Abstract
The pathophysiological and immunological characteristics of allergic immune responses are controlled by a variety of factors. We have studied the extent to which the route of sensitization influences allergen-specific IgE synthesis and local airway inflammation using a mouse model of allergic sensitization to the major birch pollen allergen Bet v 1. Sensitization of BALB/c mice with recombinant (r)Bet v 1 was performed using intraperitoneal (IP), subcutaneous (SC) or aerosol (AS) sensitization protocols. Mice were analysed for allergen-specific serum antibodies by ELISA and IgE-dependent basophil degranulation. Proliferative responses and cytokine production of splenocytes were measured upon Bet v 1 stimulation in vitro. Bronchoalveolar lavages were performed after airway challenge with aerosolized birch pollen extract for assessment of eosinophilic airway inflammation and local cytokine production in vivo. Highest allergen specific IgE levels and IgE-dependent basophil degranulation were achieved using the SC route. High IL-5 production by spleen and lung cells was associated with pronounced eosinophilia in bronchoalveolar lavages. After IP sensitization, despite giving the highest IgG levels, only low IgE levels, basophil degranulation and IL-5 production were seen. On the other hand, AS sensitization, resulting in the lowest systemic IgE and IL-5 levels, led to a comparably strong airway inflammation as the SC route. Our finding that the route of sensitization can result in a dissociation of local and systemic immune responses may contribute to a better understanding of the pathogenesis of allergic diseases and help to develop new treatment strategies.
Keywords: type I allergy, murine model, sensitization, route, local immune response, systemic immune response
INTRODUCTION
Allergic diseases are characterized by the development of pathologic immune responses against normally innocuous molecules (allergens), involving complex interactions between exogenous and genetically determined factors.
The development of allergen-specific IgE responses represents a hallmark of type I allergy. It is closely linked to allergen-specific T helper (Th) cell responses, characterized by a predominant production of type-2 cytokines (Interleukin (IL)-4, IL-5 and IL-13), as it is seen at sites of acute allergic inflammation [1]. IL-4, produced by Th2 cells, induces germ line transcription of Cɛ in human and additionally Cγ1 exons in murine B cells, leading to IgE and IgG1 antibody class switching [2], whereas IL-13 is required for optimal induction of IgE synthesis, particularly in situations where IL-4 production is low [3]. Moreover, IL-13 can induce IgE class switching of human B cells in the absence of IL-4 [4] and increased levels of IgE are found in IL-4 deficient mice carrying IL-13 transgenes [5]. Once allergen-specific IgE antibodies are bound to basophils and tissue mast cells, allergen contact leads to cross-linkage of IgE and release of histamine, leukotrienes and other mediators of acute allergic inflammation.
Allergic asthma is characterized by bronchial inflammation and airway hyperreactivity. Eosinophilic infiltration into the airways represents a hallmark of allergic asthma. Large numbers of eosinophil granulocytes can be found in bronchoalveolar lavages (BAL) [6] and induced sputum of allergic patients [7] after airway challenge with an allergen. Recruitment of eosinophils to sites of allergic inflammation is closely related to production of IL-5 by Th2 lymphocytes and activated mast cells [8]. Furthermore, allergen challenge induces local production of the chemokine CCL11 (eotaxin) by airway epithelial cells and macrophages, which selectively recruits eosinophils via its receptor CCR3 to the lungs [9]. Additionally, the CCR3/eotaxin axis contributes to further recruitment of Th2 lymphocytes to sites of allergic inflammation [10]. In asthmatic subjects, high levels of both IL-5 and eotaxin can be found in the sputum after spontaneous exacerbation [11] or allergen inhalation [12,13]. Additionally IL-13 is believed to play a central role in allergic airway inflammation and hyperreactivity [14].
Knowledge of the factors controlling allergic immune responses is essential for our understanding of the pathogenesis of allergic diseases. Important information about the pathogenesis of type I allergy is derived from animal models [15–17]. The level and quality of the immune response in such models is controlled by a variety of factors such as the dose and frequency of exposure to the allergen, the type of adjuvant [18] and the genetic background of the mouse strain [19]. We have previously shown that BALB/c mice are high IgE responders to the clinically relevant aeroallergen Bet v 1 [18]. Now we were interested in evaluating the influence of the route of sensitization on the development of IgE antibody responses and allergic airway inflammation using a mouse model of type I allergy to the major birch pollen allergen Bet v 1.
