Abstract
Although the inhibitory effect of iron on macrophage production of tumoricidal free radical nitric oxide (NO) has been reported, its possible influence on macrophage anti-tumour activity has not been established. In the present study, FeSO4 markedly reduced IFN-γ + LPS-induced NO synthesis in mouse and rat macrophages. The effect of iron coincided with the loss of macrophage cytotoxic activity against NO-sensitive C6 rat astrocytoma and L929 mouse fibrosarcoma cell lines, as measured by MTT assay for cellular respiration and the crystal violet test for cell viability. Tumour cell survival did not improve further in the presence of FeSO4 if macrophage NO release and cytotoxicity were already blocked by aminoguanidine. In accordance with the results obtained with exogenous iron, cell membrane permeable iron chelator o-phenanthroline enhanced both macrophage NO release and anti-tumour activity. Iron also down-regulated NO production and increased the viability of L929 fibrosarcoma cells stimulated with IFN-γ + LPS in the absence of macrophages. However, neither NO release nor cell viability was affected by iron addition to cultures of the C6 astrocytoma cell line. Iron was unable to prevent L929 and C6 cell death induced by the NO releasing chemicals SNP and SIN-1, indicating that iron-mediated inhibition of NO synthesis, rather than interference with its cytotoxic action, was responsible for the protection of tumour cells. Collectively, these results indicate that iron might protect tumour cells by reducing both macrophage and tumour cell-derived NO release.
Keywords: iron, nitric oxide, macrophage, tumour
INTRODUCTION
Iron is an essential nutrient for living cells that plays important roles in cellular processes such as the synthesis of DNA, RNA, and proteins, electron transport, cellular respiration, cell proliferation and differentiation, and regulation of gene expression [1]. However, both iron deficiency and iron overload can be pathogenic. Iron displays carcinogenic activity and the ability to favour tumour growth, due to catalytic effect on formation of hydroxyl radicals, promotion of tumour cell multiplication and suppression of the activity of host defense cells [2]. Accordingly, primary neoplasms develop at body sites of excessive iron deposits [3,4], and the invaded host attempts to withhold iron from the cancer cells via sequestration of the metal in newly formed ferritin [2]. Based on these observations, iron chelators have been successfully tested in the clinical setting as a component of anticancer therapy [5].
Nitric oxide is a free radical with complex biological activities, synthesized intracellularly from l-arginine by at least three different NO synthases (NOS). The endothelial and neuronal isoforms (eNOS, nNOS) are constitutively expressed and generate low amounts of NO involved in regulation of vascular tonus and neurotransmission, respectively [6]. The third isoform (iNOS) is induced by cytokines, tumour cells and microbial products mainly in macrophages, and generates large quantities of NO that have been implicated in antimicrobial and anti-tumour defence [7]. The tumoricidal action of macrophage-derived NO is mainly due to the inhibition of iron-containing enzymes and involves repression of DNA synthesis, mitochondrial respiration and the enzymes of the citric cycle in target cells [7].
There are multiple interactions between NO and iron metabolism. Through activation of iron regulatory proteins, NO controls the expression of transferin and ferritin, the critical proteins involved in iron transport and storage [8,9]. On the other hand, iron impairs the expression of macrophage iNOS and subsequent NO synthesis by inhibiting iNOS transcription [10–12]. While it has been proposed that down-regulation of macrophage NO release can contribute to iron-mediated tumour protection, this hypothesis has not been directly tested thus far.
In the present study, by using NO-sensitive tumour cell lines L929 and C6 as targets, we show that loss of macrophage anti-tumour activity in the presence of iron could indeed result from iron-mediated block of iNOS-dependent NO release.
