Abstract
A single intradermal injection of the adjuvant-oil squalene induces T cell mediated arthritis in DA rats. The chain of events leading from nonspecific provocation of the immune system to arthritis is largely unknown. Previous studies have demonstrated that lymph node (LN) cells are of pathogenic importance, i.e. cells from LNs draining the injection site can transfer arthritis to naïve DA rats. Recently we have demonstrated cellular uptake of adjuvant oil in draining lymph nodes but also that nondraining LNs become hyperplastic and harbour arthritogenic cells. Here, we aimed to determine from which time-point prior to arthritis onset arthritogenic cells appear in draining inguinal and nondraining axillary/brachial LNs, respectively. We demonstrated that the ability to transfer arthritis was strongly dependent on the time-point after adjuvant-injection with clear-cut differences between draining and nondraining LN cells. Cells harvested at day 5 postinjection (p.i) were not able to transfer arthritis, while at day 8 p.i, a first wave of arthritogenic cells appeared in draining LNs. The ability to transfer arthritis was associated with a pro-inflammatory cytokine profile as indicated by the IL-1β and IFNγ expression in cells from draining LNs. Subsequently, at day 11 p.i., just before arthritis onset, arthritogenic cells appeared also in nondraining LNs. These results shed new light on the induction of arthritic diseases, implicating a two step mechanism for the development of pathogenic cells. Firstly, a pro-inflammatory burst in responding lymphoid organs leading to a local pool of arthritogenic cells and, secondly, a transmission of arthritogenecity to other LNs and precipitation of disease in peripheral joints.
Keywords: adjuvant arthritis, adoptive transfer, DA rat, lymph node, squalene
INTRODUCTION
The recent report that mineral oil exposure can be a risk factor for developing rheumatoid arthritis (RA) [1] increases interest in experimental systems where the arthritogenic effects of oils can be delineated in a controlled fashion. A suitable experimental system is represented by RA models that are induced in arthritis-prone rat strains by a single intradermal injection of various oils, including mineral oils [2]. Another adjuvant oil is the endogenous cholesterol precursor squalene which, in similarity to other arthritogenic adjuvants, induces a T cell-mediated joint-specific inflammation in DA rats [3]. This model belongs to a group of experimentally induced inflammatory diseases where no protein or peptide antigens are coadministered. Why, when and how adjuvant oils activate arthritogenic T cells remains to be investigated.
Adjuvant oil-induced arthritis can be transferred to naive irradiated recipients with concanavalin A (con A)-stimulated lymph node cells in general, and CD4+ T cells in particular, indicating an arthritogenic role of helper T cells [4]. The transferred disease is similar to that seen in the donor animals, although somewhat milder [4]. CD8+ cells, on the other hand, do not transfer arthritis [4] and these cells may rather play a suppressive role in oil-induced joint inflammation [5].
We recently demonstrated, by ex vivo tracking of injected tritium-labelled squalene, that the joint-restricted inflammation is not due to accumulation of oil in peripheral joints or paws [6]. Instead, the majority of squalene remained at the injection site and substantial amounts were recorded in cells from hyperplastic draining lymph nodes. Intriguingly, adoptive transfers of squalene-induced arthritis (SIA) could be performed not only with cells from squalene-filled LNs draining the injection site but also from nondraining LNs containing scarce amounts of squalene [6]. It is, thus, evident that pathogenic cells appear also in LNs not harbouring the triggering adjuvant oil, suggesting that the initial arthritogenic cell activation at the site of injection and in draining lymph nodes is transmitted to nondraining lymph nodes either by migration of activated cells or by soluble factors. Such transmission could occur either during the initiation of the immune activation before precipitation of joint inflammation or after onset of arthritis symptoms.
The aim of this study was to determine from which time-point arthritogenic cells appear in inguinal LNs draining the injection site and in nondraining axillary/brachial LNs, respectively. This was done in SIA by transferring activated LN cells into naïve irradiated recipients followed by evaluation of arthritis phenotypes. We here demonstrated that the ability to transfer arthritis is strongly dependent on time-point after adjuvant-injection with clear-cut differences between draining and nondraining LN cells. To determine the differences in arthri-togenicity we analysed the balance of pro-inflammatory/anti-inflammatory cytokines between the LN cell populations at different time-points before arthritis onset as well as after established joint inflammation.
