Abstract
Human CD4+ T cells can be divided into reciprocal memory and naive T cell subsets based on their expression of CD45 isoforms and CD29/integrin beta1 subunit. To identify unique cell surface molecules on human T cells, we developed a new monoclonal antibody termed anti5H9. Binding of anti5H9 triggers a co-stimulatory response in human peripheral blood T cells. Retrovirus-mediated expression cloning has revealed that the antigen recognized by anti5H9 is identical to the tetraspanin CD9. We now show that human CD9 is preferentially expressed on the CD4+CD45RA+ naive T cell subset, and that CD9+CD45RA+ T cells respond preferentially to the recombinant beta2-glycoprotein I, compared to CD9–CD45RA+ T cells. Furthermore, anti5H9 inhibits both the recombinant beta2-glycoprotein I- and the recall antigen tetanus toxoid-specific T cell proliferation. These results suggest that the tetraspanin CD9 plays an important role in T cell activation.
Keywords: beta2-glycoprotein I, CD9, CD45RA, T cell, tetraspanin
INTRODUCTION
Human CD4+ T cell population is a heterogeneous collection of lymphocytes having different phenotypic and functional properties. These cells can be divided into functionally distinct and largely reciprocal subsets based on their differential expression of CD45 isoforms (CD45RA and CD45RO) and CD29/integrin beta1 subunit [1–8]. The CD4+CD45RO+CD29high memory (helper inducer) subset responds preferentially to soluble recall antigens such as tetanus toxoid (TT), and provides a strong helper function for IgG production by B cells [1,3]. In contrast, the CD4+CD45RA+CD29low naive (suppressor inducer) subset responds poorly to recall antigens and lacks the helper function, but this T cell subset proliferates maximally in autologous mixed lymphocyte reaction (AMLR) [2]. Among the many hypotheses regarding the biological significance of AMLR in vivo, it has been suggested that AMLR reflects a self-recognition process in the normal immune response [9,10]. In addition, it has been reported recently that CD4+CD45RA+ T cells proliferate in response to self-antigens in vitro, such as heat shock protein 60 or myelin basic protein [11,12]. These results suggest that human CD4+CD45RA+ T cells include autoreactive T cells, and it is conceivable that these cells might be involved in autoimmunity.
Many cell surface molecules have been defined and characterized by the use of specific monoclonal antibodies (MoAbs). Although many cell surface molecules preferentially expressed on CD4+CD45RO+ memory T cells have been identified, such as LFA-1, CD2 [13], CD29 [1], CD26 [14], CD82 [15] and CD43 [16], only a few molecules expressed preferentially on CD4+CD45RA+ naive T cells have been characterized thus far, including CD27 [17], CD31 [18] and CD62L [19]. In addition, in contrast to CD4+CD45RO+ T cells, the function of CD4+CD45RA+ T cells remains poorly understood.
CD9 belongs to the tetraspanin family, which consist of CD37, CD53, CD63, CD81, CD82, CD151, and a growing number of new proteins such as Tspan-1–6 and RDS/peripherin [20–22]. The tetraspanins are characterized by the presence of four conserved transmembrane regions [20–22] and are implicated in the regulation of cell–cell adhesion [23,24], cell motility [25–28], tumour cell metastasis [25–28], cell fusion [29–31], signal transduction [15,32] and cellular activation [15,32,33]. Tetraspanin is currently viewed to function as the organizer, facilitator or adaptor which assembles various molecular complexes on cell surfaces and participates in the signalling activity with associated molecules [22].
Recent gene targeting technology provides additional insight into the function of tetraspanin in vivo. Altered immune response has been reported in mice lacking CD81 or CD37 [34,35]. These results suggest that tetraspanins are involved in the regulation of the immune system. However, alterations in the immune system have not yet been observed in CD9-deficient mice, which showed reduced fertility [29–31]. In the murine system, it has been shown that CD9 is an inducer of co-stimulation and activation-induced cell death on T cells [32,33,36,37]. Meanwhile, CD9 function and distribution in human T cells remain largely unknown.
In the present study, we developed a new MoAb termed anti5H9 that reacts with human CD9, a member of the tetraspanin superfamily. Furthermore, CD9 is preferentially expressed on naive T cell population and anti5H9 inhibits both the recombinant beta2-glycoprotein I- and the recall antigen tetanus toxoid-specific T cell proliferation. These findings suggest that CD9 may play a role in the process of T cell activation.