MATERIALS AND METHODS
Reagents
The medium used for cell cultivation (culture medium) was HEPES-buffered RPMI 1640 (Flow Laboratories, Irvine, UK) supplemented with 5% fetal calf serum (ICN, Costa Mesa, CA, USA), 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 0.1% sodium pyruvate. Mouse and rat recombinant IFN-γ was obtained from PeproTech (Rocky Hill, NJ, USA). Lipopolysacharide (E. coli 055:B5, LPS), dimethyl sulphoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), FeSO4, o-phenanthroline, sulphanilamide, naphthylethylenediamine dihydrochloride, aminoguanidine, sodium nitroprusside (SNP) and 3-morpholinosydnonimine (SIN-1) were all from Sigma (St. Louis, MO, USA).
Cell cultures
The murine fibrosarcoma cell line L929 and rat astrocytoma cell line C6 were obtained from the European Collection of Animal Cell Cultures (Salisbury, UK), and grown in culture medium at 37°C in a humidified atmosphere with 5% CO2. For the experiments, C6 and L929 cells were detached by trypsinization and resuspended in culture medium. Resident macrophages were obtained from Dark Agouti (DA) rats or C57Bl/6 mice (animal facility of Institute for Biological Research, Belgrade, Serbia and Montenegro) by peritoneal lavage with cold PBS, followed by 1 h of incubation at 37°C and subsequent removal of nonadherent cells. Macrophages and/or tumour cells were incubated flat-bottom 96-well plates in 200 µl of culture medium. In all experiments, mouse or rat macrophages were seeded at 1 × 105/well or 5 × 104/well, respectively, and stimulated with mouse or rat IFN-γ (100 U/ml) and LPS (5 µg/ml). For investigating the anti-tumour action of macrophage- and chemically derived NO, tumour cells were plated at 1 × 104/well. For testing NO production by tumour cells, C6 and L929 cells were grown in 96-well plate until they reached confluence, and then stimulated with IFN-γ (250 U/ml for L929 cells and 500 U/ml for C6 cells) and LPS (5 µg/ml).
Nitrite measurement
Nitrite accumulation, an indicator of NO production, was measured using the Griess reagent [13]. Briefly, 50 µl aliquots of culture supernatants were mixed with an equal volume of Griess reagent (mixture at 1 : 1 of naphthylethylenediamine dihydrochloride and 1% sulphanilamide in 5% H3PO4), and incubated at room temperature for 10 min. The absorbance at 570 nm was measured in an automated microplate reader. Nitrite concentration (µm) was calculated from a NaNO2 standard curve.
Cell viability
Mitochondrial respiration, as an indicator of cell viability, was assessed by a colourimetric test that detects the conversion of the tetrazolium salt MTT into its formazan product by enzymes of the respiratory chain [14]. Briefly, MTT solution was added to cell cultures at the final concentration of 0.5 mg/ml and cells were incubated for an additional 1 h. Thereafter, medium was removed and cells were lysed in DMSO. The conversion of MTT to formazan, corresponding to mitochondrial respiratory activity, was monitored by automated microplate reader at 570 nm. Alternatively, cell viability was assessed by crystal violet assay that measures the number of viable adherent cells [15]. After removing nonadherent cells by repeatedly washing the cultures with PBS, cells were fixed with methanol and stained with 1% crystal violet solution at room temperature for 10 min. Plates were thoroughly washed with PBS, 33% acetic acid was added to each well and absorbance of dissolved dye, corresponding to the number of viable cells, was measured in a microplate reader at 570 nm. In both MTT and crystal violet assay, the viability of tumour cells after cocultivation with macrophages was obtained after subtracting the absorbance values of macrophages incubated alone, and expressed as percentage viability of control cultures.
Statistical analysis
Results from the representative of at least three independent experiments are presented as mean ± SD of triplicate observations. To analyse the significance of the differences between various treatments, we used analysis of variance (anova), followed by Student-Newman-Keuls test. Correlation between NO production and tumour cell viability was analysed by Spearman rank order correlation test. A P-value less than 0.05 was considered significant.
