Abstract
Behcet's disease (BD) specific peptide (p336–351) was identified within the human 60 kD heat shock protein (HSP60). Oral p336–351 induced uveitis in rats which was prevented by oral tolerization with the peptide linked to recombinant cholera toxin B subunit (CTB). This strategy was adopted in a phase I/II clinical trial by oral administration of p336–351-CTB, 3 times weekly, followed by gradual withdrawal of all immunosuppressive drugs used to control the disease in 8 patients with BD. The patients were monitored by clinical and ophthalmological examination, as well as extensive immunological investigations. Oral administration of p336–351-CTB had no adverse effect and withdrawal of the immunosuppressive drugs showed no relapse of uveitis in 5 of 8 patients or 5 of 6 selected patients who were free of disease activity prior to initiating the tolerization regimen. After tolerization was discontinued, 3 of 5 patients remained free of relapsing uveitis for 10–18 months after cessation of all treatment. Control of uveitis and extra-ocular manifestations of BD was associated with a lack of peptide-specific CD4+ T cell proliferation, a decrease in expression of TH1 type cells (CCR5, CXCR3), IFN-γ and TNF-α production, CCR7+ T cells and costimulatory molecules (CD40 and CD28), as compared with an increase in these parameters in patients in whom uveitis had relapsed. The efficacy of oral peptide-CTB tolerization will need to be confirmed in a phase III trial, but this novel strategy in humans might be applicable generally to autoimmune diseases in which specific antigens have been identified.
Keywords: oral tolerization, peptide, uveitis, Behcet's disease
INTRODUCTION
Behcet's Disease (BD) is a multisystem inflammatory disorder characterized by uveitis, oral and genital ulcers, cutaneous, vascular, joint and neurological manifestations [1–3]. It is prevalent in countries bordering the Mediterranean and in the Far East and is a significant cause of blindness in these countries. The microbial HSP65 and the homologous human-HSP60 were implicated in the aetiology of Behcet's disease as HSP65 is found in a variety of microorganisms, including Streptococcus sanguis variants which was reported as an aetiological factor in BD [4–7]. The peptide 336–351 was first identified as a T cell epitope in Britain [8,9] and the specificity in BD was confirmed in Japan [10] and Turkey [11]. Furthermore, disease activity was correlated with the T cell proliferative response to this peptide [9]. Administration of p336–351 with an adjuvant SC in Lewis rats induced uveitis [12], and this was also found with oral or nasal administration of p336–351 without an adjuvant [13]. This is in contrast to induction of tolerance by oral [14,15] or nasal administration of antigens [16]. Indeed, oral application of retinal antigen or IRBP [17,18] and nasal application of retinal antigen [19] suppressed experimental autoimmune uveoretinitis. An alternative strategy was then attempted, utilizing recombinant cholera toxin B subunit (rCTB) which enhances oral tolerance [20,21] and linking it to p336–351. The peptide-rCTB conjugate administered orally decreased significantly (P < 0·0001) the development of uveitis in rats (11/66, 16·7%), as compared with oral administration of the peptide alone (48/73, 65·8%) [22].
Tolerization with the p336–351-rCTB conjugate was now attempted in patients with BD, as a novel strategy of preventing relapses of uveitis. We adopted the therapeutic approach first introduced for evaluation of tolerance with retinal S antigen in uveitis in humans [23], by oral administration of the antigen and progressive withdrawal of the immunosuppressive drugs. Indeed, oral administration of the peptide-rCTB conjugate, 3 times weekly for 12–16 weeks, to 8 patients with BD allowed us to withdraw all immunosuppressive drugs in 5 of the 8 patients without a relapse of uveitis. Furthermore, 5 of the 6 patients had not developed relapse of uveitis if only those patients are considered who were clinically controlled by their immunosuppressive drugs prior to the peptide-CTB treatment. The clinical manifestations were correlated with significant immunological changes.
MATERIALS AND METHODS
Selection criteria, treatment regime and monitoring of patients
Patients for this study were recruited from the Uveitis Clinic at St Thomas’ Hospital. All patients were males and satisfied the International Study Group criteria for the diagnosis of Behcet's Disease [24] and all had a history of pan-uveitis requiring systemic immunosuppression for its control (Table 1). At enrolment, all patients had quiet eyes for at least 3 months and a history of relapse on attempting to reduce the dose of systemic treatment in the previous 6 months. Some patients showed signs of systemic activity, recurrent oral ulcers and folliculitis, at entry to the study. A further criterion of enrolment was that the patients should be able to attend the outpatient department at frequent intervals and are likely to comply with the treatment regime. All patients signed informed consent for the study which received local Ethical Committee approval by the St Thomas’ Research Ethics Committee.