MATERIALS AND METHODS
Animals
Female inbred Dark Agouti (DA) rats were purchased from B & K Universal, Stockholm, Sweden. The genetics and characteristics of the rat strains used are described in ‘Genetic monitoring of inbred strains of rats’[7]. Rats were kept and used under specific pathogen-free conditions at the Centre for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden. The rats were 8–12 weeks old and were delivered at least one week prior to experimental start-up. They were maintained in climate-controlled environment with 12-h light/dark cycles, housed in polystyrene cages containing wood shavings, and were given standard rodent food and water ad libitum. Experimental procedures involving animals were performed according to guidelines provided by the central board for animal experiments at the Swedish Department of Agriculture, and were approved by the Ethical Board for animal experiments in Stockholm-North.
Induction and evaluation of squalene-induced arthritis (SIA)
Arthritis was induced under anaesthesia by an intradermal injection at the base of the tail with 300 µl squalene (C30H50, Sigma Chemicals, St. Louis, MO, USA). The squalene lot was tested for protein contamination and was found to contain <0·0006% w/w protein [3].
Arthritis development was monitored using a macroscopic scoring system ranging from 0 to 4 for each of the four limbs (1, enlargement of one type of joint; 2, enlargement of two types of joints; 3, more than two types of joints involved and 4, severe arthritis in the whole paw), yielding a score of 0–16 per animal.
Adoptive transfer of arthritis
Mitogen-activated cells were prepared as follows: single cell suspensions were prepared. Lymph node (LN) cells from DA females previously injected with squalene were pooled and cultured at 1 × 107 cells/ml with 3 µg/ml Concanavalin A (con A) in Dulbecco's modified Eagle's medium supplemented with glutamine, streptomycin, d-penicillin, HEPES, β-mercaptoethanol and 10% fetal calf serum (complete DMEM). The cells were cultured at 37° in a humidified 5% CO2 atmosphere for 48 h.
Naïve recipient DA rats were gamma-irradiated with 6 Gray (Gy) from a 60Co-source (Alcyon II) and 24 h later injected intravenously through the jugular vein with mitogen-activated LN cells, either 1 × 108 axillary plus brachial LN cells or 1 × 108 inguinal LN cells, under deep anaesthesia (Hypnorm plus Dormicum). The skin was sealed with clamps. The mitogen-activated LN cells were washed three times in sterile PBS, resuspended in 1 ml cold sterile PBS and kept on ice prior to transfer.
T cell proliferation assay
LN cells were suspended at 1 × 106 cells/ml in complete DMEM, and plated in 96-well flat-bottom cell culture plates (Nunc, Roskilde, Denmark), 0·2 ml per well. Concanavalin A (conA) A was added to triplicates of wells, dissolved in PBS to the final concentration 0·7 µg/ml, which corresponds to the conA concentration/cell in the cell flasks set for the transfer experiments. As control, 10 µl PBS were added to control well triplicates. The cells were incubated for 48–52 h in 37°C and 5% CO2, and proliferating cells were labelled with 1 µCi of 3H-thymidine per well for the final 6 h before cell harvest. Incorporation of label was determined by liquid scintillation counting, using a Wallac 1450 Microbeta Trilux liquid scintillation and luminescence counter (Upplands Väsby, Sweden).
Determination of cytokine mRNA levels in lymph node cells
The quantification of cytokine mRNA levels in LN cells was performed by Real-Time PCR, as described previously [8]. Single cell suspensions of LN cells were prepared and aliquot cell pellets of 5 × 106 cells/aliquot were stored at −70°C until RNA preparation. The mRNA was extracted using TRIzol (Gibco BRL, Bethesda, MD, USA) according to the acid guanidinium thiocyanate-phenol-chloroform mixture extraction method [9]. The reverse transcription was performed on RNA samples using random hexamers (Gibco BRL) as primers and superscript reverse transcriptase (Gibco BRL), as described previously [8]. The cDNA amplification was performed using an ABI PRISM 7700 sequence detection system (Perkin-Elmer, Norwalk, CT, USA), using the 5′ nuclease method (Taq Man) with a two-step PCR protocol (95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min). All primers and probes for the cytokines interferon-γ (IFN-γ), interleukin-1β (IL-1β) and IL-4 were designed as previously described [8], except for IL-13, which primer sequences were as follows: forward 5′ (5′-> 3′: GAC AGC TGG CGG GTT CTG), reverse 3′ (5′-> 3′: GGA TGG CAT TGC AAC TGG A) and probe sequence (5′-> 3′: CAG CCC TGG AAT CCC TGA CCA ACA), and for GAPDH, which primer sequences were as follows: forward 5′ (5′-> 3′: TCA ACT ACA TGG TCT ACA TGT TCC AG), reverse 3′ (5′-> 3′: TCC CAT TCT CAG CCT TGA CTG) and probe sequence (5′-> 3′: TGA CTC TAC CCA CGG CAA GTT CAA CG). All probes were labelled with the fluorochrome FAM (carboxyfluorescein) as reporter dye and TAMRA (tetramethylrhodamine carboxyl acid) as quencher dye. The number of PCR cycles required for the detection of each transcript was defined (cycle threshold: Cτ). Serial dilutions of a standard cDNA were included to ensure linearity and reproducibility of the assay. All test samples were then diluted to lie within the linear phase of the standard curve. The quantitative value of each sample was calculated as the ratio between the amount of cytokine and the endogenous control of the house-keeping gene GAPDH.
