Abstract
Maternal anti-HPA-1a antibodies can cause severe fetal and neonatal alloimmune thrombocytopenia (FNAIT), complicated by intracranial haemorrhage (ICH). Antenatal treatment with maternal intravenous immunoglobulin (IVIG) seems to protect against ICH even when thrombocytopenia persists. The aim of this study was to investigate if anti-HPA-1a antibodies and IVIG potentially affect vascular endothelial cells (ECs) in order to identify susceptibility for ICH. Human umbilical cord endothelial cells (HUVEC) were incubated with anti-HPA-1a antibodies with or without polyclonal IVIG and evaluated for EC activation. Maternal sera with anti-HPA-1a antibodies affected neither the EC expression of intracellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and tissue factor (TF) nor the release of van Willebrand factor (vWF) or interleukin (IL)-8 nor the integrity of ECs. Maternal sera obtained after IVIG treatment and polyclonal IVIG decrease constitutive and cytokine-induced ICAM-1 and VCAM-1 expression on ECs. The results show that maternal anti-HPA-1a antibodies cause no activation or damage of ECs in this model. The clinical relevance of the de-activating properties of IVIG on EC activation with respect to ICH deserves further investigation.
Keywords: fetal or neonatal alloimmune thrombocytopenia, intracranial haemorrhage, HPA-1a, IVIG, vascular endothelium
INTRODUCTION
Fetal or neonatal alloimmune thrombocytopenia (FNAIT) is caused by transplacental transport of maternal IgG-antibodies to polymorphic human platelet specific antigens (HPA) expressed on fetal platelets. In the Caucasian population the immunodominant antigen is HPA-1a, causing 75–95% of FNAIT [1,2]. The biallelic HPA-1 is localized on glycoprotein (GP)IIIa (CD61) of the GPIIb/IIIa-complex [3,4]. This complex appears on the cell membrane of fetal platelets [5] and on the vascular endothelial cells (ECs) of the umbilical cord [3].
The estimated incidence of HPA-1a antibodies is 0·1–0·2%[6–8]. Thrombocytopenia, however, occurs in less than 10% of women with HPA-1a-antibodies [8,9]. The main complication of FNAIT is antenatal intracranial haemorrhage (ICH), which affects 7–26% of the children [1,10–10] and accounts for a high morbidity and mortality [11,13]. High-dose intravenous immunoglobulin (IVIG) weekly administered to the mother increases the fetal platelets in approximately 60% of the cases. There is clinical experience that IVIG might protect the fetus against ICH, even in cases with persistent thrombocytopenia [12,14,15], although the mechanism of this presumed protection remains unknown.
Probably additional repetitive risk factors are involved in the pathogenesis of ICH. First, the titre, IgG-subclass, or ability to activate complement of HPA-antibodies might be involved [9,10,16] or the co-existence of HLA antibodies, which enhance immune-mediated damage induced by HPA-antibodies [9,17–19]. Secondly, differences in the GPIIIa-domains recognized by anti-HPA-antibodies might influence the manifestation of the disease [4] and thirdly, as the GPIIb/IIIa-complex is expressed on ECs, binding of anti-HPA antibodies to HPA-antigen on the endothelial surface might induce vascular tissue damage [20–24].
The aim of this study was to investigate the effect of anti-HPA-1a antibodies and potential additional ICH risk factors on ECs as a model to unravel susceptibility for ICH in FNAIT. Therefore, we compared HPA-1a antigen expression on cultures of human ECs isolated from healthy newborns, we studied the effect of binding of maternal HPA-1a-antibodies on various characteristic EC functions and we examined the effect of IVIG on these EC functions.
MATERIALS AND METHODS
Monoclonal antibodies
The following monoclonal antibodies (MoAb) were used: human anti-HPA-1a-MoAb Camtran-007 (IgG1; East Anglia Blood Centre, Cambridge, UK) [25]; mouse antihuman GPIIIa (CD61) MoAb CLB-thromb/1, C17 (IgG1; Sanquin-CLB Reagents, Amsterdam, the Netherlands); mouse antihuman HLA class I MoAb W6/32 [26] (IgG2b); mouse MoAb MEM 112 (IgG1; Sanbio BV, Uden, the Netherlands) against human intracellular adhesion molecule-1 (ICAM-1, CD54); mouse MoAb 1.G11B1 (IgG1; Biosource International, Camarillo, CA, USA) against human vascular cell adhesion molecule-1 (VCAM-1, CD106); mouse MoAb TF9–10H10 (IgG1; OMNILabo International BV, Breda, the Netherlands) against the human coagulation molecule tissue factor (TF, CD142); human MoAb SN66E3 (IgM) specific for HLA-A2/A28 (class I) and human MoAb WK4E3 (IgM) specific for HLA-A (non-A1/A24, class I) [27] (from A. Mulder, department of Immunohaematology and Blood Bank, LUMC, Leiden, the Netherlands). For flow cytometry we used goat antimouse and goat antihuman phycoerythrin (PE)-conjugated immunoglobulin (Ig) (Southern Biotechnology Associates Inc., Birmingham, AL, USA).