Table 1.
Clinical features and treatment of 8 patients taking part in the phase I clinical trial
| Patient no. | Nationality | Age (years) | Duration (years) | B51 101 | Disease manifestations | Disease activity | Immuno- suppressive treatment | Dose of p336-351- CTB | Primary ocular result after all treatment withdrawn |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Middle eastern (Turkish) | 35 | 4 | + | Uveitis, RAS, pustular lesions, arthralgia | Nil | 5–7·5 mg Predn. 150 mg Azath. | 0·5 mg | No relapse maintained 24 months |
| 2 | Caucasian (Irish) | 36 | 13 | + | Uveitis, RAS, Genital ulcers, Arthralgia | Nil | 10 mg Predn. | 5 mg | No relapse maintained 18 months |
| 3 | Caucasian (English) | 35 | 1 | + | Uveitis RAS Er. Nodosum | Nil | 10–15 mg Predn.150 mg Azath. | 0·5 mg | Relapse after 10 months |
| 4 | Caucasian (Irish) | 36 | 12 | + | Uveitis, RAS Genital ulcers,Arthralgia | Nil | 10 mg Predn.100 mg Azath. | 0·5 mg | Relapse after 1 month |
| 5 | Caucasian (English) | 30 | 3 | ? | Uveitis, RAS,Genital ulcers | Nil | 10 mg Predn.1·5 mg Colchicine | 5 mg | Relapse after 2 wks |
| 6 | Middle Eastern (Iraqi) | 51 | >13 | + | Uveitis, RAS, Foliculitis, Arthralgia, Thrombophlebitis | Nil | 5 mg Predn. | 0·5 mg | Relapse within 1 day of reducing Predn. |
| 7 | Caucasian (English) | 29 | 6 | + | Uveitis, RAS, Genital ulcers, Foliculitis | Severe pustules | 15 mg Predn.400 mg Cyclosporin | 5 mg | Relapse within 2 weeks of reducing Cyclosp. |
| 8 | Caucasian (English) | 34 | 6 | − | Uveitis, RAS Genital ulcers, Foliculitis, Arthralgia | RAS and Foliculitis | 15 mg Predn.2 mg CellCept | 5 mg | Relapse within 1 day of reducing Predn. |
Predn., Prednisolone; Azath., Azathioprine; Cyclosp., Cyclosporin.
Four patients were randomly selected to start on 0·5 mg and 4 patients on 5 mg of the peptide-CTB given orally 3 times per week, dissolved in 100 ml bicarbonate solution, whilst maintaining their previous immunosuppressive drug treatment (Table 1). At week 3, the latter was reduced in a stepwise manner, according to the strategy introduced by Nussenblatt et al. [23], starting with prednisolone and then any other systemic drug (azathioprine, cyclosporin, etc.), with the aim of tailing off all treatment by week 12. During this time the tolerizing regime of peptide-CTB was maintained. The patients were then observed for a further 12 weeks at 4 weekly intervals. Subsequent follow up was based on clinical need or every 2 months. At each visit patients had a full ophthalmological examination with dilation (MRS) as well as a complete physical examination (TL). Details of ocular and systemic inflammation were recorded as described previously [25]. Patients also had optical coherence tomography images (4 mm horizontal scan length through the fovea), to determine the presence of retinal thickening or macular oedema. For the purpose of this study a relapse was defined as an increase in intraocular inflammation that required either re-starting or an increase in systemic immunosuppressive therapy. Accordingly, patients who experienced mild anterior uveitis controllable on steroid drops alone remained in the study. Eight patients were enrolled in the study (Table 1), age range 29–51 years, with 7 of the patients being 29–36 years old. All patients were HLA typed, as described before [26]. Full blood count, urea, electrolytes and liver function tests were carried out at every visit of the patients.
Preparation of GMP peptide 336–351 covalently linked to recombinant CTB
The BD peptide corresponding to residues 336–351 of the human HSP60 and with an N-terminal Cys residue was synthesized in its acetate form under GMP conditions (H-Cys-Gln-Pro-His-Asp-Leu-Gly-Lys-Val-Gly-Glu-Val-Il2-Val-Thr-Lys-AspOH acetate) (Bachem AG, Bubendorf, Switzerland). This peptide was coupled to a highly purified, recombinant CTB (rCTB) produced under GMP conditions by SBL Vaccine (Stockholm, Sweden), commonly used as a component of the internationally registered oral cholera vaccine (Dukoral). We used the bifunctional cross-linking reagent N-succinimidyl 3-(2-pyridyldithio)proportionate (SPDP) (Pierce Rockford, IL, USA), with a substitution ratio of 4–5 residues per rCTB [27]. The samples were purified by Sephadex G25 Chromatography in PBS (Amersham Biosciences, Uppsala, Sweden). The purified, eluted complexes were sterile-filtered, aliquoted, frozen and then stored. The final material was checked for sterility, composition, binding GM1 ganglioside and then tested for its safety and preventive capacity in the rat uveitis model as reported [22] before being used in the present phase I/II clinical trial.