MATERIALS AND METHODS
Antibodies
The MoAb anti5H9 was established by fusing a myeloma cell line with spleen cells of BALB/c J mice after immunization with the erythroleukaemia cell line K562 and developed hybridoma producing anti5H9 by limiting dilution method [1,2]. The isotype of anti5H9 was determined to be IgG1 kappa by the mouse MoAb isotyping kit (Amersham Biosciences, UK). The MoAbs, anti-CD3 (OKT3), anti-CD29 (4B4) [1], anti-CD45RA (2H4) [2], anti-CD45RO (UCHL-1) [3], anti-HLA-DR (L234), anti-CD82 (4F9) [15] and anti-CD28 (4B10) [1] were all purified from ascites fluid. The labelled MoAbs used were: fluorescein isothiocyanate (FITC)-conjugated anti-CD45RA (HI100, PharMingen, San Diego, CA, USA), RD1-conjugated anti-CD3 (UCHT1, Beckman Coulter, Inc., Fullerton, CA, USA), phycoerythrin (PE)-conjugated anti-CD4 (RPA-T4, PharMingen), anti-CD8 (RPA-T8, PharMingen), anti-CD45RA (2H4, Beckman Coulter), anti-CD45RO (UCHL-1, PharMingen), anti-CD62L (SK11, Immunocytometry Systems, San Jose, CA, USA), anti-CD29 (4B4, Beckman Coulter), PerCP-conjugated anti-CD4 (SK3, Immunocytometry Systems), anti-CD8 (SK1) (Immunocytometry Systems). FITC-, PE- and PerCP-conjugated control mouse IgG1, anti-CD11a and streptavidin–allophycocyanin (APC) were obtained from PharMingen. Biotinylation or FITC-conjugation of anti5H9 was performed as described previously [16]. FITC-conjugated goat antimouse Ig and control mouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Preparation of cells
Human peripheral blood mononuclear cells (PBMC) were isolated from healthy donors by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) density-gradient centrifugation. Unfractionated mononuclear cells were depleted of monocytes by adherence to plastic dishes. T cells (>95% CD3+) were then purified by E rosetting [1,2]. Further removal of monocytes from T cells was achieved by incubation with 5 mm l-leucine methyl ester HCl (Sigma) for co-stimulation assay. CD45RA+ T cells and CD45RO+ T cells were obtained from E rosette-positive cells by negative selection with anti-CD45RA (2H4), anti-CD45RO (UCHL-1) and goat antimouse IgG-conjugated immunomagnetic beads (PerSeptive Biosystems, Framingham, MA, USA) [15].
Cell lines
T cell lines (HPB-ALL and H9) and the erythroleukaemia cell line (K562) used in this study were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mm l-glutamine. An ecotropic retrovirus packaging cell line, BOSC23 (ATCC CRL 11554) was maintained in Dulbecco's modified Eagle medium (DMEM), containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 15 µg/ml hypoxanthine, 2 µg/ml aminopterin, 6 µg/ml thymidine, 250 µg/ml xanthine and 25 µg/ml mycophenolic acid. An amphotropic retrovirus packaging cell line, Phoenix (kindly provided by Dr Nolan) was maintained in DMEM, containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mm l-glutamine. A murine pre-B cell line Ba/F3 has been described previously [38,39].
Analysis and separation of cells by flow cytometry
Flow cytometric analysis was performed on an Epics XL cell sorter (Coulter Electronics, Hialeah, FL, USA) with System II software or FACSCalibur (Becton Dickinson, Franklin Lake, NJ, USA) as described previously [15]. For all samples, lymphocytes were analysed by selective gating based on the parameters of forward and side scatter. Cell sorting was fulfilled on an Epics Elite cell sorter (Coulter Electronics). In all experiments, post-sorting viability was more than 95% by Trypan blue dye exclusion. Purity of separated T cell subsets was always more than 95%.
Retrovirus-mediated expression cloning
Oligo (dT)-cDNA libraries were constructed from HPB-ALL cells, which steadily express 5H9 antigen [38,39]. Recombinant retroviruses containing the cDNA library were produced and used for infection [38,39]. In brief, packaging cell line BOSC23 cells were transfected with cDNA derived from HPB-ALL cells by Lipofectamine reagent (Gibco BRL, Grand Island, NY, USA). After 2 days, the culture supernatant containing recombinant retroviruses was harvested and used for infection of Ba/F3 cells. At 48 h after infection, the cells were subjected to flow cytometric analysis. After three cycles of sorting of the cells reactive with anti5H9, 5H9 antigen-positive cells were cloned. Genomic DNA was isolated from each clone and the integrated cDNA segment was amplified by polymerase chain reaction (PCR) using primers to retroviral vector pMX [40]. The PCR reaction was performed for 38 cycles (30 s at 96°C, 30 s at 58°C and 4 min at 72°C) with the LA PCR kit version 2 (Takara Shuzo Co. Ltd, Tokyo, Japan). The resulting PCR fragments were subcloned into the TA cloning vector pCR2·1 (Invitrogen) and sequenced using the PRISM dye terminator cycle sequencing ready reaction kit (Applied Biosystems, UK) and the ABI 373S sequencing system. All sequences of the cDNA were determined by constructing deletion mutant clones. Furthermore, the retroviral vector inserted the cloned cDNA was constructed again and transfected to H9 cells, which did not express the CD9 molecule, with amphotropic packaging cell line Phoenix cells in the same manner.
Western blotting
Cells were lysed with lysis buffer (25 mm HEPES, 150 mm NaCl, 5 mm MgCl2, 2 mm phenylmethylsulfonylfluoride (PMSF), 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin A, 2 mm NaF, 1% CHAPS). The cell lysates were denatured with sample buffer with or without 5% 2-mercaptoethanol at 95°C for 5 min. The samples were subjected to SDS-PAGE and electrotransferred onto PVDF membranes (Millipore, Bedford, MA, USA). The blots were blocked with 5% skimmed milk in Tris-buffer solution containing 0·05% Tween-20 (TBS-T) for 1 h at room temperature. The blots were washed three times with TBS-T and incubated for 1 h at room temperature in TBS-T with 1 µg/ml anti5H9. After washing three times, the blots were incubated with horseradish peroxidase-conjugated goat antimouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), and the bands were visualized according to the chemiluminescent reaction method (ECL Western blotting detection reagents, Amersham Biosciences). The blots were then exposed to X-ray film for a few minutes.