RESULTS
FeSO4 down-regulates IFN-g + LPS-induced NO synthesis in macrophages
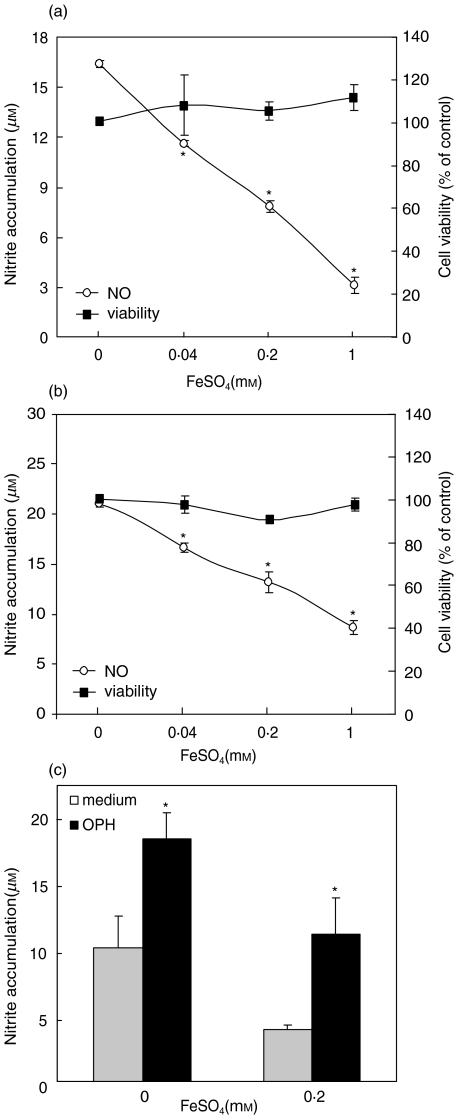
Compared to low, almost undetectable levels of nitrite in unstimulated cultures (<3 µm), stimulation of murine and rat macrophages with IFN-γ + LPS caused significant increase of nitrite accumulation in cell culture supernatants (Fig. 1a,b). The observed rise in nitrite concentration was almost completely blocked with aminoguanidine (from 14.2 ± 0.6 – 2.3 ± 0.3 and from 12.6 ± 0.8 – 3.9 ± 0.5 for murine and rat macrophages, respectively), a fairly selective inhibitor of inducible NOS isoform [16], thus confirming that nitrite accumulation resulted from iNOS-mediated NO release. Treatment with FeSO4 in a dose-dependent manner decreased the amount of NO produced by IFN-γ + LPS-activated macrophages (Fig. 1a,b). The viability of macrophages was not affected by FeSO4, as determined by MTT assay for mitochondrial respiration (Fig. 1a,b). The observed NO reduction by FeSO4 was due to ferrous ion (Fe2+), since cell-permeable Fe2+ chelator o-phenanthroline abolished FeSO4 effect (Fig. 1c). Accordingly, o-phenanthroline increased macrophage NO production in the absence of exogenously added FeSO4 (Fig. 1c), suggesting a role for endogenous, presumably intracellular iron in down-regulation of NO synthesis.
Fig. 1.
Effect of iron on IFN-γ + LPS-induced NO production in macrophages. (a) Mouse macrophages or (b) rat macrophages were stimulated with IFN-γ + LPS, in the presence or absence of different concentrations of FeSO4. (c) Mouse macrophages were stimulated with IFN + LPS, in the presence or absence of FeSO4 and/or o-phenanthroline (OPH; 0·2 mm). (a–c) Nitrite concentration was determined after 48 h, and macrophage viability was assessed at the same time by MTT test (control viability of 100% refers to macrophage cultures without FeSO4); *P < 0·05 refers to cell cultures without FeSO4 (a,b) or o-phenanthroline (c).