Serum samples and IVIG
Maternal serum samples were selected from a panel of mothers with FNAIT due to anti-HPA-1a-antibodies (n = 19). The patient's characteristics are listed in Table 1. Most mothers (n = 15) were treated with freeze-dried Immunoglobulin I.V.® (human Ig; Sanquin-CLB). Three mothers were treated with liquid Ivegam® (Sanquin-CLB) (cases 6, 7, 9; Table 1) and one with Octagam® (human Ig, OCTA pharma, Brussels, Belgium) (case 17; Table 1). IVIG was administered weekly (1 g/kg/body weight) with a median period of 5 weeks (3–8 weeks). Samples of the IVIG products used were collected simultaneously with maternal sera, which were taken before the first gift and after several weeks of IVIG treatment, and both stored at –70°C. The hospital's ethics committee approved the study and informed consent was obtained in each case. Presence of HPA-1a-antibodies in the maternal sera was detected by the monoclonal antibody-specific immobilization of platelet antigens (MAIPA) test [2,28] or solid phase-enzyme-linked immunosorbent assay (ELISA) to platelet glycoproteins and HLA class I antigens (GTI-PAK 12, GTI, Brookfield, WI, USA). In addition, the sera were tested for presence of HLA antibodies by a standard complement-dependent cytotoxicity assay and a solid phase ELISA (Quikscreen, GTI) (Table 1).
Table 1. Clinical characteristics of maternal serum samples in HUVEC experiments.
| Obstetric history | Index pregnancy | |||||
|---|---|---|---|---|---|---|
| case* | ICH in previous pregnancyaa –/+b | Platelet count previous childc (< or >50 × 109/l) –/+b | Titre of HPA-1a-Absd pre-IVIGf (1)e | Titre of HPA-1a-Absd post-IVIGf (1)e | HLA-Absd +/–/±b | Platelet count in index pregnancyc (< or >50 × 109/l) –/+b |
| 1 | + | – | 64 | 40 | – | – |
| 2 | – | – | 80 | 128 | + | – |
| 3 | – | – | 10 | 32 | + | + |
| 4 | + | – | 40 | 64 | – | – |
| 5 | + | – | 10 | 8 | + | + |
| 6 | – (†) | – | 40 | 32 | ± | – |
| 7 | – | – | 4 | 32 | ± | – |
| 8 | – | – | 32 | 16 | – | – |
| 9 | – | – | 4 | 4 | – | – |
| 10 | – | – | 128 | 32 | – | – |
| 11 | – | – | 32 | 32 | – | + |
| 12 | – | – | 40 | 16 | – | + |
| 13 | – | – | 10 | 4 | – | + |
| 14 | – | – | 32 | 16 | – | + |
| 15 | – | + | 32 | 16 | – | + |
| 16 | +(†) | – | 32 | 32 | – | + |
| 17 | – | – | 40 | 16 | ± | + |
| 18 | – | – | 4 | 4 | ± | + |
| 19 | – | – | 64 | 8 | – | + |
Serum sample of mothers that have been treated intravenously with immunoglobulin G (IVIG) during the pregnancy of a child at risk of alloimmune thrombocytopenia;
occurrence of intracranial haemorrhage (ICH) in a previous pregnancy due to severe alloimmune thrombocytopenia; Abs = antibodies;
+ = yes or presence, –= no or absence, ± = weak presence;
platelet count at birth, <50 = –, >50 = +;
anti-HPA-1a- or HLA class I-antibodies;
titre of antibodies, for example 1 in 32;
pre-IVIG = serum sample taken before start of IVIG treatment, post-IVIG = serum sample taken after IVIG-treatment;
= deceased.
Endothelial cells
HUVECs and HUAECs were isolated from human umbilical cord veins and arteries, respectively, from healthy newborns with blood group O or A, as described by Beekhuizen et al. [29]. ECs were cultured on 0·75% gelatine-coated (Clostridiopeptidase A, Sigma Chemicals Co., St Louis, Missouri, USA) tissue culture dishes (Falcon; Becton Dickinson BV, Alphen a/d Rijn, the Netherlands) in EC growth medium (ECGM) consisting of M199 (Biowhittaker Europe, Verviers, Belgium), supplemented with 10% 56°C heat-inactivated AB serum from non-immunized donors (HuSi), 1 mm/ml l-glutamine (Flow Laboratories, Irvine, UK), 0·1 mg/ml EC growth factor from calf hypothalamus, 5 U/ml heparin, 0·1 mg/ml streptomycin (Gist-brocades NV, Delft, the Netherlands), 100 U/ml fungizone (amphotericin B, Squibb BV, Rijswijk, the Netherlands) and 100 U/ml penicillin (Brocades Pharma BV, Leiderdorp, the Netherlands) in a 5% CO2 incubator at 37°C. At confluence these primary cultures were harvested with 0·05% (w/v) trypsin (Difco Laboratories, Detroit, Michigan, USA) and 0·01% (w/v) EDTA (Roche Diagnostics BV, Almere, the Netherlands) in phosphate buffered saline (PBS) and grown to confluence (i.e. secondary cultures) in gelatine-coated culture dishes or culture plates (Corning Costar Europe, Badhoevedorp, the Netherlands). Experiments were performed with primary, secondary or tertiary post confluent monolayers of ECs. HPA-1a and -b and HLA class I typing was performed on secondary cultures of HUVEC after trypsinization by PCR with sequence-specific primers [30,31].