Immunological investigations
Reagents.
For analytical purposes HSP65 was prepared from M. bovis as described elsewhere [28]. The peptide 336–351 and control peptide 136–151 were synthesized in the Hansen's Disease Laboratory (Centre for Disease Control, Atlanta, GA, USA) as described before [7].
T cell proliferative responses.
PBMC were separated from defibrinated blood by density gradient centrifugation. The cells were cultured (105 cells/well) with the optimal concentration of p336–351, p136–151, HSP65, Concanavalin A (Sigma) and without any antigen in quadruplicates for 5 days in 10% autologous serum, as described before [7,8]. The cultures were pulsed with tritiated thymidine (18·5 mBq per well), harvested and then assessed for 3H thymidine incorporation by liquid scintillation counting. The results were assessed in stimulation indices, defined as the ratio of counts per minute of antigen stimulated to unstimulated cultures.
Studies of cell surface markers by flow cytometry.
Phenotypic analysis of cell surface molecules was carried out by diluting whole blood (1 : 1) with phosphate-buffered saline, 1% bovine serum albumin and 0·1% sodium azide (PBA); 50 µl of diluted blood was then mixed with 5 µl of the appropriate fluorochrome-conjugated antibodies. The cells were then treated as described before [8] and analysed on a Coulter XL counter (Coulter, Oxford, UK). Cells for analysis were gated by cell size (forward and size scatter), so as to analyse the lymphocyte population, except for CD40 expression the cells were gated on monocytes. Variation in the performance of the cytometer was controlled by calibration with fluorescent labelled beads (Flow-Check, Coulter) every time the cytometer was used. Fluorescent labelled IgG isotype controls (DAKO, UK) were used to identify nonspecific binding of antibody.
Cytometric bead analysis of IFN-γ and TNF-α.
The human Th1/Th2 Cytokine Cytometric Bead Array method (BD Biosciences Pharmingen, San Diego, CA, USA) was used to assay TNF-α, IFN-γ IL-2, IL-4, IL-5, IL-10 concentrations in culture supernatants from PBMC stimulated with either p336–351 or HSP65 for 3 days (as above). The assays were performed according to the manufacturer's instructions and analysed in a BD Calibur flow cytometer with data analysis carried out by the BD CBA Analysis software.
Statistical analysis
The paired Student's t-test was used to analyse the sequential immunological data, pre- and post-tolerization in each group of patients. The Mann–Whitney nonparametric test was used to evaluate the data between the patients responding and not responding to the tolerization regime.
RESULTS
Clinical findings
The investigation was completed on 8 patients, all of whom were maintained prior to being enlisted to this clinical trial, on a minimal effective dose of immunosuppressive drugs. Any attempt over the previous 6 months to decrease prednisolone by even 2·5 mg per day had precipitated a relapse in uveitis. About 3 weeks after starting oral tolerization with p336–351-CTB, all immunosuppressive drugs were gradually withdrawn over a period of 6–9 weeks. None of the patients reported any adverse effect after administration of p336–351-CTB, and their blood pressure, haematological indices and blood chemistry showed no significant changes. However, 3 of the 8 patients showed a relapse of uveitis within 2–8 weeks of withdrawing the immunosuppressive drugs, whereas in the remaining 5 patients the immunosuppressive drugs were completely withdrawn without relapse of uveitis (Table 1). Of the 3 patients who experienced a relapse of uveitis 2 showed muco-cutaneous disease activity at the start of the trial, with recurrent aphthous stomatitis and extensive pustular skin lesions. Thus, considering only those patients who showed no disease activity at the start of the trial, 5 of the 6 patients were controlled by the peptide-CTB treatment, after the immunosuppressive drugs had been withdrawn (Table 1). The third patient who relapsed was much older (51 years) than the other patients (29–39 years) and had long-standing BD (>13 years). The 5 patients who responded to the tolerizing regime, showed no evidence of relapsing uveitis or muco-cutaneous lesions, except 1 patient who had occasional oral ulceration.