Co-stimulation assay
Monoclonal antibodies were immobilized to wells of 96-well flat-bottomed microculture plates (Corning Glass Works, Corning, NY, USA) at appropriate concentrations in a final volume of 0·1 ml. Purified T cells at 2·0 × 105 cells/well were cultured in RPMI-1640 medium supplemented with 10% FBS at 37°C in humidified atmosphere with 5% CO2 for the indicated period. Proliferation of T cells was assessed by incorporation of [3H]-thymidine in the last 13 h of cultures. The results were expressed as mean cpm ± s.d. of triplicate cultures.
T cell proliferation assay
Antigen-induced T cell proliferation was assayed by Hattori's methods with some modification [41]. Briefly, purified CD9+CD45RA+ T cells or CD9–CD45RA+ T cells were cultured with or without antigen in the presence of 6% of macrophages in 96-well round-bottomed culture plates for 7 days, and then proliferation was assessed by incorporation of [3H]-thymidine in the last 13 h of cultures. Recombinant beta2-glycoprotein I (GPI) (10 µg/ml) produced by Escherichia coli expression system or tetanus toxoid (TT, 5 ng/ml) (purchased from Calbiochem, CA, USA) was added to the cultures. In parallel, phytohaemagglutinin (5 µg/ml) was used to stimulate T cells to evaluate the non-specific responsiveness of T cells.
Statistics
Student's t-test or Welch's t-test was used to determine whether the difference between control and sample was statistically significant (P < 0·05 being significant).
RESULTS
5H9 antigen is identical to a tetraspanin family molecule, human CD9
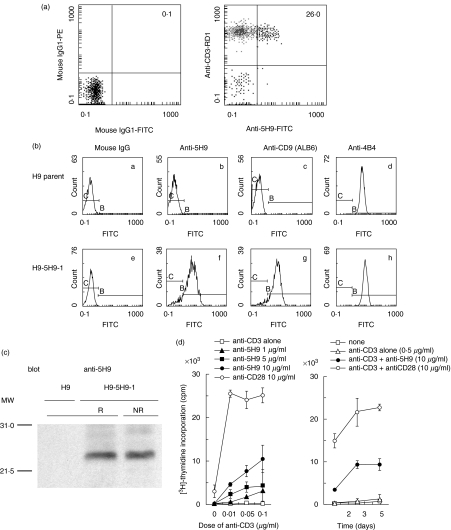
In an attempt to identify novel cell surface molecules on human T cells, we developed a variety of hybridoma clones and selected a clone producing a monoclonal antibody strongly reactive to human peripheral blood T cells, termed anti5H9 (5H9+CD3+ 26·6 ± 9·5%, n = 5) (Fig. 1a). To characterize the 5H9 antigen, we performed cDNA cloning of the 5H9 antigen from a cDNA library of HPB-ALL constitutively expressing the 5H9 antigen by the retrovirus-mediated expression cloning system, and then sequenced the isolated cDNA. As a result of a homology search on the DDBJ/EMBL/GenBank database, it was determined that the nucleotide sequence of cDNA encoding the 5H9 antigen is identical to that of human CD9, a member of the tetraspanin superfamily, containing 684 base pairs (bp) of open reading frame of CD9 cDNA [DDBJ databases; nucleotide sequence data of CD9 are available in the DDBJ databases under Accession number(s) AB079244]. The isolated cDNA was again inserted into the retrovirus vector (pMX-5H9-1) and the gene product of the cDNA was expressed on the CD9-negative T cell line H9 (H9–5H9-1). The reactivity of anti-CD9 MoAbs to this cell line was subsequently analysed by flow cytometry. As shown in Fig. 1(b), H9–5H9-1 cells bound specifically to both ALB-6 (a commercially available anti-CD9 MoAb) and anti5H9, whereas these MoAbs did not react with parent H9 cells. The irrelevant MoAb, anti-CD29 (4B4), reacted with H9–5H9-1 as well as parent H9 cells. Next, Western blot analysis of cell lysate derived from H9–5H9-1 cells showed that a single band with molecular size of 24 kDa was detected by anti5H9 under both reducing and non-reducing conditions (Fig. 1c). Furthermore, co-stimulation assays for anti5H9-mediated comitogenic response in human peripheral blood T cells showed that anti5H9 could induce a dose-dependent co-mitogenic effect with a submitogenic dose of anti-CD3, whereas anti5H9 alone did not induce T cell proliferation, as demonstrated by a representative experiment shown in Fig. 1d. Based on the above results, we concluded that the 5H9 antigen is identical to human CD9, a member of the tetraspanin superfamily, and that anti5H9 can provide co-stimulatory signal to human peripheral T cells.
Fig. 1.