Pretreatment of HUVEC
Confluent secondary cultures of HPA-1a-positive HUVECs in 24-well tissue culture plates (cell density about 2 × 105 cells per well), were incubated for 24 h in ECGM with 10% heat-inactivated FCS (Live Technologies BV, Breda, the Netherlands) to replace possible traces of human Ig. Then cultures were incubated in duplicate in 300 µl plain incubation medium (ECGM/10% fresh non-heat-inactivated AB HuS) or ECGM supplemented with 10% fresh non-heat-inactivated maternal serum (cases 1–6; Table 1) taken before (pre-IVIG serum) or after IVIG treatment (post-IVIG serum), 10% polyclonal IVIG (30 g/l) used as treatment or mixtures of pretreatment sera with IVIG, 3 µg/ml anti-HPA-1a MoAb (optimal signal/background-concentration of a range of 0·3–3 µg/ml) or 2 µg/ml anti-HLA class-I MoAb (W6/32). 500 U/ml rHu-tumour necrosis factor (TNF)-α (R&D Systems, Abingdon, UK) served as positive control [32,33]. In some experiments ECs were (pre)activated by incubation for 12–16 h with 50 U/ml rHu-interferon (IFN)-γ (TNO, Rijswijk, the Netherlands). Unstimulated ECs were exposed only to plain incubation medium (i.e. ECGM with non-heat-inactivated HuS). Maternal sera and HUVECs were selected for ABO-compatibility. For evaluation of HLA antibodies in EC activation HLA-A2 typed HUVEC cultures and human MoAbs SN66E3 or WK4E3 recognizing HLA-A2 were used. To study the effect on EC activation of crosslinking of HPA-1a antigens on the surface of ECs, HUVECs were incubated for 30 min with anti-HPA-1a MoAb Camtran-007, washed and incubated for 30 min with goat antihuman Ig. After 24 h of incubation, supernatants of cell cultures were collected and stored at –70°C. ECs were trypsinized and prepared for flow cytometry.
Flow cytometric analysis of endothelial surface expression
HUVEC or HUAEC cultures were trypsinized, collected in cold (4°C) PBS with 10% heat-inactivated FCS, washed in cold PBS with 1% inactivated FCS (wash buffer) and incubated for 15 min with 1% goat serum in wash buffer. After washing in cold wash buffer, cells were incubated for 30 min with MoAb, 3 µg/ml for anti-HPA-1a and 1 µg/ml for ICAM-1, VCAM-1 and TF MoAb. Anti-CD61 MoAb was used as reference for GPIIIa expression. After two washes, ECs were incubated with 2 µg/ml of the appropriate goat-PE-conjugated Ig for 30 min and washed again. Then 5000 cells were analysed by flow cytometry, using a FACScan flow cytometer (Becton Dickinson). ECs treated with PE-conjugated-Ig alone served as control to set background fluorescence.
Analysis of interleukin (IL)-8, VWF and LDH release
In thawed supernatants, harvested after various treatments, IL-8 was measured by ELISA using the Pelikine Compact™ human IL-8 ELISA kit (Sanquin-CLB). vWF was measured by ELISA according to the method described by Kamphuisen et al. [34]. A standard concentration curve for vWF was prepared with vWF isolated from a pool of normal human plasma, and was found to be linear from 0·035 to 2·24 U/ml vWF antigen. Presence of LDH activity to determine EC damage, resulting in loss of EC monolayer integrity, was analysed using a commercial LDH-MPR2-kit (Roche Diagnostics BV) [35]. Data were calculated according to the formula: percentage specific release = [(experimental release – spontaneous release)/(maximal release – spontaneous release) × 100%], where maximal release was produced by 10 min incubation of the cells with Triton 2% in PBS and spontaneous release by a sample of ECGM before EC culture.
Statistical analysis
SPSS version 10·0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data expressed are the means ± s.e.m. of individual experiments with ECs from different donors, unless stated otherwise. Differences between the results of the various experiments were analysed after logarithmic transformation by means of anova, paired-samples t-test, independent-samples t-test and linear regression. The level of significance was set at P < 0·05.
RESULTS
HPA-1a-antigen expression on HUVEC and HUAEC
HPA-1a on HUVEC is expressed invariably among 31 randomly chosen healthy newborns. The MFI ± s.e.m. representing HPA-1a surface expression is 81·2 ± 7·9 and that representing CD61 expression is 37·2 ± 3·2. A significant association (95% CI = 0·6–1·3; P < 0·001) was observed between both antigens. As could be expected, no HPA-1a negative HUVECs were found. The level of expression of HPA-1a and CD61 is not influenced by subculture procedures (data not shown). The HPA-1a surface expression on HUAEC is 68·5 ± 16·9 (MFI ± s.e.m., n = 4), being slightly lower than that on HUVEC, whereas the CD61 expression on HUAEC is 65·1 ± 27·7 (n = 4), which is higher than its expression on HUVEC (data not shown).