In the second phase of the trial (Table 1) 2 of the 5 patients developed uveitis within one month of discontinuing the peptide-CTB regime and 2–3 months after total withdrawal of immunosuppressive treatment. The remaining 3 patients remained free of uveitis and other manifestations of BD for a period of 10–18 months after discontinuing the tolerization regime and withdrawal of all immunosuppressive drugs. None of the patients developed retinal ischaemia over the follow-up period of up to 2 years. However, one patient (No. 3) had ischaemic retinal involvement before the start of the tolerizing regime and he was free of any clinical manifestations for 10 months after discontinuing all treatment.
Optical coherence tomography images showed normal anatomical features of the fovea during remission, in contrast to gross macular oedema and retinal thickening in a relapse of uveitis. Retinal thickness correlated directly with visual acuity; in remission 20/20, in exacerbation 20/200.
Immunological changes
All but 1 patient expressed the HLA B51·101 allotype (Table 1).
T cell proliferation
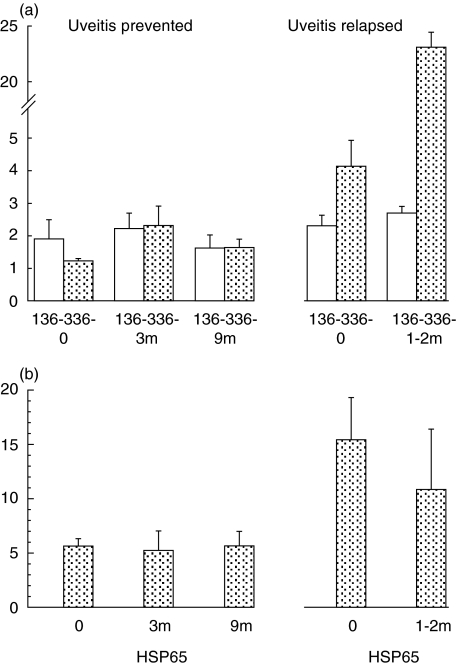
Stimulation with the BD specific peptide 336–351 showed SI < 2 before the tolerizing regime started in the 5 patients in whom the development of uveitis was prevented (mean ± sem 1·24 ± 0·1). In contrast, all 3 patients with relapsing uveitis showed a SI > 2 (4·1 ± 0·9) before the start of the tolerizing regime and this was significantly higher (P < 0·05) than the SI in the other group (Fig. 1). This was consistent with disease activity in 2 of the 3 patients in the latter group, as shown in Table 1 (Nos. 7 and 8). Sequential monitoring of the T cell proliferative response stimulated by the control p136–151 showed no significant changes in the proliferative responses at 3 or 9 months (Fig. 1a). The response to p336–351 showed no significant change at 3 or 9 months, despite discontinuing all the immunosuppressive drugs. However, in the 3 patients with relapsing uveitis 1–2 months after the tolerizing regime was initiated, and the doses of pre-existing immunosuppressive drugs were decreased, the p336–351 response rose from a mean of 4·1 (±0·9) to 23·1 (±18·0), as compared with the control peptide 136–151 from 2·3 (±0·3) to 2·7 (±0·2). Whilst we cannot exclude the possibility that decreasing the dosage of the immunosuppressive drugs might have been responsible for the raised SI, this was not found in the 5 patients in whom complete withdrawal of drugs was accomplished under the tolerizing regime of p336–351-CTB. A significant increase in the SI was found between the 2 groups of patients before (1·2 ± 0·1 compared with 4·1 ± 0·9; P < 0·05) and 1–3 months after the tolerizing regime was started (2·5 ± 0·3 compared with 23·1 ± 18; P < 0·05), altough immunosuppressive drugs were decreased in both groups of patients. The tolerizing regime was discontinued in these 3 patients and the immunosuppressive treatment restarted.
Fig. 1.
T cell proliferative responses stimulated with (a) p336–351 ( ) or 136–151 (□) and (b) HSP65 before (
) or 136–151 (□) and (b) HSP65 before ( ) and after tolerance was completed (3 months) and at the end of observaiton (9+ months) in 5 patients in whom uveitis was prevented and 3 patients with relapsing uveitis.
) and after tolerance was completed (3 months) and at the end of observaiton (9+ months) in 5 patients in whom uveitis was prevented and 3 patients with relapsing uveitis.
The T cell proliferative response to HSP65 was raised before the tolerizing regime was started in all 8 patients, but significantly higher mean SI were found in relapsing uveitis (15·4 ± 4·8) than in the group in which uveitis was prevented (5·6 ± 0·8), which is consistent with disease activity in the former group (Fig. 1b). These levels remained unchanged after the tolerizing regime was started, though a fall in the SI was noted in the group of patients with relapsing uveitis. Thus, a rise in p336–351 response and a fall in HSP65 response was observed in the group with relapsing uveitis which is not consistent with a decrease in immunosuppressive drugs.