5H9 antigen is identified to be the CD9 molecule. (a) Reactivity of anti5H9 MoAb to human peripheral blood T cells. Two-colour staining analysis of freshly isolated human PBMC was performed using FITC-conjugated anti5H9, RD1-conjugated anti-CD3. Numbers indicate the relative percentages of positive cells within a quadrant. The result is representative of five separate experiments. (b) Reactivity of anti-CD9 MoAbs on H9 cells transfected with isolated cDNA of the 5H9 antigen. H9 parent cells as a negative control (a–d) or H9–5H9-1 cells transfected with isolated cDNA of 5H9 antigen (e–h) were stained with mouse IgG (a and e), anti5H9 (b and f), ALB6 (anti-CD9) (c and g), 4B4 (anti-CD29) (d and h) and FITC-conjugated goat antimouse IgG. (c) Western blotting of 5H9 antigen. H9–5H9-1 cells were lysed in lysis buffer. The lysates were separated on 12% SDS-PAGE under reducing (R) or non-reducing (NR) condition, and then immunoblotting was carried out with anti5H9 (blot). The positions of molecular weight markers are indicated on the left (MW). (d) Co-stimulatory effect of anti5H9 with the immobilized submitogenic dose of anti-CD3 on human peripheral blood T cells. Left: dose–response curve; right: time–course curve. Purified T cells at 2·0 × 105 cells/well were stimulated with immobilized suboptimal concentration of anti-CD3 (OKT3) and the indicated concentrations of anti5H9 or anti-CD28 (4B10). Proliferation was assessed by [3H]-thymidine (1 µCi/well) incorporation assay in the last 13 h of cultures. The result is representative of three independent experiments and expressed as mean cpm ± s.d. of triplicate cultures.
CD9 is expressed preferentially on the human CD4+CD45RA+ T cell population
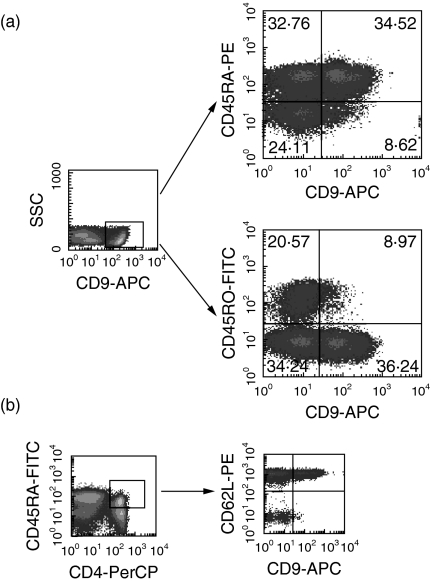
We next examined the expression of CD9 on subsets of human peripheral blood T cells by flow cytometric analysis using anti5H9. According to data from assays involving two-colour staining of human PBMC, the expression level of CD9 on T cells was relatively modest, with the population of CD9+ T cells being relatively small. CD9 was expressed on both CD4+ and CD8+ T cells (CD9+CD4+ 12·9 ± 9·0%, CD9+CD8+ 14·1 ± 2·4%, respectively). In addition, we performed four-colour staining of human PBMC. As shown by a representative staining pattern (Fig. 2a), CD9 was expressed preferentially on the CD4+CD45RA+ naive subset within human T cells (CD4+CD45RA+CD9+ 22·6 ± 10·2%versus CD4+CD45RO+CD9+ 4·9 ± 3·7%, n = 5, P < 0·05). Moreover, as shown in Fig. 2b, almost all the CD4+CD45RA+CD9+ T cells express l-selectin (CD62L), which is the homing receptor and is also a marker for naive T cells, while displaying a low level of CD29 (data not shown). These results indicate that CD9 is expressed preferentially on the subset of human CD4+CD45RA+ naive T cells.
Fig. 2.
Preferential expression of CD9 on CD4+CD45RA+ human naive T cells. CD9 defined by anti5H9 is present on a subpopulation of human peripheral blood T cells. Four-colour staining analysis of freshly isolated human PBMC was performed using FITC-conjugated anti-CD45RO, PE-conjugated anti-CD45RA, PerCP-conjugated anti-CD4, biotinylated anti5H9 and APC-conjugated streptavidin (a) and FITC-conjugated anti-CD45RA, PerCP-conjugated anti-CD4, PE-conjugated anti-CD62L, biotinylated anti5H9 and APC-conjugated streptavidin (b). Numbers indicate the relative percentages of positive cells within a quadrant. The result is representative of five separate experiments.
CD9+CD45RA+ T cells respond maximally to beta2-GPI
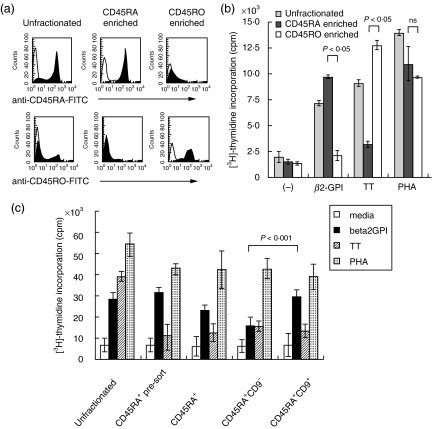
We next focused on the property of CD9+ T cells within the CD4+CD45RA+ T cell subset. To address the issue, we assessed the difference in proliferative response between CD9+CD45RA+ and CD9–CD45RA+ cells, as it has been suggested that CD4+CD45RA+ T cells contain the responding population to autoantigens [11,12]. We first examined the proliferative response of CD45RA+ and CD45RO+ T cells to recombinant beta2-GPI. Beta2-GPI is the most common antigenic target molecule in the antiphospholipid syndrome (APS) and is regarded as an autoantigen. In vitro, it has been reported that human CD4+ T cells from healthy individuals as well as APS patients with HLA-DR53 respond to recombinant beta2-GPI [41]. CD45RA+ T cells and CD45RO+ T cells were purified from peripheral blood of healthy donors with HLA-DR53 by negative selection (CD45RA+ enriched T cells >95% positive, CD45RO+ enriched T cells >95% positive, Fig. 3a). As shown in Fig. 3b, we demonstrated a difference in the relative responsiveness of these two T cell subsets to a model of autoantigen or a recall antigen, as detected by T cell proliferation assay. CD45RA+ and CD45RO+ T cells responded preferentially to the recombinant beta2-GPI and the recall antigen TT, respectively (Fig. 3b). In contrast, no significant difference was observed in the proliferative response to the non-specific stimulation of phytohaemagglutinin (PHA). After immunostaining with FITC-conjugated anti5H9, CD45RA+ T cells were sorted into CD9+ and CD9– cells by flow cytometry, and then the proliferative response of CD9+CD45RA+ T cells and CD9–CD45RA+ T cells to beta2-GPI was determined by T cell proliferation assay. As shown in Fig. 3c, CD9+CD45RA+ T cells incorporated significantly more [3H]-thymidine than CD9–CD45RA+ T cells in response to beta2-GPI (28977 ± 3859 cpm versus 15816 ± 409, P < 0·001). In contrast, no difference was observed in the responsiveness of each population to PHA (38820 ± 5880 cpm versus 42576 ± 4615 P = 0·8). Additionally, the responses of all CD45RA+ populations to TT were lower than that of unfractionated T cells. Therefore we could not assess the response of CD9+ or CD9− populations in CD45RA+ T cells to the recall antigen TT. These results suggest that the CD45RA+ naive T cell population that expresses CD9 is the one that responds preferentially to beta2-GPI.