MATERIALS AND METHODS
Animals
Female, 7-week-old BALB/c mice were obtained from Charles River (Sulzfeld, Germany). The experiments were approved by the Animal Experimentation Ethics Committee of the University of Vienna and the Ministry of Education, Science and Culture.
Antigens
Recombinant (r)Bet v 1 was obtained from Biomay GmbH (Vienna, Austria). Birch pollen (BP) (Allergon AB, Engelholm, Sweden) was used for preparation of an aqueous extract and total protein content was determined as previously described [18].
Experimental design
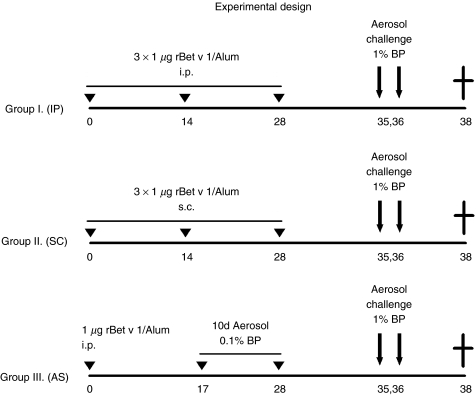
To compare the influence of the route of sensitization on the allergic immune responses, three different approaches were followed. One group of mice (group I) (n = 10) was sensitized via the intraperitoneal (IP) route by three IP injections with 1 µg of rBet v 1 adsorbed to 2 mg Al(OH)3 (Serva, Heidelberg, Germany) in a total volume of 150 µl. Another group (group II) of mice (n = 8) was subcutaneously (SC) injected three times with the same amount of antigen adsorbed to 2 mg Al(OH)3; the third group (group III) of mice (n = 7) was sensitized via an aerosol of BP extract solution (aerosol sensitization, AS), as previously described [18]. Briefly, mice were exposed 10 times to aerosolized 0·1% BP extract (4 ml of 1 mg/ml) in an aerosol chamber, subsequent to an initial priming by a single IP injection with 1 µg rBet v 1/Al(OH)3(Fig. 1).
Fig. 1.
Experimental design. BALB/c mice were sensitized to Bet v 1 via three different routes. Group I (IP) received three intraperitoneal injections of 1 µg rBet v 1/Al(OH)3, group II (SC) three subcutaneous injections of 1 µg rBet v 1/Al(OH)3, and group III (AS) was exposed 10 times to an aerosol of 0·1% birch pollen (BP) extract solution, subsequent to a single IP injection with 1 µg rBet v 1/Al(OH)3. For induction of allergic airway inflammation mice were aerosol challenged twice with 1% BP extract solution. At sacrifice (day 38), bronchoalveolar lavages were taken for analysis of eosinophilic inflammation and cytokine analysis (IL-5, eotaxin). Spleens were removed for in vitro proliferation and cytokine assays. Blood samples for measurement of Bet v 1-specific antibody levels were taken on day 0 and 37.
For induction of allergic airway inflammation mice were challenged twice (day 35 and 36) with an aerosol of a 1% BP extract solution (4 ml of 10 mg/ml).
Sampling
Blood samples were taken by tail bleeding at day 0 from untreated mice and one day before the mice were killed (day 37, Fig. 1). Sera were prepared for measurement of allergen-specific antibodies and performance of rat basophil release assays, and stored at –20°C until analysis.
Spleens were removed under sterile conditions and cell suspensions were prepared as previously described [18]. Briefly, the organs were homogenized, filtered through sterile nylon cell strainers and red blood cells were lysed. The cells were washed and re-suspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 0·1 mg/ml gentamicin, 2 mm glutamine and 50 µm 2-mercaptoethanol.
Bronchoalveolar lavages (BAL) were performed using a blunted 23 Gauge needle. Lungs were flushed twice with 0·8 ml ice-cold PBS and approximately 1·4 ml of clear BAL fluid was recovered for assessment of eosinophilic airway inflammation and local cytokine production (IL-5, eotaxin).