Iron-mediated reduction of NO synthesis coincides with the loss of macrophage anti-tumour activity
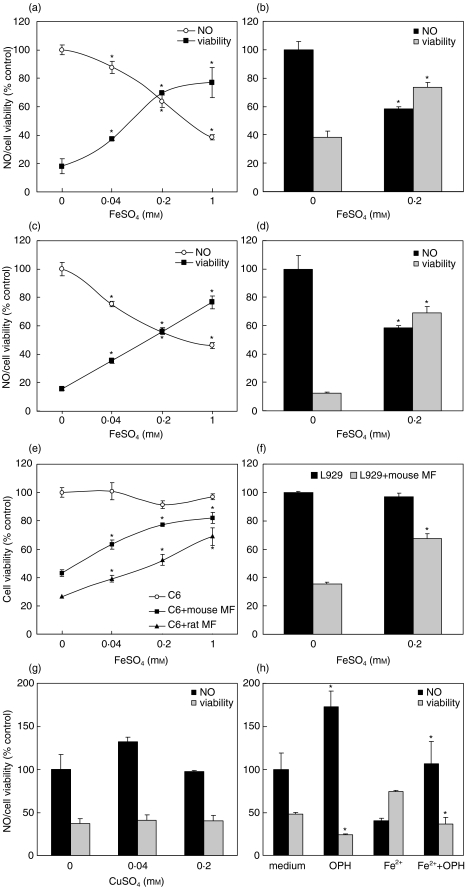
It is well known that NO released by activated macrophages acts as powerful antiproliferative and proapoptotic signal for tumour cells [17]. Thus, we next examined the influence of FeSO4 on tumour cell survival in cocultures of macrophages and NO-sensitive tumour cell lines L929 or C6. As expected, both rat and mouse macrophages, activated with IFN-γ + LPS, significantly reduced cellular respiration in cultures of C6 astrocytoma or L929 fibrosarcoma cells (Fig. 2a–d). During incubation with macrophages, tumour cells lost their processes and become round and detached from the well surface. The observed changes in mitochondrial respiration and cell morphology were paralleled by the loss of tumour cell ability to exclude trypan blue (not shown), therefore confirming macrophage cytotoxic effect. In accordance with the data obtained with macrophages alone (Fig. 1), the treatment with FeSO4 markedly decreased NO production in macrophage-tumour cell cocultures (Fig. 2a–d). This effect of FeSO4 coincided with significant dose-dependent improvement of tumour cell survival, as judged by both MTT test (Fig. 2a–d) and crystal violet assay (Fig. 2e,f). The presence of FeSO4 did not affect growth of tumour cell lines in the absence of macrophages (Fig. 2e,f); similar results were obtained with MTT assay (data not shown). Tumour cells were unlikely to significantly contribute to NO synthesis and subsequent anti-tumour activity, since both C6 and L929 cells produced only negligible amounts of NO (less than 3 µm) at low cell number (1 × 104/well) used for cocultivation with macrophages. The treatment with CuSO4 neither decreased NO production nor rescued tumour cells from macrophage-mediated cytotoxicity (Fig. 2g), suggesting that the observed protection of tumour cells was indeed mediated by Fe2+. This was further confirmed by finding that cell membrane-permeable Fe2+ chelator o-phenanthroline blocked FeSO4-mediated effects on NO production and tumour cell viability in macrophage-tumour cell cocultures (Fig. 2h). Interestingly, o-phenanthroline by itself, in the absence of exogenously added iron, markedly augmented NO production and reduced tumour cell viability (Fig. 2h), thus indicating a down-regulatory role for intracellular iron and/or trace amounts of extracellular iron in macrophage NO-mediated tumoricidal action.
Fig. 2.
Iron down-regulates macrophage anti-tumour activity. (a–d) Co-cultures of murine (a,b,e,f) or rat macrophages (c–e) with C6 (a,c,e) or L929 (b,d,f) cells were stimulated with IFN-γ + LPS, in the presence or absence of different concentrations of FeSO4. (g) Rat macrophages were incubated with C6 cells and stimulated with IFN-γ + LPS, in the presence or absence of CuSO4. (h) Co-cultures of murine macrophages and L929 cells were stimulated with IFN-γ + LPS, in the presence or absence of FeSO4 (0·2 mm) and o-phenanthroline (OPH; 0·2 mm). (a–h) After 48 h of incubation, nitrite concentration (a–d,g,h) was measured, and cell viability was determined by MTT (a–d,g,h) or crystal violet (e,f) assay. Control value for NO production (100%) represents macrophage NO release in the absence of FeSO4. Control value for the cell viability (100%, with standard deviations less than 10% in a–d,g,h) refers to viability of tumour cells incubated in the absence of macrophages; *P < 0·05 refers to cell cultures without FeSO4 (a–f) or o-phenanthroline (h).