Effect of maternal sera on EC functions
Post-confluent monolayers of HUVEC were incubated with the maternal pre-IVIG sera for 24 h and evaluated by light microscopy. No morphological changes of individual cells or cobblestone-like organization of the cell monolayer were observed (not shown). In contrast, incubation of HUVEC cultures for 24 h with 500 U/ml rHu-TNF-α results in a significant number of cells with an elongated or spindle-shaped appearance, indicating cellular activation (data not shown) [36]. Possible cytolysis of ECs was measured by the release of cytosolic LDH into the culture supernatant. LDH release by untreated, i.e. unstimulated, monolayers of HUVEC was very low and was not increased after 24 h exposure to maternal anti-HPA-1a antibodies, anti-HPA-1a MoAb (3 µg/ml) or 500 U/ml rHu-TNF-α[37]. Slightly elevated concentrations of cytosolic LDH were measured only after incubation with a high dose (104 U/ml) rHu-TNF-α (data not shown). In addition, none of the antibodies, either maternal or monoclonal, caused an increase of the basal level of secretion of vWF, i.e. 0·3 ± 0·03 U/ml in 24 h, confirming the absence of EC damage [38,39]. Next, we explored the possible effect of maternal sera on EC activation, reflected by increase in surface expression of adhesion molecules ICAM-1 and VCAM-1 [32,40], pro-coagulant molecule TF [39] and secretion of the proinflammatory chemokine IL-8 [41]. Unstimulated HUVEC expresses a moderate constitutive level of ICAM-1 (MFI ± s.e.m. of 37·5 ± 7·8; n = 5), a very low level of VCAM-1 (MFI ± s.e.m. of 17·7 ± 3·1; n = 5) and negligible amounts of TF (MFI ± s.e.m. of 12·1 ± 1·4; n = 3) on the cell surface. The cells secrete about 5·7 ± 1·5 ng/ml IL-8 (n = 5) per well. All these values remained unchanged after incubation of ECs from eight different donors with maternal anti-HPA-1a sera with or without concomitant anti-HLA class I antibodies (cases 1–6; Table 1) (data not shown). Similarly, incubation of HLA-A2 positive, HPA-1a positive HUVEC (n = 3) with anti-HPA-1a MoAb in combination with human anti-HLA-A2 MoAb revealed no effect on the expression of ICAM-1 and VCAM-1 (data not shown). Cross-linking of HPA-1a antigens on the surface of ECs by anti-HPA-1a plus anti-Hu-Ig did not change the amount of released IL-8 (5·5 ± 0·4 ng/ml) within 24 h of culture (data not shown). When the same EC cultures, however, were exposed to 500 U/ml rHu-TNF-α, surface expression of TF significantly increased to 72·0 ± 0·7 MFI ± s.e.m. (P = 0·02; n = 3) within 6 h. The expression of ICAM-1 and VCAM-1 increased significantly to 154·3 ± 34·0 and 232·7 ± 74·8 MFI ± s.e.m., respectively (P = 0·00; n = 5) within 24 h and IL-8 levels also increased about sevenfold up to 42·5 ± 6·4 ng/ml (P = 0·00; n = 5) within 24 h after stimulation with TNF-α[42].
Effect of IVIG on EC functions
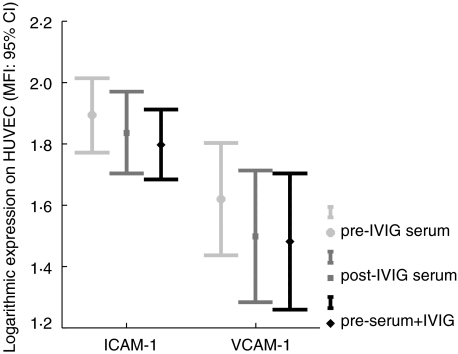
Exposure of monolayers of HUVEC for 24 h to medium with 10% IVIG results in levels of endothelial ICAM-1, VCAM-1 or TF expression that are in the same range as levels of unstimulated HUVEC. Individual differences in IVIG activity were excluded by testing nine different batches of polyclonal IVIG (data not shown). However, a consistent slight but significant decrease in ICAM-1 (P = 0·006; n = 16) and VCAM-1 expression (P = 0·02; n = 16) with different maternal post-IVIG sera was shown compared to maternal pre-IVIG sera (Fig. 1). This effect was not associated with either a clinical response or a failure to raise the fetal platelet count by IVIG treatment. The inhibitory effect of post-IVIG treatment sera on endothelial ICAM and VCAM expression could be confirmed in vitro by co-incubation of maternal pre-IVIG sera with 10% IVIG, resulting in a decrease of endothelial ICAM-1 (P = 0·002, n = 16) and VCAM-1 (P = 0·023, n = 16) surface expression (Fig. 1). Neither IVIG nor maternal post-IVIG sera had an effect on endothelial TF expression (data not shown).
Fig. 1.
The effect of in vivo and of in vitro addition of IVIG on the expression of endothelial adhesion molecules. Secondary and tertiary monolayers of HUVEC were incubated for 24 h with incubation medium supplemented with 10% maternal sera either taken before (pre-IVIG serum), or after several weeks of IVIG treatment just prior to delivery (post-IVIG serum) and with 10% maternal pre-IVIG sera coincubated with polyclonal IVIG (preserum + IVIG). The cell cultures were analysed by flow cytometry for ICAM-1 and VCAM-1 surface expression. Values represent MFI (95% C.I) after logarithmic transformation of 16 experiments with ECs from different healthy newborns.