Allergen-specific serum antibody levels
Allergen-specific antibody levels were determined by ELISA, as previously described [18]. Microtitre plates (Nunc-Immuno Plate) were coated with rBet v 1 (2 µg/ml). Serum samples were diluted 1/1000 for IgG1, 1/500 for IgG2a and 1/10 for IgE. Rat anti-mouse Immunoglobulin (Ig)G1, IgG2a, and IgE antibodies (1 µg/ml, Pharmingen, San Diego, CA, USA) were applied and peroxidase-conjugated mouse anti-rat IgG antibodies (1/1000; Jackson, Immuno Laboratories Inc, West Grove, PA, USA) were used for detection. The rat anti-mouse IgE antibody was tested for cross-reactivity with mouse IgG1 using a monoclonal purified mouse IgG1 (BIP-1) [20]. Antibody levels were expressed as arbitrary ELISA-units, which were calculated from a standard curve obtained with a standard serum pool of rBet v 1 immunized mice.
Rat basophil leukaemia (RBL) cell degranulation
The allergenic antibody serum activity was determined as previously described [21]. Briefly, RBL-2H3 cells were plated in 96 well tissue culture plates (4 × 104 cells per well) and passively sensitized by incubation with serum samples of the respective experimental groups or serum of naïve control mice in final dilutions of 1/10, 1/30, 1/90 and 1/270 for two hours. Unbound antibodies were removed by two washings with Tyrode's buffer and degranulation was induced by addition of rBet v 1 (0·3 µg/ml). The supernatants were analysed for β-hexosaminidase content by incubation with 80 µm 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide (Sigma) in citrate buffer (0·1 m, pH 4·5) and fluorescence was measured at λex: 360 nm/λem: 465 nm using a fluorescence microplate reader (Cytofluor 2350, Millipore, MA, USA). Results are reported as the percentage of total β-hexosaminidase release after addition of 1% Triton X-100.
Allergen specific stimulation of splenocytes
Proliferative responses.
For determination of allergen-specific lympho-proliferation, spleen cell suspensions of the experimental groups and naïve mice were stimulated with or without rBet v 1 (2 µg/well) for 5 days and lymphocyte proliferation was measured by 3H-thymidine incorporation as previously described [18]. For epitope mapping experiments, pools of spleen cells of the respective experimental groups were stimulated with overlapping dodecapeptides, covering the whole sequence of Bet v 1, as previously described [22].
Cytokine production.
Cytokine production upon in vitro re-stimulation with rBet v 1 was measured in spleen cell cultures of the different experimental groups and naïve mice. Splenocytes were cultured in 48-well flat bottom plates at a concentration of 5 × 106 cells/500 µl with or without rBet v 1 (10 µg/well). Supernatants were taken after 48 h for measurement of IL-4, IL-5, IL-13, eotaxin and interferon (IFN)-γ.
Cytokine analysis
IFN-γ was measured by ELISA as previously described [23]. All other cytokines were measured with commercially available mouse ELISA kits; IL-4 and IL-5 (Endogen, Woburn, MA, USA), IL-13 and eotaxin (R & D Systems, MN, USA), with sensitivities of <5 pg/ml for IL-4 and IL-5, <1·5 pg/ml for IL-13 and <3 pg/ml for eotaxin.
Eosinophilic airway inflammation
To evaluate allergic airway inflammation after allergen airway challenge, BAL fluid was collected two days after aerosol challenge with 1% BP extract, and 100 µl were spun onto microscope slides at 110 g for 3 min (Shandon Cytospin®, Shandon Southern Instruments, USA), dried overnight at room temperature and stained with a blood smear staining set (Hemacolor®, Merck, Darmstadt, Germany). At least 200 cells per slide were counted and differentiated by light microscopy according to standard morphologic criteria and the fraction of eosin-positive cells was determined and expressed as the percentage of total BAL cells.
Local cytokine production in the airways
BAL fluids of the respective groups were analysed for IL-5 and eotaxin by ELISA.
Statistics
Mann–Whitney U-test was used for all statistical analysis. Level of significance was set at 5% for all comparisons.
RESULTS
Allergen-specific serum antibody levels
The highest IgG1 (P < 0·01, Fig. 2a) and IgG2a levels (P < 0·05, Fig. 2b) were induced via the IP route, compared to animals sensitized via the SC route or the airways (AS).
Fig. 2.
Serum antibody responses. Allergen-specific antibody levels (IgG1, IgG2a and IgE) were measured in sera of mice sensitized with rBet v 1 intraperitoneally (IP), subcutaneously (SC) or via an aerosol (AS). Data are reported as individual values using arbitrary ELISA units. Horizontal bars represent the mean value of each group. **P < 0·01, *P < 0·05 as determined by Mann–Whitney U-test.