Statistical methods
Fischer's exact test or Student's t-test (unpaired) was used as indicated in the figure legends and tables. P-values below 0·05 were considered significant.
RESULTS
Appearance of arthritogenic LN populations in pre-arthritic animals
In previous studies, transfer experiments were performed with cells taken from arthritic animals. Here, we determined the arthritogenic potential of inguinal and axillary/brachial LN cells from prearthritic rats (days 5, 8, 11 post injection: p.i) with LN cells from arthritic animals (day 14 p.i).
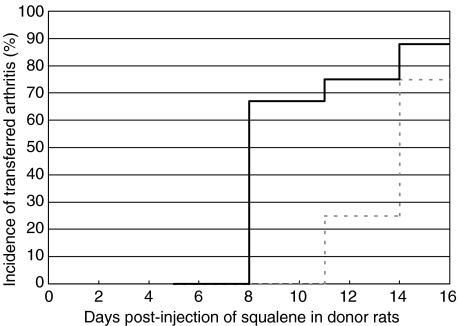
As shown in Table 1, all donor rats displayed arthritis at day 14 p.i. while no signs of joint inflammation were observed at day 11 p.i. or earlier time-points. At day 14, pooled cells from arthritic animals yielded a high penetrance, both in the group receiving inguinal LN cells (7/8; 88%) as well as the group receiving axillary/brachial LN cells (3/4; 75%) [Fig. 1]. At day 11 p.i. a 25% penetrance was achievable with the axillary/brachial LN cells but if collected at earlier time-points these cells were not arthritogenic. However, the inguinal LNs harboured arthritogenic cells both at day 11 and 8 p.i. but not at day 5, yielding a time interval between day 8 and 11 where inguinal but not axillary/brachial LN cells were able to transfer arthritis.
Table 1. Individual arthritis score for all donor rats at the time-point of sacrifice.
| Day postinjection | No of arthritic rats/ total rat donors | Arthritis clinical score |
|---|---|---|
| 5 | 0/18 | – |
| 8 | 0/36 | – |
| 11 | 0/18 | – |
| 14 | 18/18 | 1, 2, 4, 5, 5, 6, 6, 7, 7, 8,8, 8, 8, 8, 10, 10, 11, 12 |
Fig. 1.
Transferred arthritis incidence in recipient rats, illustrated as incidence (%) curves. Black line represents rats receiving inguinal lymph node cells, and dotted grey line represents rats receiving cells originating from the axillary/brachial lymph nodes. Number of rats receiving cells from inguinal lymph nodes are 9, 15, 8 and 8 for day 5, 8, 11 and 14, respectively; and the number of rats receiving cells from axillary/brachial lymph nodes are 3, 6, 4 and 4 for day 5, 8, 11 and 14, respectively. At day 8, the incidence was significantly higher in the group of animals receiving inguinal lymph node cells compared to animals receiving axillary/brachial lymph node cells (P < 0·05, Fischer's exact test).
Other arthritis-linked phenotypes such as arthritis maximum score and day of onset were also analysed and presented in Table 2. Here, the max score values for rats receiving inguinal LN cells taken at day 14 p.i. were significantly higher than for those receiving axillary/brachial LN cells. Day of arthritis onset did not differ, neither between time-points for cell transfer nor between origins of LN cells.