Fig. 3.
Differential response of CD45RA+ and CD45RO+ T cells to recombinant beta2-GPI and tetanus toxoid (TT). (a) CD45RA+ T cells and CD45RO+ T cells were enriched by negative selection, and their purity was analysed by flow cytometry; unfractionated: pre-enrichment, CD45RA enriched: CD45RA+ T cells were enriched (>95% positive), CD45RO enriched: CD45RO+ T cells were enriched (>95% positive). (b) The proliferative response to beta2-GPI or TT was assessed with incorporation of [3H]-thymidine. Media alone (–), beta2-GPI (5 µg/ml), TT (5 ng/ml) and PHA (5 µg/ml) were used. The data are expressed as mean cpm ± s.d. of triplicate samples. Representative data of three separate experiments are shown. (c) Comparison between proliferative response of CD9+CD45RA+ T cells and CD9–CD45RA+ T cells to beta2-GPI (5 µg/ml), TT (5 ng/ml) and PHA (5 µg/ml) by T cell proliferation assay; unfractionated (unfractionated T cells population), CD45RA+ pre-sort (a population without anti5H9 treatment for cell sorting), and CD45RA+ (a population with anti5H9 treatment for cell sorting) T cells were also examined. The data are expressed as mean cpm ± s.d. of sextuple samples. Representative data of three separate experiments are shown.
Effect of anti5H9 on T cell proliferation induced by beta2-GPI
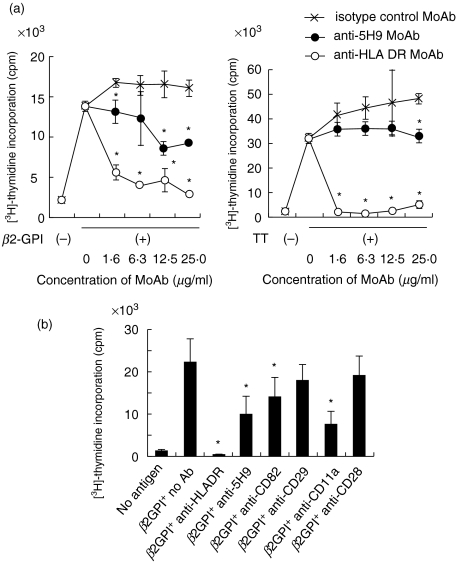
Finally, to assess the potential involvement of CD9 molecule in the preferential responsiveness of CD4+CD45RA+ T cells to beta2-GPI, the effect of anti5H9 on the proliferation of T cells to beta2-GPI was investigated. As shown in Fig. 4a, anti5H9 significantly inhibited both beta2-GPI- and TT-specific T cell proliferation in a dose-dependent manner, and anti-HLA DR (anti-L243) completely inhibited both responses. It has been reported that MoAbs against co-stimulatory molecule CD5, CD44 and CD11a, apart from CD28, all induce apoptosis of once-activated naive T cells [37]. Because it has also been reported that another tetraspanin molecule CD82 as well as CD9 induces apoptosis [42], we examined the effects of various MoAbs on beta2-GPI induced T cell proliferation. In addition to anti5H9 (anti-CD9), anti-CD11a and anti-CD82 significantly inhibited the beta2-GPI-induced T cell proliferation, while anti-CD28 and anti-CD29 did not (Fig. 4c). These results suggest that CD9 itself appears to play a key role in the specific proliferative response of naive and memory T cells to beta2-GPI and TT, respectively.
Fig. 4.
Effect of anti5H9 on T cell proliferation induced by recombinant beta2-GPI and TT. (a) The effect on proliferative response to recombinant beta2-GPI (5 µg/ml) (left panel) and TT (5 ng/ml) (right panel) was assessed with various concentrations of MoAb, isotype-matched control MoAb (anti4B4), anti5H9 and anti-HLA DR MoAb (anti-L243). Data are expressed as mean cpm ± s.d. of triplicate samples. Representative data of three independent experiments are shown. *Significant inhibition compared to the culture with isotype-matched control MoAb (P < 0·05). (b) Inhibitory effect of anti5H9 (15 µg/ml) on beta2-GPI-induced T cell proliferation was compared with those of various MoAbs (15 µg/ml). Data are expressed as mean cpm ± s.d. of sextuple samples. Representative data of three independent experiments are shown. *Significant inhibition compared to the culture without MoAb (P < 0·05).