Effect of IVIG on cytokine-activated EC
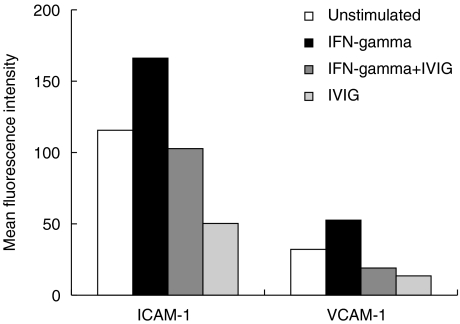
The inhibitory effect of IVIG on ICAM-1 and VCAM-1 expression on non-activated ECs was explored further with cytokine-activated ECs. Monolayers of HUVEC were incubated with 500 U/ml rHu-TNF-α alone or TNF-α mixed with IVIG for 24 h. ICAM-1 and VCAM-1 surface expression after stimulation with rHu-TNF-α increased to 134·7 ± 14·4 and 152·9 ± 20·7 MFI ± s.e.m., respectively. In the presence of IVIG this activation was reduced slightly to values of 123·4 ± 13·0 for ICAM-1 (P = 0·4, n = 16) and 122·3 ± 17·8 for VCAM-1 (P = 0·2, n = 16) (data not shown). In a similar experiment we evaluated the IVIG effect on the response of HUVEC to rHu-IFN-γ, a proinflammatory cytokine that increased expression of endothelial adhesion molecules [32]. A suboptimal dose of 50 U/ml rHu-IFN-γ and a short incubation period of 12–16 h were chosen intentionally to prevent overstimulation of ECs. Stimulation of HUVEC with this dose of rHu-IFN-γ up-regulates the ICAM-1 expression with 31% (115·6–166·2 MFI) and the VCAM-1 expression with 42% (32·1–52·6), whereas co-incubation of rHu-IFN-γ and IVIG completely inhibits rHu-IFN-γ-induced up-regulation to values lower than the expression on unstimulated ECs (P = 0·005 for ICAM-1 and p = 0·001; n = 3) (Fig. 2). When HUVEC cultures were preincubated instead with rHu-IFN-γ and subsequently incubated with maternal post-IVIG sera, the rHu-IFN-γ-induced increase of surface ICAM-1 and VCAM-1 expressions reduced to values of constitutive expression on unstimulated cells. Similarly, co-incubation of maternal pre-IVIG serum and IVIG resulted in inhibition of the rHu-IFN-γ-induced surface expression of ICAM-1 and VCAM-1 (data not shown).
Fig. 2.
Effect of IVIG on rHu-IFN-γ-induced expression of endothelial adhesion molecules. Secondary monolayers of HUVEC were incubated for 24 h with plain incubation medium (unstimulated ECs, representing constitutive endothelial ICAM-1 and VCAM-1 expression), or plain incubation medium supplemented with 50 U/ml rHu-IFN-γ, or with rHu-IFN-γ and polyclonal IVIG (IFN-γ+IVIG) or with polyclonal IVIG product (IVIG). The cells were analysed by flow cytometry for ICAM-1 and VCAM-1 expression. Values represent MFI of a representative experiment of three experiments with ECs from different healthy newborns.
DISCUSSION
The aim of this study was to evaluate a possible interaction between maternal anti-HPA-1a antibodies with or without IVIG and vascular endothelium in order to understand susceptibility for ICH in FNAIT and a beneficial effect of IVIG. Induction of vascular tissue damage by HPA-antigen–antibody complexes has been suggested, resulting in either rupture of cerebral vessels [43] or stimulation of factors of the coagulation pathway, resulting in thrombosis in peri-ventricular vessels and subsequent ischaemia [24]. Variation in the degree of this vascular damage by genetic heterogeneity of ECs or properties of the antiplatelet antibodies might explain why ICH occurs in only a proportion of patients. In the model we used [29,40], HUVEC constitutively expressed significant numbers of the vascular adhesion molecule ICAM-1, very low numbers of VCAM-1 molecule and did not express the procoagulant molecule TF [32,44]. Several stimulatory or injurious agents can induce activation of HUVEC, associated with production of proinflammatory factors and coagulation factors, such as IL-8, vWF and TF, or increase of adhesion molecule expression, including ICAM-1 and VCAM-1, which can promote leucocyte adhesion [32,40,41]. This process leads to extravasation of leucocytes and loss of endothelial integrity [32]. In vivo injured or activated endothelium was shown to bind and aggregate platelets, resulting in activation of coagulation pathways and thrombus formation by binding of vWF [45,46].
Because GPIIIa, the membrane carrier of HPA-1a antigen, is expressed on ECs, we confirmed endothelial expression of the HPA-1a antigen and GPIIIa on 35 umbilical cord veins and arteries. The differences found in HPA-1a expression among individuals were very small and unlikely to offer an explanation for susceptibility for ICH, although the number of cords investigated was limited. Neither monoclonal anti-HPA-1a nor different maternal pre-IVIG treatment sera with various titres of anti-HPA-1a antibodies, or an obstetric history with and without ICH, showed an effect on activation or damage of ECs in comparison to sera from non-immunized AB donors. Maternal anti-HPA-1a antibodies containing concomitant HLA-antibodies or mixed with monoclonal allele-specific HLA-antibodies did not result in measurable vascular injury or activation, despite cross-linking of HPA-1a antigens on HUVEC, which could have enhanced an undetectable weak signal. These results contrast with the results obtained with vasculitis-associated antibodies. Such antibodies have been shown capable of up-regulation of endothelial ICAM-1 expression [47], as well as VCAM-1 expression and release of vWF [40,48]. It is possible, however, that not the antibody–antigen interaction in vasculitis sera but the concomitant expression of cytokines activate ECs. The role of HLA-antibodies in activation or modulation of ECs remains controversial in various studies [49,50].
Susceptibility for ICH was not explained by an interaction of antibodies with antigens on vascular endothelium. The HUVEC model, however, is an artificial model and vascular damage in vivo might occur after more prolonged exposure to antibodies or by a more complex interaction in the presence of accessory factors, provided by granulocytes, monocytes, proinflammatory mediators, platelets and pro-coagulants. Cerebral vascular endothelium may differ from HUVEC, because periventricular vessels have been demonstrated to have a high expression of Fc-IgG receptors [51] and because heterogeneity of HPA-1a expression in different cerebral vessels [4] could not be excluded.