Table 2. Arthritis-linked phenotypes in naive irradiated animals receiving lymph node (LN) cells of different origins taken at different days postsqualene injection.
| Lymph node cells taken at X days postsqualene injection | ||||
|---|---|---|---|---|
| Arthritis-linked phenotype | Day 5 | Day 8 | Day 11 | Day 14 |
| Incidence percentage (no. of animals) | ||||
| Axillary/brachial LN | 0 (0/3) | 0 (0/6)* | 25 (1/4) | 75 (3/4) |
| Inguinal LN | 0 (0/9) | 67 (10/15) | 75 (6/8) | 88 (7/8) |
| Max score† | ||||
| Axillary/brachial LN | – | – | 2 | 1 (1; 2)* |
| Inguinal LN | – | 1·5 (1; 5) | 2 (1; 5) | 3 (1; 4) |
| Day of onset† | ||||
| Axillary/brachial LN | – | – | 10 | 11 (5; 11) |
| Inguinal LN | – | 11 (8; 25) | 9 (9; 19) | 10 (10; 11) |
Values are presented as median (min; max) of arthritic animals only.
P < 0·05 compared to animals receiving inguinal LN cells, using the Fischer's exact test for incidence and unpaired Student's T-test for max score, respectively.
Hyperplasia in lymph nodes after squalene injection
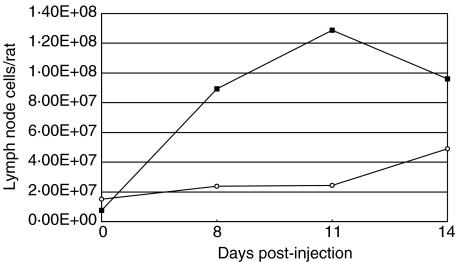
Squalene was injected intradermally at the base of the tail and 8, 11 and 14 days p.i. lymph nodes (LNs) were dissected out and cells were counted. Normal rats were also sacrificed and lymph node cells were harvested. As illustrated in Fig. 2, the cell numbers in draining inguinal LNs revealed a prominent hyperplasia already at day 8 p.i. while in axillary/brachial LNs, the prearthritic hyperplasia was less pronounced, implying that cells are recruited and/or activated to a higher extent in inguinal LNs. In arthritic animals (day 14 p.i), however, lymphoplasia was observed also in the nondraining axillary/brachial LNs. For inguinal LNs the cell numbers were highest at day 11 p.i and decreased at the day of onset (day 14 p.i), whereas there is an increase for the axillary/brachial LNs throughout the study.
Fig. 2.
Lymph node cell numbers/rat taken at different time-points post-squalene injection at the base of the tail. ▪ inguinal lymph nodes, draining the injection site; ○ nondraining axillary/brachial lymph nodes. Data represents lymph node cell numbers from pooled individuals. Number of rats representing each time-point are 5, 18, 18 and 18 for day 0, 8, 11 and 14, respectively.
Comparison of cytokine gene expression in draining and nondraining LNs
LN cells were analysed for mRNA expression of the cytokines IFN-γ, IL-1β, IL-4 and IL-13, respectively, using semiquantitative real-time PCR. The relative amounts of cytokine mRNA levels were determined by calculation of a ratio versus the house-keeping gene GAPDH. To investigate the relative dominance of TH1 versus the TH2 response, we calculated an additional ratio of IFN-γ/IL-4. When comparing the cytokine expression between cells from the different LNs, we observed a stronger expression of the pro-inflammatory cytokine, IL-1β and the TH1-promoting cytokine, IFN-γ in draining inguinal LNs both in prearthritic animals (day 5 p.i) as well as in established arthritis at day 14 [Table 3]. Also the IFN-γ/IL-4 ratio was higher in draining LNs compared to nondraining LNs at both time-points. IL-4 mRNA was observed in both axillary/brachial and inguinal LNs already at day 5 p.i with a somewhat higher expression in the draining LNs, while the opposite was observed after disease onset. IL-13 was not detected in unstimulated cells at the early time-point but was clearly detected in arthritic animals with a higher expression in axillary/brachial LNs.