In terms of IgE, there was a tendency towards higher IgE levels in SC sensitized mice, which however, was not significant (Fig. 2c). No cross reactivity of the rat anti-mouse IgE antibody with mouse IgG1 was detected using a monoclonal mouse IgG1 (BIP-1) [20] (data not shown).
Rat basophil leukaemia (RBL) cell degranulation
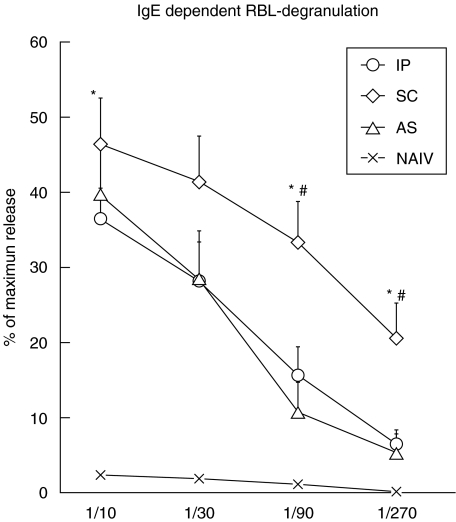
The highest allergenic activity of sera was found in mice sensitized via the SC route. IgE dependent degranulation, indicated by β-hexosaminidase release from the RBL-2H3 cells, was up to four-fold stronger than after IP (P < 0·05) and AS (P < 0·05) sensitization. Naïve control sera showed significantly lower release at all dilutions, indicating the specificity of the assay also at high dilutions (Fig. 3).
Fig. 3.
Rat basophil (RBL) degranulation assay. RBL cells were preincubated with sera of naïve control mice (NAIV) or mice sensitized with rBet v 1 intraperitoneally (IP), subcutaneously (SC) or via an aerosol (AS) in dilutions of 1/10, 1/30, 1/90 and 1/270. Degranulation of RBL cells loaded with mouse IgE was induced with rBet v 1 (0·3 µg/ml), and β-hexosaminidase release was measured and reported as the mean percentage of maximum release induced with 1% Triton X-100. Error bars indicate SEMs. *significantly higher than IP (P < 0·05), ♯significantly higher than AS (P < 0·05), as determined by Mann–Whitney U-test. At any dilution β-hexosaminidase release was significantly stronger with sera from the experimental groups (IP, SC, AS) compared to sera of naive mice (NAIV, not indicated).
Allergen specific stimulation of splenocytes
Proliferative responses.
Highest proliferative responses upon in vitro re-stimulation with rBet v 1 were seen with spleen cells from mice immunized via the SC route (Stimulation index (SI) = 2·2 ± 0·2 (SEM), whereas IP (SI = 1·6 ± 0·2 SEM) and AS (SI = 1·6 ± 0·2 SEM) sensitization led to lower proliferative responses. In comparison to spleen cells from naive mice (n = 13) stimulated with rBet v 1 (SI = 1·12 ± 0·11 SEM), proliferative responses of mice from all three experimental groups could be considered significant (P < 0·05). No differences between the groups were found in epitope mapping experiments, revealing a major T cell epitope at the C terminus of the Bet v 1 molecule as previously described [22,24].
Cytokine production.
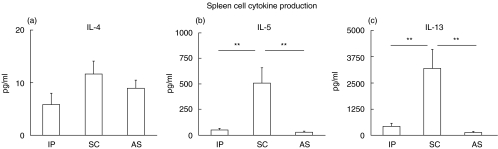
Spleen cells of mice sensitized via the SC route displayed the highest production of Th2 cytokines after rBet v 1 stimulation in vitro, compared to spleen cells from mice sensitized via the IP or AS route. IL-5 production of spleen cells (Fig. 4b) was 10-fold higher than in IP sensitized (P < 0·01), and 18-fold higher than in AS sensitized animals (P < 0·01). IL-13 levels (Fig. 4c) were 7 timers higher than after IP (P < 0·01) and 26 times higher than after AS sensitization (P < 0·01). Only low levels of IL-4 were produced by splenocytes of all groups with a tendency towards highest levels in the SC immunized group (Fig. 4a). IL-4 production of all three groups was significantly higher than in naïve mice stimulated with rBet v 1 (0·16 pg ± 0·19 SEM, P < 0·05). Mice sensitized via an aerosol (AS) generally showed the lowest spleen cell cytokine production (Fig. 4). The chemokine eotaxin was not detectable in any of the groups after 48 h stimulation with the allergen (data not shown). IFN-γ levels were low in all three groups (IP: 13·13 ± 3·8 pg/ml, SC: 9·53 ± 3·7 pg/ml, AS: 9·8 ± 3·1 pg/ml) indicating a Th2 pronounced immune response after sensitization via all three routes.