A consistent observation was the significant lower expression of ICAM-1 and VCAM-1 on ECs incubated with maternal post-IVIG treatment sera. This inhibitory effect of IVIG treatment on the constitutive expression of unstimulated ECs was also observed in vitro by co-incubation of maternal pre-IVIG treatment sera with IVIG. In addition, a suppressive effect of IVIG was shown on cytokine-activated ECs, using TNF-α or a suboptimal dose of IFN-γ (50 U/ml). Preliminary results show that the expression of adhesion markers on ECs was abolished and even reduced below the constitutive levels of unstimulated ECs in the presence of IVIG. Similar observations of blockade of TNF-α-induced expression of adhesion molecules by IVIG were described in vascular and inflammatory diseases, such as atherosclerosis [52] and Kawasaki disease [53]. The greater inhibitory effect of IVIG on IFN-γ-induced activation could be the result of the observed presence of anti-IFN-γ antibodies in IVIG [54], although a similar inhibitory effect was seen after pre-activation of ECs with IFN-γ. Nevertheless, these observations are suggestive of a dampening effect of IVIG on EC activation. Whether this effect is of any relevance to susceptibility for ICH requires further investigations.
In summary, the HPA-1a antigen is constitutively and invariably expressed on human ECs of the umbilical cord veins and arteries. Neither the binding of maternal anti-HPA-1a antibodies or human monoclonal antibodies against anti-HPA-1a, nor the binding of polyclonal or monoclonal anti-HLA-antibodies affects normal endothelial functions. Interestingly, in vivo and in vitro IVIG-treatment leads to a smal, but consistent and significant decrease in the expression of the constitutive or TNF-α- and IFN-γ-induced expression of adhesion molecules ICAM-1 and VCAM-1. The clinical relevance of this observation deserves further investigation.
Acknowledgments
The authors gratefully acknowledge K. Armour of the East Anglia Blood Centre for construction of the monoclonal antibody Camtran 007, W. Verduyn for HPA-PCR typing of the HUVEC and HUAEC, M. D. Witvliet for serological quantitative HPA- and HLA-antibody assays and H. Putter for his expertise on the statistical analysis of the results.
REFERENCES
- 1.Mueller-Eckhardt C, Kiefel V, Grubert A, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–6. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 2.Porcelijn L, Kanhai HH. Diagnosis and management of fetal platelet disorders. In: Rodeck CH, Whittle MJ, editors. Fetal medicine: basic science and clinical practice. London: Churchill Livingstone; 1999. pp. 805–15. [Google Scholar]
- 3.Leeksma OC, Zandbergen-Spaargaren J, Giltay JC, van Mourik JA. Cultured human endothelial cells synthesize a plasma membrane protein complex immunologically related to the platelet glycoprotein IIb/IIIa complex. Blood. 1986;67:1176–80. [PubMed] [Google Scholar]
- 4.Valentin N, Visentin GP, Newman PJ. Involvement of the cysteine-rich domain of glycoprotein IIIa in the expression of the human platelet alloantigen, PlA1: evidence for heterogeneity in the humoral response. Blood. 1995;85:3028–33. [PubMed] [Google Scholar]
- 5.Gruel Y, Boizard B, Daffos F, Forestier F, Caen J, Wautier JL. Determination of platelet antigens and glycoproteins in the human fetus. Blood. 1986;68:488–92. [PubMed] [Google Scholar]
- 6.Durand-Zaleski I, Schlegel N, Blum-Boigard C, Uzan S, Dreyfus M, Kaplan C Immune Thrombocytopenia Working Group. Screening primiparous women and newborns for fetal/neonatal alloimmune thrombocytopenia: a prospective comparison of effectiveness and costs. Am J Perinatol. 1996;13:423–31. doi: 10.1055/s-2007-994382. [DOI] [PubMed] [Google Scholar]
- 7.Ahya R, Turner ML, Urbaniak SJ. Fetomaternal alloimmune thrombocytopenia; SNAIT Study team. Transfusion Apheresis Sci. 2001;25:139–45. doi: 10.1016/s1473-0502(01)00102-1. [DOI] [PubMed] [Google Scholar]
- 8.Murphy MF, Williamson LM, Urbaniak SJ. Antenatal screening for fetomaternal alloimmune thrombocytopenia: should we be doing it? Vox Sang. 2002;83:409–16. doi: 10.1111/j.1423-0410.2002.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 9.Williamson LM, Hackett G, Rennie J, et al. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 10.Muller JY, Reznikoff-Etievant MF, Patereau C, Dangu C, Chesnel N. [Neonatal alloimmune thrombopenia. Clinical and biological study of 84 cases] Presse Med. 1985;14:83–6. [in French] [PubMed] [Google Scholar]
- 11.Kaplan C, Daffos F, Forestier F, Morel MC, Chesnel N, Tchernia G. Current trends in neonatal alloimmune thrombocytopenia: diagnosis and therapy. In: Kaplan-Gouet C, Schlegel N, Salmon C, McGregor J, editors. Platelet immunology: fundamental and clinical aspects. Paris, France: Colloque INSERM/John Libbey Eurotext; 1991. pp. 267–78. [Google Scholar]
