocyte membrane responses. Blood. 1984;64:131–8. [PubMed] [Google Scholar]
- 21.Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology. 1987;157:421–30. doi: 10.1016/0042-6822(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 22.Scheiblauer H, Reinacher M, Tashiro M, Rott R. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis. 1992;166:783–91. doi: 10.1093/infdis/166.4.783. [DOI] [PubMed] [Google Scholar]