DISCUSSION
In this study, we demonstrated that the newly developed anti5H9 MoAb targets human CD9, which is expressed preferentially on CD4+CD45RA+ naive T cells. Furthermore, this T cell population responds preferentially to recombinant beta2-GPI, with anti5H9 MoAb being able to inhibit this beta2-GPI-induced T cell proliferation and also TT-induced T cell proliferation, suggesting that the tetraspanin CD9 plays a important role both in the self-antigen- and recall antigen-induced T cell activation.
CD9 was described originally as a cell surface glycoprotein expressed on human pre-B cells and platelets but not on T cells [43,44]. While our preliminary experiments have demonstrated that several other anti-CD9 MoAbs hardly reacted with human peripheral blood T cells as detected by flow cytometric analysis (e.g. anti-ALB6 9·9%, anti-CLB 2·5%, anti72B6 0·9%), anti5H9 displayed a higher level of reactivity (anti5H9 26·6%). Moreover, others reported that murine CD9 defined by anti-KMC8·8 is only slightly expressed on murine T cells and B cells [45]. In contrast, it has been reported recently that murine CD9 recognized by anti9D3 is expressed on the cell surface of almost all T cells and B cells derived from murine thymus, spleen and lymph node [32,33,36]. Potential explanations for this observed discrepancy may involve differences in the relative affinity of anti-CD9 MoAbs or the specific epitope recognized by the various MoAbs. With our unique anti-CD9 MoAb, termed anti5H9, we have demonstrated clearly that CD9 is also expressed on human T cells, being restricted predominantly to the CD4+CD45RA+ naive T cell subset.
Concerning the role of tetraspanins in lymphocyte function, it has been shown that cross-linking of CD81 or CD82 on lymphocytes delivers co-stimulatory signals leading to cytokine production and modulation of cellular proliferation [15,24,46,47]. It has been also reported that murine CD9 defined by anti9D3 is a co-stimulatory molecule and is involved in activation-induced cell death [32,33,36]. Our present data show that CD9 also triggers a co-stimulatory signal in human T cells. However, the intensity of anti5H9-mediated T cell co-stimulation is relatively weaker than that induced by anti-CD28 in human T cells, potentially explained by the fact that CD9 expression is restricted to a selected T cell subset, while CD28 is present on almost all T cells. Moreover, we could not demonstrate clearly activation-induced cell death following CD9-mediated co-stimulation, in contrast to murine CD9 (data not shown). Thus, although there may be differences between human and murine T cells regarding CD9-associated apoptosis, our data would suggest that CD9 has a role in both human and murine T cell activation. While additional studies are required for the identification of a putative ligand for CD9 involved in T cell co-stimulation, it has already been reported that pregnant-specific glycoprotein 17 is a natural ligand for CD9 [48].
Our studies have focused on the functional property of CD9+ T cells within the CD4+CD45RA+ T cell subset. It is reported that the CD4+CD45RA+ naive T cell subset proliferates maximally in the AMLR [2] and responds to self-antigens, such as heat shock protein 60 [11] or myelin basic protein [12]. Several attempts to identify autoreactive T cells have been reported previously [11,12,41,49]. Generally, most autoreactive T cells are deleted in the thymus by negative selection. However, some cells manage to evade the thymic selection process and migrate into the peripheral tissues in both patients with autoimmune diseases and healthy individuals. Hattori et al. have shown that CD4+ T cells restricted by the HLA-DR53 alleles respond to reduced or recombinant beta2-GPI but not native beta2-GPI in both healthy individuals and patients with APS by presentation of its cryptic peptides [41]. Our present work using the same recombinant beta2-GPI also showed that CD45RA+-enriched T cells, but not CD45RO+-enriched T cells, proliferated preferentially in response to recombinant beta2-GPI, hence confirming that CD4+CD45RA+ T cells contain beta2-GPI-reactive T cells. We demonstrated further that CD9+CD4+CD45RA+ T cells represent the T cell population that responds maximally to recombinant beta2-GPI. CD9 expression thus appears to be preferential to naive T cells with relatively high responsiveness to self-antigens. Meanwhile, the self-reactivity that causes autoimmune diseases is believed to be an integral physiological aspect of immunity based on the hypothesis that alterations in T cell-activation thresholds by self-ligands facilitate positive selection and regulate the level of self-reactivity in the periphery [50]. It is currently unclear whether CD9+CD45RA+ T cells play a role in pathogenic or physiological conditions. Regardless, our findings that anti-CD9 inhibited beta2-GPI-mediated T cell response suggest that the CD9 molecule itself appears to be involved in the regulation of autoreactive T cells. As shown in Fig. 4c, anti-CD11a and anti-CD82 as well as anti-CD9 had an inhibitory effect on the beta2-GPI-induced T cell proliferation, while anti-CD28 and anti-CD29 did not exhibit a similar effect. Although murine anti-CD9, anti-CD82 and anti-CD11a have been reported to trigger activation-induced cell death after co-stimulation [37,42], we could not detect it clearly after co-stimulation induced by anti5H9 (data not shown). Therefore, it is possible that anti-CD9 blocks beta2-GPI-induced T cell activation by interfering with the cognitive interaction between antigen-presenting cells and T cells. Future work will determine the precise mechanisms involved in this process. This is the first study that demonstrates the functional significance of the tetraspanin CD9 expressed on human T cells. Further determination of the mechanism of co-stimulation through CD9, and identification of the CD9-ligand for co-stimulation, may lead to a detailed understanding of the regulation of autoreactive T cells and may provide insights into the development of immunotherapy for autoimmune diseases.