- 12.Bussel JB, Berkowitz RL, Lynch L, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin. a randomized trial of the addition of low-dose steroid to intravenous gamma-globulin. Am J Obstet Gynecol. 1996;174:1414–23. doi: 10.1016/s0002-9378(96)70582-3. [DOI] [PubMed] [Google Scholar]
- 13.Spencer JA, Burrows RF. Feto-maternal alloimmune thrombocytopenia: a literature review and statistical analysis. Aust NZ J Obstet Gynaecol. 2001;41:45–55. doi: 10.1111/j.1479-828x.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 14.Kornfeld I, Wilson RD, Ballem P, Wittmann BK, Farquharson DF. Antenatal invasive and noninvasive management of alloimmune thrombocytopenia. Fetal Diagn. 1996;11:210–7. doi: 10.1159/000264304. [DOI] [PubMed] [Google Scholar]
- 15.Radder C, Brand A, Kanhai H. A less invasive treatment strategy to prevent intracranial hemorrhage in fetal and neonatal alloimmune thrombocytopenia. Am J Obstet Gynecol. 2001;185:683–8. doi: 10.1067/mob.2001.116727. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan C, Daffos F, Forestier F, et al. Management of alloimmune thrombocytopenia: antenatal diagnosis and in utero transfusion of maternal platelets. Blood. 1988;72:340–3. [PubMed] [Google Scholar]
- 17.Kelton JG. Platelet and red cell clearance is determined by the interaction of the IgG and complement on the cells and the activity of the reticuloendothelial system. Transfus Med Rev. 1987;1:75–84. doi: 10.1016/s0887-7963(87)70008-x. [DOI] [PubMed] [Google Scholar]
- 18.King KE, Kao KJ, Bray PF, et al. The role of HLA antibodies in neonatal thrombocytopenia: a prospective study. Tissue Antigens. 1996;47:206–11. doi: 10.1111/j.1399-0039.1996.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 19.Mawas F, Wiener E, Williamson LM, Rodeck CH. Immunoglobulin G subclasses of anti-human platelet antigen 1a in maternal sera: relation to the severity of neonatal alloimmune thrombocytopenia. Eur J Haematol. 1997;59:287–92. doi: 10.1111/j.1600-0609.1997.tb01688.x. [DOI] [PubMed] [Google Scholar]
- 20.Leeksma OC, Giltay JC, Zandbergen-Spaargaren J, Modderman PW, van Mourik JA, dem Borne AE. The platelet alloantigen Zwa or PlA1 is expressed by cultured endothelial cells. Br J Haematol. 1987;66:369–73. doi: 10.1111/j.1365-2141.1987.tb06925.x. [DOI] [PubMed] [Google Scholar]
- 21.Giltay JC, Brinkman HJ, von dem Borne AE, van Mourik JA. Expression of the alloantigen Zwa (or P1A1) on human vascular smooth muscle cells and foreskin fibroblasts: a study on normal individuals and a patient with Glanzmann's thrombasthenia. Blood. 1989;74:965–70. [PubMed] [Google Scholar]
- 22.Trent RJ, Clancy RL, Danis V, Basten A. Immune complexes in thrombocytopenic patients: cause or effect? Br J Haematol. 1980;44:645–54. doi: 10.1111/j.1365-2141.1980.tb08719.x. [DOI] [PubMed] [Google Scholar]
- 23.Kay HH, Hage ML, Kurtzberg J, Dunsmore KP. Alloimmune thrombocytopenia may be associated with systemic disease. Am J Obstet Gynecol. 1992;166:110–1. doi: 10.1016/0002-9378(92)91840-7. [DOI] [PubMed] [Google Scholar]
- 24.Khouzami AN, Kickler TS, Callan NA, Shumway JB, Perlman EJ, Blakemore KJ. Devastating sequelae of alloimmune thrombocytopenia: an entity that deserves more attention. J Matern Fetal Med. 1996;5:137–41. doi: 10.1002/(SICI)1520-6661(199605/06)5:3<137::AID-MFM8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Griffin HM, Ouwehand WH. A human monoclonal antibody specific for the leucine-33 (P1A1, HPA-1a) form of platelet glycoprotein IIIa from a V gene phage display library. Blood. 1995;86:4430–6. [PubMed] [Google Scholar]
- 26.Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J Immunol. 1979;123:342–9. [PubMed] [Google Scholar]
- 27.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class I molecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Hum Immunol. 1997;53:90–7. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 28.Kiefel V, Santoso S, Weisheit M, Mueller-Eckhardt C. Monoclonal antibody-specific immobilization of platelet antigens (MAIPA). a new tool for the identification of platelet-reactive antibodies. Blood. 1987;70:1722–6. [PubMed] [Google Scholar]
- 29.Beekhuizen H, van Furth R. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J Vasc Res. 1994;31:230–9. doi: 10.1159/000159048. [DOI] [PubMed] [Google Scholar]
- 30.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 31.Rozman P, Drabbels J, Schipper RF, Doxiadis I, Stein S, Claas FH. Genotyping for human platelet-specific antigens HPA-1-2-3-4 and -5 in the Slovenian population reveals a slightly increased frequency of HPA-1b and HPA-2b as compared to other European populations. Eur J Immunogenet. 1999;26:265–9. doi: 10.1046/j.1365-2370.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 32.Beekhuizen H, van de Gevel JS. Endothelial cell adhesion molecules in inflammation and postischemic reperfusion injury. Transplant Proc. 1998;30:4251–6. doi: 10.1016/s0041-1345(98)01405-5. [DOI] [PubMed] [Google Scholar]