Fig. 4.
Spleen cell cytokine production. Spleen cells of mice sensitized with rBet v 1 intraperitoneally (IP), subcutaneously (SC) or via an aerosol (AS) were re-stimulated in vitro with rBet v 1 for 48 h. Cytokine levels (IL-4, IL-5 and IL-13) were measured in supernatants by ELISA. Data shown are mean values ± SEMs. **P < 0·01, as determined by Mann–Whitney U-test.
Local cytokine production in the airways
The highest local IL-5 production after airway challenge was detected in BAL fluids of SC (43·0 ± 25·0 pg/ml) and AS (27·2 ± 12·5 pg/ml) sensitized animals (Fig. 5b). IL-5 levels in BALs of IP sensitized mice were only low (4·8 ± 2·3 pg/ml). Local eotaxin production was highest in BALs of SC (15·1 ± 7·1 pg/ml) and AS (4·9 ± 3·0 pg/ml) sensitized mice, but remained undetectable in mice sensitized via the IP route (Fig. 5c).
Fig. 5.
Allergic airway inflammation. To evaluate allergic airway inflammation, mice were immunized with rBet v 1 intraperitoneally (IP), subcutaneously (SC) or via an aerosol (AS) and exposed to two consecutive aerosol challenges with 1% BP extract. Bronchoalveolar lavage (BAL) fluids were collected 48 h after challenge and evaluated for the percentage of eosinophil granulocytes of total BAL cells by light microscopy. Cytokine levels in BAL fluids (IL-5 and eotaxin) were measured by ELISA. Data shown are mean values ± SEMs. **P < 0·01, *P < 0·05 as determined by Mann–Whitney U-test.
Eosinophilic airway inflammation
According to the local IL-5 and eotaxin production, the highest percentages of eosinophilic granulocytes in BAL after challenge with aerosolized BP extract solution were found after SC (29·5%) and AS (23·0%) sensitization. On the other hand, in IP sensitized mice the percentage of eosinophils in BAL fluid was significantly lower (4·9%, Fig. 5a).
DISCUSSION
In this study we investigated the impact of different routes of sensitization on allergen-specific immune responses and allergic airway inflammation, using the major birch pollen allergen Bet v 1. We showed that the SC route was the most effective route for eliciting high IgE levels (Figs 2 and 3), preferential production of Th2 cytokines in spleen cells (Fig. 4), as well as allergic airway inflammation (Fig. 5). The latter was also induced after aerosol sensitization, although the systemic humoral and cellular Th2 biased immune responses were low. In case of IP sensitization IgG1 and IgG2a levels were profoundly induced, but IgE dependent basophil degranulation was only low and airway inflammation least expressed.
A major requirement for an experimental model of allergic sensitization is that it should mimic typical immunological features of human type I allergy/asthma, such as the presence of allergen-specific IgE, Th2 cytokines (i.e. IL-4, IL-5 and IL-13), and eosinophilic infiltration of the lungs. These characteristics were best achieved after immunization via the subcutaneous route. In this case not only the IgE levels were highest (Fig. 2), but also the IgE-dependent degranulation of rat basophils, equivalent to allergic manifestations in vivo, was most strongly expressed (Fig. 3). This was the case, even though IL-4 – a crucial cytokine for IgG1 and IgE class switch of murine B cells [2]- did not significantly differ between the SC-, AS- and IP-immunized mice and was generally measurable at low concentrations, but still within the detection limit of the ELISA (Fig. 4). However, since IL-13, known to selectively increase IgE antibody levels in vivo[5], was preferentially up-regulated in SC immunized mice, this might be good enough to explain the high IgE vs. relatively low IgG1 levels in this group. Our assumption is supported by a recent paper by Herrick et al. [25] showing that Th2 responses and airway inflammation after SC sensitization were inducible in IL-4 knock out mice and dependent on a sufficient production of IL-13. Another example for showing that the sub/epicutaneous route is favourable in inducing allergic immune responses was given by Nelde et al. [26] using ovalbumin as an antigen. In this study high IgE levels were also reached after airway sensitization via intranasal antigen instillation, which is in contrast to our findings where airway sensitization was less effective compared to the SC route. This might be explained by the fact that intranasal antigen instillation may deliver higher amounts of antigen than aerosol application to the sites of antigen uptake (personal observation).