In summary, our data indicate that the tetraspanin CD9 was preferentially expressed on the population of CD4+CD45RA+ T cells. Meanwhile, the anti-CD9 antibody 5H9 provided a co-stimulatory signal to human peripheral T cells and inhibited both the recombinant beta2-GPI- and the recall antigen TT-specific T cell proliferation, indicating that the CD9 molecule plays an important role in T cell activation. Elucidating the putative ligand for the CD9 molecule involved in T cell co-stimulation as well as the molecular mechanisms by which anti5H9 inhibits T cell activation will be necessary for a complete understanding of this interesting molecule.
Acknowledgments
This work was supported in part by National Institutes of Health grants AR33713, and by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health, Labor and Welfare of Japan. N. H. Dang is supported in part by a grant from the M. D. Anderson Cancer Center Physician–Scientist Program and the Gillson Longenbaugh Foundation. We thank Dr R. Mukasa, Dr T. Ohtsuki and Ms A. Kuribara for helpful discussion and technical support.
REFERENCES
- 1.Morimoto C, Letvin NL, Boyd AW, et al. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985;134:3762–9. [PubMed] [Google Scholar]
- 2.Morimoto C, Letvin NL, Distaso JA, Aldrich WR, Schlossman SF. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985;134:1508–15. [PubMed] [Google Scholar]
- 3.Smith SH, Brown MH, Rowe D, Callard RE, Beverley PC. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd CE, Morimoto C, Wong LL, Schlossman SF. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987;166:1758–73. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi T, Schlossman SF, Morimoto C. The T4 molecule differentially regulating the activation of subpopulations of T4+ cells. J Immunol. 1987;139:665–71. [PubMed] [Google Scholar]
- 6.Morimoto C, Matsuyama T, Rudd CE, Forsgren A, Letvin NL, Schlossman SF. Role of the 2H4 molecule in the activation of suppressor inducer function. Eur J Immunol. 1988;18:731–7. doi: 10.1002/eji.1830180512. [DOI] [PubMed] [Google Scholar]
- 7.Sanders ME, Makgoba MW, Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988;9:195–9. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- 8.Horgan KJ, Tanaka Y, Shaw S. Postthymic differentiation of CD4 T lymphocytes: naive versus memory subsets and further specialization among memory cells. Chem Immunol. 1992;54:72–102. [PubMed] [Google Scholar]
- 9.Smolen JS, Chused TM, Novotny EA, Steinberg AD. The human autologous mixed lymphocyte reaction. III. Immune circuits. J Immunol. 1982;129:1050–3. [PubMed] [Google Scholar]
- 10.Scheinecker C, Machold KP, Majdic O, Hocker P, Knapp W, Smolen JS. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7–1 and B7–2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–73. [PubMed] [Google Scholar]
- 11.Ramage JM, Young JL, Goodall JC, Gaston JS. T cell responses to heat-shock protein 60: differential responses by CD4+ T cell subsets according to their expression of CD45 isotypes. J Immunol. 1999;162:704–10. [PubMed] [Google Scholar]
- 12.Muraro PA, Pette M, Bielekova B, McFarland HF, Martin R. Human autoreactive CD4+ T cells from naive CD45RA+ and memory CD45RO+ subsets differ with respect to epitope specificity and functional antigen avidity. J Immunol. 2000;164:5474–81. doi: 10.4049/jimmunol.164.10.5474. [DOI] [PubMed] [Google Scholar]
- 13.Sanders ME, Makgoba MW, Sharrow SO, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–7. [PubMed] [Google Scholar]
- 14.Morimoto C, Torimoto Y, Levinson G, et al. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989;143:3430–9. [PubMed] [Google Scholar]
- 15.Nojima Y, Hirose T, Tachibana K, et al. The 4F9 antigen is a member of the tetra spans transmembrane protein family and functions as an accessory molecule in T cell activation and adhesion. Cell Immunol. 1993;152:249–60. doi: 10.1006/cimm.1993.1285. [DOI] [PubMed] [Google Scholar]
- 16.Mukasa R, Homma T, Ohtsuki T, et al. Core 2-containing O-glycans on CD43 are preferentially expressed in the memory subset of human CD4 T cells. Int Immunol. 1999;11:259–68. doi: 10.1093/intimm/11.2.259. [DOI] [PubMed] [Google Scholar]
- 17.Sugita K, Tanaka T, Doshen JM, Schlossman SF, Morimoto C. Direct demonstration of the CD27 molecule involved in the negative regulatory effect on T cell activation. Cell Immunol. 1993;152:279–85. doi: 10.1006/cimm.1993.1288. [DOI] [PubMed] [Google Scholar]
- 18.Torimoto Y, Rothstein DM, Dang NH, Schlossman SF, Morimoto C. CD31, a novel cell surface marker for CD4 cells of suppressor lineage, unaltered by state of activation. J Immunol. 1992;148:388–96. [PubMed] [Google Scholar]
- 19.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–95. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 20.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–94. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 21.Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 22.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–42. [PubMed] [Google Scholar]