Phenotypic analysis
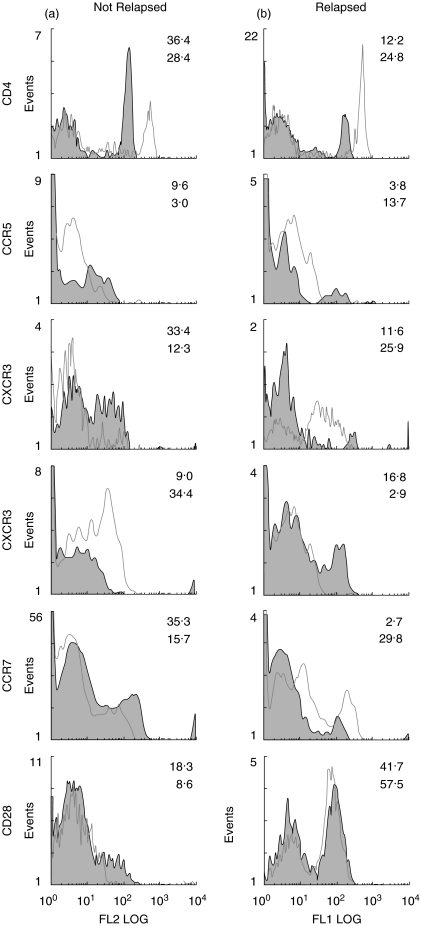
Sequential monitoring of the flow cytometric changes (Table 2) before and after tolerization showed a significant decrease in the proportion of CD4+ cells in 4/5 subjects with a remission, from a mean of 37·3 (±3·0)% to 29·5 (±3·7)% over the 3 months of p336–351-CTB administration which decreased further to 22·0 (±2·5)%, 6–9 months after all treatment had ceased t = 5·25, P = 0·01) (Table 2). In contrast, patients who developed relapsing uveitis showed 28·6 (±10·5)% CD4+ cells before which increased to 46·1 (±12·7)% during administration of the tolerizing regime (Table 2). Similar inverse relationships between the 2 cohorts was found in the expression of CCR5 and CXCR3 which are markers of TH1+ cells, CCR7 a memory T cell homing to lymph nodes and CXCR4 which with CCR7 is up-regulated in dendritic cell maturation (Table 2, Fig. 2). CD28 and CD40 which are costimulatory molecules binding B7 and CD40L, respectively, showed similar changes but only CD40 reached the 5% level of significance (Table 2). Less marked differences were found with TCR γδ+, CCR6+ and CD86+ cells between the 2 groups of patients and none were significant (Table 2). This was surprising for TCRγδ was implicated in the pathogenesis of Behcet's disease. CD8, CD45RA, CD45RO and CCR3 showed little or no difference between the 2 groups of patients.
Table 2.
Phenotypic analysis of PBMC in the two groups of patients with BD in whom uveitis was either prevented or relapsed during and after the change from immunosuppressive drugs to p336–351-CTB tolerizing regime
| Uveitis not relapsed (n = 5) (mean ± sem) | Uveitis relapsed (n = 3) (mean ± sem) | |||||||
|---|---|---|---|---|---|---|---|---|
| After | After | |||||||
| Cell marker | Change | Before | 3 months† | 6–9 months‡ | Change | Before | 1–2 months | |
| Inverse relationship | ||||||||
| 1 | CD4 | Decrease | 37·3 (3·0) | 29·5 (3·7) | 22·0 (2·5)** | Increase | 28·6 (10·5) | 46·1 (12·7) |
| 2 | CCR5 | Decrease | 7·8 (1·8) | 4·0 (1·0)* | 3·8 (1·4) | Increase | 7·9 (2·2) | 10·2 (3·5) |
| 3 | CXCR3 | Decrease | 19·0 (4·3) | 12·5 (1·6) | 14·8 (4·1) | Increase | 14·4 | 26·6 |
| 4 | CCR7 | Decrase | 32·6 (6·6) | 31·8 (6·0) | 2·4 (1·0)* | Increase | 6·4 | 31·4 |
| 5 | CXCR4 | Decrease | 21·7 (5·9) | 23·1 (7·1) | 27·6 (3·1) | Decrease | 24·3 (15·6) | 5·1 (3·2) |
| 6 | CD28 | Decrease | 27·7 (10·4) | 14·3 (4·3) | 8·8 (2·8) | Increase | 30·1 (12·5) | 31·4 (15·7) |
| 7 | CD40 | Decrease | 42·2 (8·9) | 32·8 (4·6) | 19·8 (2·3)* | Increase | 25·3 (5·6) | 53·4 (9·4) |
| No inverse relationship | ||||||||
| 8 | TCR-γδ | Decrease | 5·0 (0·9) | 3·1 (1·1) | 4·3 (1·2) | No change | 2·9 (0·8) | 1·7 (0·7) |
| 9 | CCR6 | Decrease | 9·0 (1·8) | 3·7 (1·8) | 6·4 (1·3) | Nochange | 2·6 (0·4) | 1·5 (0·6) |
| 10 | CD86 | Increase | 9·6 (2·6) | 16·4 (2·1) | 11·6 (5·4) | Decrease | 4·5 (2·0) | 4·9 (3·0) |
at the end of tolerisation;
3 patients who showed no relapse up to 10 months;
P = 0·05;
P = 0·01. CD8, CD45RA, CD45RO, CCR3 showed no distinguishing pattern.