Eosinophilic inflammation is a hallmark of allergic asthma. IL-5 and CCL11 (eotaxin) are important for the attraction and survival of eosinophils at sites of allergic inflammation [8,9]. In accordance with the high IgE antibody levels and systemic Th2 prone cytokine production, the IL-5 and eotaxin levels in BAL fluids were also highest after SC immunization (Fig. 5). Similarly, high IL-5 levels in BAL fluids, although lowest in spleen cell cultures, and enhanced eotaxin levels were found in AS sensitized mice after airway challenge. Consecutively, eosinophilic infiltration of the airways was comparably high as in SC sensitized mice. CCL11 (eotaxin) was only detectable in the BAL but not in spleen cell cultures of SC or AS immunized mice, probably reflecting the local production of eotaxin by airway epithelial cells in response to Th2 cytokines produced by local Th2 cells [27].Thus, the dissociation of local and systemic Th2 responses in AS sensitized mice indicates the establishment of a local Th2 response predominantly in the airways as previously described after aerosol sensitization with OVA [28]. Contrary to that, in IP sensitized mice the allergic immune responses were most reflected at the systemic site and not locally in the airways.
In order to address the question which factors might influence the differences of the immunological responses following these distinct routes of immunization, we compared the T cell epitope recognition pattern between the different groups. However, no differences in the epitope distribution were found. Most likely the influence of the microenvironment plays an important role in our model. Depending on the nature of the antigen presenting cell at the site of antigen contact, the resulting immune responses can differ in their quality. In this respect it has been shown that dendritic cells in the skin and the epithelium of the mucosa of the respiratory tract favour Th2 responses [29,30] as it excessively occurs in allergic diseases such as atopic dermatitis or allergic asthma, whereas macrophages (found in large numbers in the peritoneal cavity) tend to favour the induction of Th1/Th0 immune responses [31]. Our findings might be explained in a similar way.
In summary, the present study examined the influence of the route of allergic sensitization on systemic and local immune responses and inflammation. Besides the methodological description of our results, these findings might be taken into account when performing preclinical studies in mice on allergy and allergy treatment. Additionally, our study might give information about the influence of antigen delivery on the efficacy of a potential antiallergy treatment/vaccine.
Acknowledgments
The authors wish to thank Mrs Schwarz for dedicated animal care and Ms Hatak for perfect technical assistance. The work was supported by a grant from the Austrian Fund for Science and Research (SFB F01814).
REFERENCES
- 1.Hamid QA, Minshall E. In situ detection of cytokines in allergic inflammation. Adv Exp Med Biol. 1996;409:327–35. doi: 10.1007/978-1-4615-5855-2_46. [DOI] [PubMed] [Google Scholar]
- 2.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–70. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 3.de Vries JE. The role of IL-13 and its receptor in allergy and inflammatory responses. J Allergy Clin Immunol. 1998;102:165–9. doi: 10.1016/s0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- 4.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig) E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aalbers R, Kauffman HF, Vrugt B, Koeter GH, de Monchy JG. Allergen-induced recruitment of inflammatory cells in lavage 3 and 24 h after challenge in allergic asthmatic lungs. Chest. 1993;103:1178–84. doi: 10.1378/chest.103.4.1178. [DOI] [PubMed] [Google Scholar]
- 7.Gauvreau GM, Watson RM, O'Byrne PM. Kinetics of allergen-induced airway eosinophilic cytokine production and airway inflammation. Am J Respir Crit Care Med. 1999;160:640–7. doi: 10.1164/ajrccm.160.2.9809130. [DOI] [PubMed] [Google Scholar]