- 33.Klein CL, Bittinger F, Kohler H, et al. Comparative studies on vascular endothelium in vitro. 3. Effects of cytokines on the expression of E-selectin, ICAM-1 and VCAM-1 by cultured human endothelial cells obtained from different passages. Pathobiology. 1995;63:83–92. doi: 10.1159/000163938. [DOI] [PubMed] [Google Scholar]
- 34.Kamphuisen PW, Houwing-Duistermaat JJ, van Houwelingen HC, Eikenboom JC, Bertina RM, Rosendaal FR. Familial clustering of factor VIII and von Willebrand factor levels. Thromb Haemost. 1998;79:323–7. [PubMed] [Google Scholar]
- 35.Wacker WEC, Ulmer DD, Vallee BL. Metalloenzymes and myocardial infarction; malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956;255:449–56. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- 36.Stolpen AH, Guinan EC, Fiers W, Pober JS. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986;123:16–24. [PMC free article] [PubMed] [Google Scholar]
- 37.Schuger L, Varani J, Marks RM, Kunkel SL, Johnson KJ, Ward PA. Cytotoxicity of tumor necrosis factor-alpha for human umbilical vein endothelial cells. Lab Invest. 1989;61:62–8. [PubMed] [Google Scholar]
- 38.Blann AD. Soluble markers of endothelial cell function. Clin Hemorheol Microcirc. 1997;17:3–11. [PubMed] [Google Scholar]
- 39.Pearson JD. Endothelial cell function and thrombosis. Baillières Clin Haematol. 1994;7:441–52. doi: 10.1016/s0950-3536(05)80092-7. [DOI] [PubMed] [Google Scholar]
- 40.Dupuy E, Herbert JM, Giraudeau V, Quere I, Zini JM, Tobelem G. Induction of endothelial cell adhesion molecules by serum and immunoglobulins G from a patient with vasculitis and monoclonal gammapathy: potential relevance to vasculitis. Thromb Haemost. 1998;80:477–80. [PubMed] [Google Scholar]
- 41.Rot A, Hub E, Middleton J, et al. Some aspects of IL-8 pathophysiology. III. Chemokine interaction with endothelial cells. J Leukoc Biol. 1996;59:39–44. doi: 10.1002/jlb.59.1.39. [DOI] [PubMed] [Google Scholar]
- 42.Paleolog EM, Delasalle SA, Buurman WA, Feldmann M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Br J Haematol. 1994;84:2578–90. [PubMed] [Google Scholar]
- 43.Naidu S, Messmore H, Caserta V, Fine M. CNS lesions in neonatal isoimmune thrombocytopenia. Arch Neurol. 1983;40:552–4. doi: 10.1001/archneur.1983.04050080052009. [DOI] [PubMed] [Google Scholar]
- 44.Veltrop MH, Beekhuizen H, Thompson J. Bacterial species- and strain-dependent induction of tissue factor in human vascular endothelial cells. Infect Immun. 1999;67:6130–8. doi: 10.1128/iai.67.11.6130-6138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caen JP, Rosa JP. Platelet–vessel wall interaction: from the bedside to molecules. Thromb Haemost. 1995;74:18–24. [PubMed] [Google Scholar]
- 46.Udvardy M, Bodolay E, Szegedi G, Harsfalvi J, Boda Z, Rak K. Alterations of primary haemostasis in mixed connective tissue disease (MCTD) Thromb Res. 1991;63:281–6. doi: 10.1016/0049-3848(91)90131-f. [DOI] [PubMed] [Google Scholar]
- 47.Johnson PA, Alexander HD, McMillan SA, Maxwell AP. Up-regulation of the endothelial cell adhesion molecule intercellular adhesion molecule-1 (ICAM-1) by autoantibodies in autoimmune vasculitis. Clin Exp Immunol. 1997;108:234–42. doi: 10.1046/j.1365-2249.1997.3741271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai KN, Leung JC, Lai KB, Lai FM, Wong KC. Increased release of von Willebrand factor antigen from endothelial cells by anti-DNA autoantibodies. Ann Rheum Dis. 1996;55:57–62. doi: 10.1136/ard.55.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith JD, Yacoub MH, Rose ML. Endothelial cell activation by sera containing HLA antibodies is mediated by interleukin-1. Transplantation. 1998;66:1229–37. doi: 10.1097/00007890-199811150-00019. [DOI] [PubMed] [Google Scholar]
- 50.Bian H, Reed EF. Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163:1010–8. [PubMed] [Google Scholar]
- 51.Peress NS, Siegelman J, Fleit HB. High avidity periventricular IgG-Fc receptor activity in human and rabbit brain. Clin Immunol Immunopathol. 1987;42:229–38. doi: 10.1016/0090-1229(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 52.Ronda N, Bernini F, Giacosa R, et al. Normal human IgG prevents endothelial activation induced by TNFalpha and oxidized low-density lipoprotein atherogenic stimuli. Clin Exp Immunol. 2003;133:219–26. doi: 10.1046/j.1365-2249.2003.02215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu C, Poirier B, Van Huyen JP, et al. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. Am J Pathol. 1998;153:1257–66. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toungouz M, Denys C, Dupont E. Blockade of proliferation and tumor necrosis factor-alpha production occurring during mixed lymphocyte reaction by interferon-gamma-specific natural antibodies contained in intravenous immunoglobulins. Transplantation. 1996;62:1292–6. doi: 10.1097/00007890-199611150-00020. [DOI] [PubMed] [Google Scholar]