Fig. 2.
Phenotypic analysis of six cell surface markers by flow cytometry in two representative patients undergoing tolerizing regime, in one of whom uveitis was prevented in contrast to the other in whom uveitis relapsed.
Cytometric bead analysis of IFNγ and TNF-α
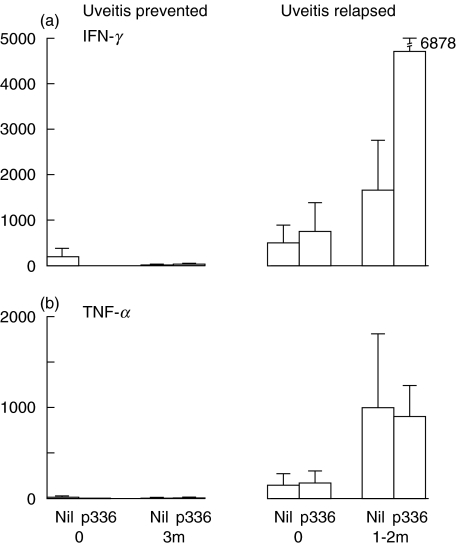
Specific stimulation of PBMC with p336–351 induced negligible production of IFN-γ in those patients in whom the tolerizing regime prevented uveitis, both before and 3 months after the immunosuppressive drugs were withdrawn (Fig. 3a). In contrast, increased concentration of IFN-γ was found in the group of patients in whom uveitis relapsed, even before tolerization started; without stimulation (504 ± 411 pg/ml) and with p336–351 stimulation (758 ± 636 pg/ml). This increased greatly to 1665 ± 1111 without stimulation and to 4723 (±2639) pg/ml with p336–351 stimulation 1–2 months after tolerization was initiated and the immunosuppresive drugs were in the process of being decreased (Fig. 3a). However, despite the obvious quantitative differences, the 5% level of significance was not reached, because of the small number of patients in each group and great variation between the patients. The results with TNF-α were similar, except that there was little difference between the unstimulated and p336–351 stimulated production of TNF-α in patients with relapsed uveitis (Fig. 3b).
Fig. 3.
The effect of stimulating PBMC with p336–351 on the production of (a) IFN-γ and (b) TNF-α, evaluated by cytometric bead analysis.
DISCUSSION
The rationale of this therapeutic trial was that if oral administration of p336–351 linked to rCTB were to induce effective tolerization regime in BD, then stepwise decrease of immunosuppressive drugs might be achieved, as was found with oral administration of retinal S antigen [23]. In the present phase I/II clinical trial oral administration of peptide 336–351 linked to the tolerizing rCTB [20–22] prevented relapse of uveitis in 5 of 8 patients with BD after all immunosuppressive drugs were withdrawn. The 3 patients who were taking the p336–351-CTB for more than 3 weeks developed relapsing uveitis only after decreasing the dosage of prednisolone from 15 mg to either 12·5 or 7·5 mg in 2 patients and from 5 to 2·5 mg daily in 1 patient. Indeed, 6 months prior to starting the trial, a decrease in the dosage of prednisolone by 2·5 mg per day resulted in a relapse of uveitis. Thus, the natural history of the disease in the 5 protected patients argues against this being a chance finding and is strongly supported by the associated immunological changes.
The finding that 2 of 3 patients who suffered a relapse of uveitis using this tolerizing regimen had not been controlled with immunosuppressive drugs before tolerization was initiated and showed evidence of muco-cutaneous disease is consistent with the experimental evidence in animals [22]. This showed that BD-specific conjugate prevented the development of uveitis in rats only if it was started before the disease was induced. These results are also consistent with the experimental findings that there is a critical threshold between naïve and memory T cells which may govern the ease of tolerance induction; the greater the memory T cell pool the more difficult it is to induce tolerance [29]. Control of disease activity with immunosuppressive drugs and hence disease severity, and disease chronicity are two criteria which will need attention in the planning of a phase III trial. In the secondary objective 3 of the 5 patients have been maintained without a relapse of uveitis 10–18 months after the immunosuppressive and tolerization treatment were discontinued. The remaining 2 patients relapsed within 1 month of cessation of the tolerization regimen. Furthermore, only 1 of the 5 responding patients developed recurrent oral ulcers, and arthralgia of the knees improved in 2 of 3 patients. Folliculitis or erythema nodosum in 2 patients or genital ulcers in 1 patient did not recur. The possibility that a decrease in immunosuppression might precipitate long-term sequelae, such as retinal vascular occlusion was not observed. The significance of these results will have to be verified in a double-masked phase III trial.