- 8.O'Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J Allergy Clin Immunol. 2001;108:503–8. doi: 10.1067/mai.2001.119149. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–8. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd CM, Delaney T, Nguyen T, et al. CC chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–74. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SW, Kim do J, Chang HS, et al. Association of interleukin-5 and eotaxin with acute exacerbation of asthma. Int Arch Allergy Immunol. 2003;131:283–90. doi: 10.1159/000072140. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Kleimberg J, Marini M, et al. Kinetics of eotaxin expression and its relationship to eosinophil accumulation and activation in bronchial biopsies and bronchoalveolar lavage (BAL) of asthmatic patients after allergen inhalation. Clin Exp Immunol. 1998;114:137–46. doi: 10.1046/j.1365-2249.1998.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulakvelidze I, Inman MD, Rerecich T, O'Byrne PM. Increases in airway eosinophils and interleukin-5 with minimal bronchoconstriction during repeated low-dose allergen challenge in atopic asthmatics. Eur Respir J. 1998;11:821–7. doi: 10.1183/09031936.98.11040821. [DOI] [PubMed] [Google Scholar]
- 14.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr Opin Pulm Med. 2003;9:21–7. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Marsella R, Olivry T. Animal models of atopic dermatitis. Clin Dermatol. 2003;21:122–33. doi: 10.1016/s0738-081x(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg-Feig H, Justice JP, Keane-Myers A. Animal models of allergic asthma. Curr Allergy Asthma Rep. 2003;3:70–8. doi: 10.1007/s11882-003-0015-8. [DOI] [PubMed] [Google Scholar]
- 17.Helm RM. Food allergy animal models: an overview. Ann N Y Acad Sci. 2002;964:139–50. doi: 10.1111/j.1749-6632.2002.tb04139.x. [DOI] [PubMed] [Google Scholar]
- 18.Wiedermann U, Jahn-Schmid B, Fritsch R, et al. Effects of adjuvants on the immune response to allergens in a murine model of allergen inhalation: cholera toxin induces a Th1-like response to Bet v, 1 the major birch pollen allergen. Clin Exp Immunol. 1998;111:144–51. doi: 10.1046/j.1365-2249.1998.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorham JD, Guler ML, Steen RG, et al. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci USA. 1996;93:12467–72. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarolim E, Tejkl M, Rohac M, et al. Monoclonal antibodies against birch pollen allergens: characterization by immunoblotting and use for single-step affinity purification of the major allergen Bet v 1. Int Arch Allergy Appl Immunol. 1989;90:54–60. doi: 10.1159/000235000. [DOI] [PubMed] [Google Scholar]
- 21.Repa A, Grangette C, Daniel C, et al. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine. 2003;22:87–95. doi: 10.1016/s0264-410x(03)00528-0. [DOI] [PubMed] [Google Scholar]
- 22.Bauer L, Bohle B, Jahn-Schmid B, et al. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin Exp Immunol. 1997;107:536–41. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkler B, Baier K, Wagner S, et al. Mucosal tolerance as therapy of type I allergy: intranasal application of recombinant Bet v 1, the major birch pollen allergen, leads to the suppression of allergic immune responses and airway inflammation in sensitized mice. Clin Exp Allergy. 2002;32:30–6. doi: 10.1046/j.0022-0477.2001.01214.x. [DOI] [PubMed] [Google Scholar]
- 24.Wiedermann U, Herz U, Baier K, et al. Intranasal treatment with a recombinant hypoallergenic derivative of the major birch pollen allergen Bet v 1 prevents allergic sensitization and airway inflammation in mice. Int Arch Allergy Immunol. 2001;126:68–77. doi: 10.1159/000049496. [DOI] [PubMed] [Google Scholar]
- 25.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–75. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelde A, Teufel M, Hahn C, et al. The impact of the route and frequency of antigen exposure on the IgE response in allergy. Int Arch Allergy Immunol. 2001;124:461–9. doi: 10.1159/000053781. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Xia Y, Nguyen A, et al. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999;162:2477–87. [PubMed] [Google Scholar]
- 28.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002;169:4788–96. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 29.Banfield CC, Callard RE, Harper JI. The role of cutaneous dendritic cells in the immunopathogenesis of atopic dermatitis. Br J Dermatol. 2001;144:940–6. doi: 10.1046/j.1365-2133.2001.04179.x. [DOI] [PubMed] [Google Scholar]
- 30.Upham JW, Stumbles PA. Why are dendritic cells important in allergic diseases of the respiratory tract? Pharmacol Ther. 2003;100:75–87. doi: 10.1016/s0163-7258(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz J, Assenmacher M, Radbruch A. Regulation of T helper cell cytokine expression: functional dichotomy of antigen-presenting cells. Eur J Immunol. 1993;23:191–9. doi: 10.1002/eji.1830230130. [DOI] [PubMed] [Google Scholar]