Table 3. Comparison of mRNA cytokine levels in unstimulated single-cell suspensions taken at 5 or 14 days postsqualene injection. Lymph node cells from 18 rats were pooled at each time-point, respectively.
| Pre-conA cytokine levels | ||
|---|---|---|
| 5 | 14 | |
| IL-1β/GAPDH | ||
| Axillary/brachial | 0·001 | 0·089 |
| Inguinal | 0·185 | 1·140 |
| IFN-γ/GAPDH | ||
| Axillary/brachial | 0·002 | 0·021 |
| Inguinal | 0·028 | 0·184 |
| IL-4/GAPDH | ||
| Axillary/brachial | 0·867 | 1·891 |
| Inguinal | 1·530 | 0·310 |
| IL-13/GAPDH | ||
| Axillary/brachial | U.V. | 0·194 |
| Inguinal | U.V. | 0·098 |
| IFN-γ/IL-4 | ||
| Axillary/brachial | 0·002 | 0·011 |
| Inguinal | 0·019 | 0·594 |
| IFN-γ/IL-13 | ||
| Axillary/brachial | N.D. | 0·106 |
| Inguinal | N.D. | 1·878 |
N.D: not determined; UV: undetectable values
The expression of cytokine mRNA after conA-stimulation
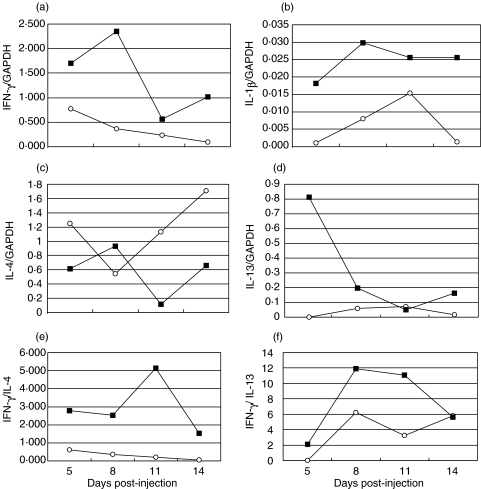
Since conA activation of LN cells is critical for successful transfer of arthritis, we also analysed the cytokine gene expression after mitogen stimulation. As illustrated in Fig. 3a,b, the relative IFN-γ and IL-1β mRNA levels were higher in the inguinal LN cells compared to the axillary/brachial LN cells at all time-points analysed. On the other hand, the levels of the anti-inflammatory cytokine IL-4 were lower in the inguinal LN cells throughout the time interval from day 5 to day 14 p.i. (Fig. 3c). In contrast, another TH2-associated cytokine, IL-13 was immensely elevated in conA stimulated inguinal LN cells at day 5 p.i. compared to axillary/brachial LN cells (Fig. 3d) while at other time-points the IL-13 levels were either low or undetectable by our real-time PCR technique. In accordance with a predominant TH1 profile, the IFN-γ/IL-4 and IFN-γ/IL-13 ratios were consequently higher in the inguinal LN cells than in axillary/brachial LNs (Fig. 3e,f).
Fig. 3.
Cytokine mRNA levels in 5 × 106 con A-stimulated lymph node (LN) cells, taken at different time-points postsqualene injection at the base of the tail. ▪ pooled inguinal LN cells; ○ pooled axillary/brachial LN cells. Number of rats pooled at each time-point are 18, 36, 18 and 18 for day 5, 8, 11 and 14, respectively.
The proliferation response of inguinal and axillary/brachial LN cells
Inguinal and axillary/brachial LN cells were tested in a standard 3H-thymidine assay resulting in a similar responsiveness to conA-stimulation by all cell populations regardless of LN origin or time-point for sampling (data not shown).
DISCUSSION
Experimental arthritis serve as a useful tool in studies of mechanisms underlying RA pathogenesis, particularly when investigating events that occur prior to arthritis onset since such studies in human arthritic conditions are difficult to perform. Yet, the primary events and/or cells leading to joint inflammation have not been fully elucidated, although both T cells and macrophages are considered to play important roles [10,11]. Neither have the mechanisms for adjuvant induction of arthritis been fully understood.