All but 1 patient expressed the HLA-B51·101 haplotype [30–32] which argues against this haplotype affecting the response to the tolerizing regime. The T cell proliferative responses to p336–351 were significantly raised only in patients with relapsing uveitis who started with a SI of 4·1 ± 0·8 and increased to 23·1 (±18·0) with the relapse of disease manifestations. The specificity of the response to p336–351 was demonstrated with little or no stimulation of T cell proliferation with the unrelated HSP60 peptide 136–151. Furthermore, a number of immune parameters showed significant differences between the 2 groups of patients. Tolerance appeared to be associated with a decrease in TH1 type of cells expressing CCR5+ and CXCR3+, central memory T cells expressing CCR7 and decrease or absent IFN-γ and TNF-α production. There was no significant p336–351 specific T cell response, and a decrease was found in the proportion of cells expressing the costimulatory CD28 and CD40 molecules. These results are consistent with TH2 type of cells being involved in oral tolerance (generating IL-4 and IL-10) [14]. The decrease in CCR7+ cells argues in favour of tolerization of central memory cells, as demonstrated recently in mice [29]. Furthermore, blockade or decrease in the costimulatory CD40 and CD28 molecules promote tolerance and memory cells are less susceptible to tolerization than naïve cells [33,34].
This novel oral tolerization regime, used for the first time in humans, that prevented relapse of uveitis and other manifestations of BD has to be confirmed by a phase III double-masked trial. However, in addition to safety of administration of the peptide-CTB conjugate, we have defined selection criteria of BD patients for the tolerization strategy; they should be adequately controlled with immunosuppressive drugs, so as to be free of disease activity before tolerization is started and the disease should be of short duration. The novel peptide-rCTB oral tolerance strategy might be applicable to a number of autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis and other types of uveitis in which induction of oral tolerance has not been achieved by using an antigen alone.
Acknowledgments
This work was supported by a grant from the Iris Fund for the Prevention of Blindness, the Swedish Science Council (Medicine), the Institute of Medical Science, Kawasaki, Japan, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Tristol AB linked the peptide to rCTB provided by SBL Vaccin.
REFERENCES
- 1.Lehner T. Behcet's Disease. In: Ledingham JGG, Warrell DA, editors. Oxford Textbook of Medicine. Oxford: Oxford University Press; 2003. pp. 1050–3. [Google Scholar]
- 2.Sakane T, Takeno M, Suzuki M, Inaba G. Behcet's Disease. New Engl J Med. 1999;341:1284–91. doi: 10.1056/NEJM199910213411707. [DOI] [PubMed] [Google Scholar]
- 3.Pickering MC, Haskard DO. Behcet's syndrome. J R Coll Physicians Lond. 2000;34:169–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima Y. Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the antigens in Behcet's Disease – a multi-centre study. J Rheumatol. 1989;16:506–11. [PubMed] [Google Scholar]
- 5.Isogai E, Ohno K, Takehashi K. Close association of Streptococcus sanguis uncommon serotypes with Behcet's disease. Bifidobact Microflora. 1990;9:27–41. [Google Scholar]
- 6.Kaneko S, Suzuki N, Yamashita N. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behcet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–12. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behcet's disease. Infect Immun. 1991;59:1434–41. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pervin K, Childerstone A, Shinnick T, Mizushima R, van der Zee R, Hasan A, Vaughan R, Lehner T. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behcet's disease. J Immunol. 1993;151:2273–82. [PubMed] [Google Scholar]
- 9.Hasan A, Fortune F, Wilson A, et al. Role of γδ T cells in patho-genesis and diagnosis of Behcet's disease. Lancet. 1996;347:789–94. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki N, Sakane T. Characterisation of heat shock protein specific T cells in patients with Behcet's disease. Rev Rheum. 1996;63:531–63. [Google Scholar]
- 11.Direskeneli H, Eksioglu-Demiralp E, Yavuz S, Ergun T, Shinnick T, Lehner T, Akogl T. T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behcet's disease. J Rheumatol. 2000;27:708–13. [PubMed] [Google Scholar]