- 23.Shaw AR, Domanska A, Mak A, et al. Ectopic expression of human and feline CD9 in a human B cell line confers beta 1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem. 1995;270:24092–9. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- 24.Lagaudriere-Gesbert C, Le Naour F, Lebel-Binay S, et al. Functional analysis of four tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in costimulation, cell adhesion, and migration: only CD9 upregulates HB-EGF activity. Cell Immunol. 1997;182:105–12. doi: 10.1006/cimm.1997.1223. [DOI] [PubMed] [Google Scholar]
- 25.Miyake M, Koyama M, Seno M, Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31–15, which inhibits cell motility. J Exp Med. 1991;174:1347–54. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyake M, Nakano K, Ieki Y, et al. Motility related protein 1 (MRP-1/CD9) expression: inverse correlation with metastases in breast cancer. Cancer Res. 1995;55:4127–31. [PubMed] [Google Scholar]
- 27.Miyake M, Nakano K, Itoi SI, Koh T, Taki T. Motility-related protein-1 (MRP-1/CD9) reduction as a factor of poor prognosis in breast cancer. Cancer Res. 1996;56:1244–9. [PubMed] [Google Scholar]
- 28.Mori M, Mimori K, Shiraishi T, et al. Motility related protein 1 (MRP1/CD9) expression in colon cancer. Clin Cancer Res. 1998;4:1507–10. [PubMed] [Google Scholar]
- 29.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–21. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 30.Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–4. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 31.Kaji K, Oda S, Shikano T, et al. The gamete fusion process is defective in eggs of CD9-deficient mice. Nat Genet. 2000;24:279–82. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- 32.Tai XG, Yashiro Y, Abe R, et al. A role for CD9 molecules in T cell activation. J Exp Med. 1996;184:753–8. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai XG, Toyooka K, Yashiro Y, et al. CD9-mediated costimulation of TCR-triggered naive T cells leads to activation followed by apoptosis. J Immunol. 1997;159:3799–807. [PubMed] [Google Scholar]
- 34.Miyazaki T, Muller U, Campbell KS. Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 1997;16:4217–25. doi: 10.1093/emboj/16.14.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knobeloch KP, Wright MD, Ochsenbein AF, et al. Targeted inactivation of the tetraspanin CD37 impairs T-cell-dependent B-cell response under suboptimal costimulatory conditions. Mol Cell Biol. 2000;20:5363–9. doi: 10.1128/mcb.20.15.5363-5369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CS, Yashiro Y, Tai XG, et al. Differential involvement of a Fas-CPP32-like protease pathway in apoptosis of TCR/CD9-costimulated, naive T cells and TCR-restimulated, activated T cells. J Immunol. 1998;160:5790–6. [PubMed] [Google Scholar]
- 37.Yashiro Y, Tai XG, Toyo-oka K, et al. A fundamental difference in the capacity to induce proliferation of naive T cells between CD28 and other co-stimulatory molecules. Eur J Immunol. 1998;28:926–35. doi: 10.1002/(SICI)1521-4141(199803)28:03<926::AID-IMMU926>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura T, Onishi M, Kinoshita S, Shibuya A, Miyajima A, Nolan GP. Efficient screening of retroviral cDNA expression libraries. Proc Natl Acad Sci USA. 1995;92:9146–50. doi: 10.1073/pnas.92.20.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onishi M, Kinoshita S, Morikawa Y, et al. Applications of retrovirus-mediated expression cloning. Exp Hematol. 1996;24:324–9. [PubMed] [Google Scholar]
- 40.Deng HK, Unutmaz D, Kewa I, Ramani VN, Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 41.Hattori N, Kuwana M, Kaburaki J, Mimori T, Ikeda Y, Kawakami Y. T cells that are autoreactive to beta2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 2000;43:65–75. doi: 10.1002/1529-0131(200001)43:1<65::AID-ANR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 42.Ono M, Handa K, Withers DA, Hakomori S. Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation. Cancer Res. 1999;59:2335–9. [PubMed] [Google Scholar]
- 43.Kersey JH, LeBien TW, Abramson CS, Newman R, Sutherland R, Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981;153:726–31. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowell BL, Tuck FL, Borowitz MJ, LeBien TW, Metzgar RS. Phylogenetic distribution of a 24,000 dalton human leukemia-associated antigen on platelets and kidney cells. Dev Comp Immunol. 1984;8:187–95. doi: 10.1016/0145-305x(84)90022-3. [DOI] [PubMed] [Google Scholar]
- 45.Oritani K, Wu X, Medina K, et al. Antibody ligation of CD9 modifies production of myeloid cells in long- term cultures. Blood. 1996;87:2252–61. [PubMed] [Google Scholar]
- 46.Lebel-Binay S, Lagaudriere C, Fradelizi D, Conjeaud H. CD82, member of the tetra-span-transmembrane protein family, is a costimulatory protein for T cell activation. J Immunol. 1995;155:101–10. [PubMed] [Google Scholar]
- 47.Shibagaki N, Hanada K, Yamaguchi S, Yamashita H, Shimada S, Hamada H. Functional analysis of CD82 in the early phase of T cell activation: roles in cell adhesion and signal transduction. Eur J Immunol. 1998;28:1125–33. doi: 10.1002/(SICI)1521-4141(199804)28:04<1125::AID-IMMU1125>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 48.Waterhouse R, Ha C, Dveksler GS. Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med. 2002;195:277–82. doi: 10.1084/jem.20011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13:687–98. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]