Our experimental model, using only the endogenous adjuvant squalene to induce arthritis in rats, fulfils at least 4 of the ACR diagnostic criteria set for RA, and is thus a useful model for the human disease. It also shares other features with RA, as it is dependent on genes both within and outside the MHC [3,12]. The role of MHC genes has been implicated by comparing disease susceptibility between MHC congenic rat strains indirectly suggesting a role of T cell–receptor/MHC interactions, even though no antigen recognized by the T-cell receptor has been identified [3,13–15]. The importance of T-cells in adjuvant oil-induced arthritides has been demonstrated by two different experimental approaches. Firstly, administering monoclonal Abs directed against the T cell-receptor into animals with oil-induced arthritides almost completely abolish the active inflammation [3,14,16]. Secondly, transfer of mineral oil-induced arthritis to naive recipients can be performed by purified LN T cells, particularly CD4+ T cells, derived from arthritic donor rats [4,17]. With these previous studies in mind, it is reasonable to conclude that the ability to transfer arthritis from prearthritic animals in our experimental model is closely linked to the presence of activated T cells in LNs, most likely of the CD4 subtype.
We have previously demonstrated successful transfer of SIA using LN cells from arthritic animals to naive recipients [6]. Intriguingly, arthritis could be transferred even with cells originating from LNs not draining the injection site even though these LNs accumulated minimal amount of squalene compared to the inguinal LN cells draining the site of injection [6]. In the present study, we demonstrated that inguinal LNs cells were more potent in transferring arthritis since these cells induced a more severe disease in recipients than was observed for cells from axillary/brachial LNs. Although axillary/brachial LN cells from arthritic animals transferred a milder arthritis, the incidence was close to 100% with the same day of onset as the inguinal LN cells. However, at earlier time-points before disease onset only draining LNs could produce arthritis with a high penetrance. These data indicate a ‘transfer window’, where the arthritogenic cells arrive to or develop in inguinal LNs draining the injection site and subsequently, just before the first macroscopic signs of joint inflammation are noted, the arthritogenicity is transmitted to other nondraining LNs.
It is, thus, clear from our data that the time-point for collecting cells for arthritis transfer experiments is crucial. We hypothesized that the time-kinetics of expressed T cell-and macrophage-derived cytokines in LNs determines the ability to transfer arthritis from prearthritic animals. The cytokine profile was, however, not clearly correlated with arthritogenicity, although it was evident that the more pathogenic inguinal LNs displayed higher levels of IL-1β and IFN-γ than the axillary/brachial LNs.
Analysis of the cytokine expression in transferred cells, i.e. after conA stimulation indicated that arthritogenic inguinal LN cells display an overall cytokine profile marking a pro-inflammatory phenotype. When comparing the different cell populations, it was evident that inguinal lymph node cells expressed higher levels of cytokines associated with a type 1 inflammatory responses, i.e. IL-1β and IFN-γ, throughout the time course from the earliest time-point at day 5 to disease onset at day 14. We also observed a more pronounced skewing of the cytokine balance towards TH1 in transferred cells from these LNs as indicated by the higher mRNA ratio of IFN-γ/IL-4 and IFN-γ/IL-13. These results may explain the higher potency of inguinal LNs to transfer arthritis since several previous studies have reported that TH1-associated cytokines strongly promote the type 1 inflammatory response in experimental arthritis, while TH2-associated cytokines may play a down-modulatory role [18–20]. At day 5, when arthritis could not be transferred the pro-inflammatory cytokines were possibly overwhelmed by high IL-13 expression in the conA-stimulated cells, thereby attenuating the arthritogenic cells residing in the inguinal LN. The appearance of arthritogenic cells in nondraining axillary/brachial LNs after arthritis onset was associated with increased lymphoplasia but not, however, with a type1-skewed cytokine profile. Why axillary/brachial lymph node cells, despite the lack of an evident TH1-bias at this time-point, are capable of transferring a mild arthritis remains to be explained. No direct relationship between TH1 and arthritogenicity can therefore be concluded.
In summary, the adjuvant injection induced a faster appearance of arthritogenic cells in draining LNs, most likely due to a relatively early deposition of squalene in those LNs as a result of transportation by responder cells from the injection site through the draining lymphatics. The arthritogenic capacity of nondraining LN cells could be explained by indirect activation, either by transport of arthritogenic cells from draining to nondraining LNs or by transmission of arthritogenicity through soluble factors. This study demonstrates the presence of pathogenic cells in draining lymph nodes prior to being present in nondraining lymph nodes and provides new understanding of how adjuvants, despite no known immunogenic properties, can provoke immune activation resulting in arthritic disease.