- 12.Stanford MR, Kasp E, Whiston R, et al. Heat shock protein peptides reactive in patients with Behcet's disease are uveitogenic in Lewis rats. Clin Exp Immunol. 1994;97:226–31. doi: 10.1111/j.1365-2249.1994.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W, Hasan A, Wilson A, et al. Experimental mucosal induction of uveitis with the 60kD HSP derived peptide aa 336–351. Eur J Immunol. 1998;28:2444–55. doi: 10.1002/(SICI)1521-4141(199808)28:08<2444::AID-IMMU2444>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Weiner H. Oral tolerance immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–41. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 15.Mowat AM, Vinney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderton S, Burkhart C, Metzler B, Wraith D. Mechanisms of central and peripheral T-cell tolerance: lessons from experimental models of multiple sclerosis. Immunol Rev. 1999;169:123–37. doi: 10.1111/j.1600-065x.1999.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 17.Nussenblatt RB, Caspi RR, Mahdi R, Chan C-C, Roberge F, Lider O, Weiner HL. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral induction of tolerance with S-antigen. J Immunol. 1990;144:1689–95. [PubMed] [Google Scholar]
- 18.Rizzo LV, Miller-Rivero NE, Chan C-C, Wiggert B, Nussenblatt RB, Caspi RR. Interleukin-2 treatment potentiates induction of oral tolerance in a murine model of autoimmunity. J Clin Invest. 1994;94:1668–72. doi: 10.1172/JCI117511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dick AD, Cheng YF, Liversidge J, Forrester JV. Intranasal administration of retinal antigens suppresses retinal antigen-induced experimental autoimmune uveoretinitis. Immunology. 1994;82:625–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J-B, Holmgren J, Czerkinsky C. Cholera toxin B subunit: An efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–9. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J-B, Holmgren J, Czerkinsky C. Oral administration of cholera toxin B subunit conjugated to myelin basic protein protects against experimental autoimmune encephalomyelitis by inducing transforming growth factor-β-secreting cells and suppressing chemokine expression. Int Immunol. 2000;12:1449–57. doi: 10.1093/intimm/12.10.1449. [DOI] [PubMed] [Google Scholar]
- 22.Phipps PA, Stanford MR, Sun J-B, et al. Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit. Eur J Immunol. 2003;33:224–32. doi: 10.1002/immu.200390025. [DOI] [PubMed] [Google Scholar]
- 23.Nussenblatt RB, Gery I, Weiner HL. Treatment of uveitis by oral administration of retinal antigens: results of a phase I/II randomised masked trial. Am J Ophthalmol. 1997;123:583–92. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]
- 24.International Study Group for Behcet's Disease. Criteria for diagnosis of Behcet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 25.Dumonde DC, Kasp-Growchowska E, Graham EM, Sanders MD, Faure JP, de Kozak Y, Tuyen VV. Anti-retinal autoimmunity and circulating immune complexes in patients with retinal vasculitis. Lancet. 1982;ii:787–92. doi: 10.1016/s0140-6736(82)92679-4. [DOI] [PubMed] [Google Scholar]
- 26.Bunce M, Barnado MC, Proctor J, Marsh SG, Vilchers C, Welsh KI. High resolution of HLA-C typing by PCR-SSP. identification of allelic frequencies and linkage disequilibria in 604 unrelated random UK Caucasoids and a comparison with serology. Tissue Antigens. 1997;50:100–11. doi: 10.1111/j.1399-0039.1997.tb02847.x. [DOI] [PubMed] [Google Scholar]
- 27.Czerkinsky C, Russell MW, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody response in salivary glands and extramucosal tissues. Infect Immun. 1999;57:1072–8. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehlert A, Young DB. Biochemical and antigenic characterisation of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989;3:125–30. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–95. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno S, Nakayama E, Sugiura N, Itakura K, Aoki K. Specific histocompatibility antigens associated with Behcet's disease. Am J Ophthalmol. 1975;80:636–41. doi: 10.1016/0002-9394(75)90394-3. [DOI] [PubMed] [Google Scholar]
- 31.Yazici H, Akohan G, Yalcin B, Muftuoglu A. The high prevalence of HLA B5 in Behcet's disease. Clin Exp Immunol. 1977;30:259–61. [PMC free article] [PubMed] [Google Scholar]
- 32.Lehner T, Batchelor JR, Challacombe SJ, Kennedy L. An immunological basis for the tissue involvement in Behcet's syndrome. Immunology. 1979;37:895–900. [PMC free article] [PubMed] [Google Scholar]
- 33.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4– T cells. J Immunol. 2000;164:265–72. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 34.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–9. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]