Acknowledgments
The authors are grateful to Ulrica Ribbhammar and Liselotte Bäckdahl, for help with irradiation of animals and dissection of organs. This work was supported by grants from the Swedish Medical Research Council, King Gustav V's 80th Birthday Jubilee Foundation, Nanna Svartz′ Foundation, Åke Wiberg Foundation, Alex & Eva Wallström Foundation and the Swedish Rheumatism Association. J.C.L. is in receipt of a Fellowship from the Swedish Medical Research Council.
REFERENCES
- 1.Klareskog L, Lorentzen JC, Padyukov L, et al. Genes and environment in arthritis: can RA be prevented? Arthritis Res. 2002;4:S31–6. doi: 10.1186/ar566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleinau S, Erlandsson H, Holmdahl R, et al. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmunity. 1991;4:871–80. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson BC, Jansson ÅM, Larsson A, et al. The endogenous adjuvant squalene can induce a chronic T-cell-mediated arthritis in rats. Am J Pathol. 2000;156:2057–65. doi: 10.1016/S0002-9440(10)65077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svelander L, Müssener Å, Erlandsson-Harris H, et al. Polyclonal Th1 cells transfer oil-induced arthritis. Immunology. 1997;91:260–5. doi: 10.1046/j.1365-2567.1997.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansson ÅM, Lorentzen JC, Bucht A. CD8+ cells suppress oil-induced arthritis. Clin Exp Immunol. 2000;120:532–6. doi: 10.1046/j.1365-2249.2000.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm BC, Svelander L, Bucht A, et al. The arthritogenic adjuvant squalene does not accumulate in joints, but gives rise to pathogenic cells in both draining and non-draining lymph nodes. Clin Exp Immunol. 2002;127:430–5. doi: 10.1046/j.1365-2249.2002.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhouse DD, Festing MFW, Hasan S, et al. Stuttgart: Gustaf Fischer Verlag; 1990. Genetic Monitoring of Inbred Strains of Rats. [Google Scholar]
- 8.Svelander L, Holm BC, Bucht A, et al. Responses of the rat immune system to arthritogenic adjuvant oil. Scand J Immunol. 2001;54:599–605. doi: 10.1046/j.1365-3083.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Kinne RW, Brauer R, Stuhlmuller B, et al. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmdahl R, Lorentzen JC, Lu S, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202. doi: 10.1034/j.1600-065x.2001.1840117.x. [DOI] [PubMed] [Google Scholar]
- 12.Holm BC, Xu HW, Jacobsson L, et al. Rats made congenic for Oia3 on chromosome 10 become susceptible to squalene-induced arthritis. Hum Mol Genet. 2001;10:565–72. doi: 10.1093/hmg/10.6.565. [DOI] [PubMed] [Google Scholar]
- 13.Vingsbo C, Jonsson R, Holmdahl R. Avridine-induced arthritis in rats; a T cell-dependent chronic disease influenced both by MHC genes and by non-MHC genes. Clin Exp Immunol. 1995;99:359–63. doi: 10.1111/j.1365-2249.1995.tb05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vingsbo C, Sahlstrand P, Brun JG, et al. Pristane-induced arthritis in rats. Am J Pathol. 1996;149:1675–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Lorentzen JC, Klareskog L. Susceptibility of DA rats to arthritis induced with adjuvant oil or rat collagen is determined by genes both within and outside the major histocompatibility complex. Scand J Immunol. 1996;44:592–8. doi: 10.1046/j.1365-3083.1996.d01-354.x. [DOI] [PubMed] [Google Scholar]
- 16.Holmdahl R, Goldschmidt TJ, Kleinau S, et al. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 1992;76:197–202. [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinau S, Klareskog L. Oil-induced arthritis in DA rats: Passive transfer by T cells but not with serum. J Autoimmunity. 1993;6:449–58. doi: 10.1006/jaut.1993.1037. [DOI] [PubMed] [Google Scholar]
- 18.Müssener Å, Klareskog L, Lorentzen JC, et al. TNF-α dominates cytokin mRNA expression in lymphoid tissues of rats developing collagen- and oil-induced arthritis. Scand J Immunol. 1995;42:128–34. doi: 10.1111/j.1365-3083.1995.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 19.Mattsson L, Lorentzen JC, Svelander L, et al. Immunization with alum-collagen II complex suppresses the development of collagen-induced arthritis in rats by deviating the immune response. Scand J Immunol. 1997;46:619–24. doi: 10.1046/j.1365-3083.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 20.Wendling U, Paul L, van der Zee R, et al. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10- producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–7. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]