Iron inhibits macrophage tumoricidal action and tumour cell autotoxicity by blocking NO release
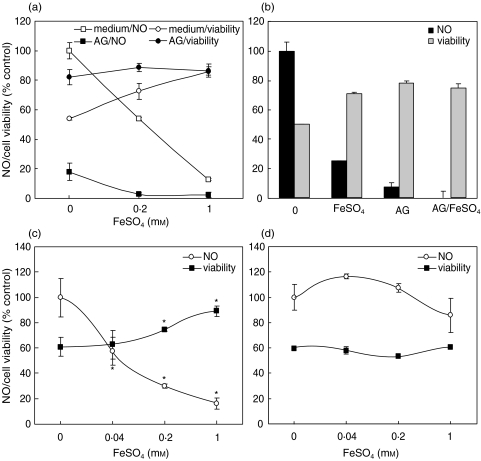
A strong and highly significant negative correlation was observed between NO production and tumour cell viability in macrophage-tumour cell cocultures treated with FeSO4 (r = −0·91 or r = −0·85 for cocultures of tumour cells with mouse or rat macrophages, respectively; P < 0·001), suggesting a causal relationship between reduced NO release and tumour cell protection by iron. To further confirm that iron might have protected tumour cells by reducing macrophage NO release, the effect of Fe2+ was investigated in the presence of iNOS inhibitor aminoguanidine. Expectedly, aminoguanidine abolished macrophage NO production and markedly attenuated their tumoricidal action (Fig. 3a,b). However, although FeSO4 by itself partly rescued the tumour cells from macrophage cytotoxicity, it did not further improve tumour cell viability when macrophage NO release was completely blocked by aminoguanidine (Fig. 3a,b). Next, we examined the influence of Fe2+ on NO production and tumour cell viability in the experimental setting in which tumour cells were the source of NO. To that effect, confluent L929 or C6 cells were stimulated with LPS + 2·5-fold (250 U/ml) or 5-fold (500 U/ml) higher dose of IFN-γ respectively. Under these conditions, both L929 and C6 cells produced significant amounts of NO (>10 µm, in comparison with <1 µm in unstimulated cultures) and down-regulated their respiration in NO-dependent manner [18,19]. FeSO4 markedly reduced NO production and augmented cell respiration in L929 cultures (Fig. 3c), suggesting that FeSO4 might rescue tumour cells from NO-mediated auto-toxicity. Interestingly, this effect was cell-specific, since FeSO4 had no influence on NO production and viability of C6 cells (Fig. 3d).
Fig. 3.
Iron protects tumour cells by down-regulating macrophage and tumour cell NO release. (a,b) Murine macrophages were incubated with C6 (a) or L929 (b) cells and stimulated with IFN-γ + LPS in the presence or absence of aminoguanidine (AG; 2 mm) and FeSO4 (0·2 mm ive (b)). (c,d) Confluent L929 (c) or C6 (d) cells were stimulated with IFN-γ + LPS, in the presence or absence of various concentrations of FeSO4. (a–d) Nitrite concentration and cell viability were determined after 48 h. Control value (100%) for NO production represents macrophage or tumour cell NO release in the absence of AG or FeSO4. Control value for the cell viability (100 ± 5·2%) refers to viability of tumour cells incubated in the absence of macrophages or IFN-γ + LPS (c,d); *P < 0·05 refers to cultures without FeSO4.
Iron does not affect NO generation and tumoricidal activity of chemically derived NO
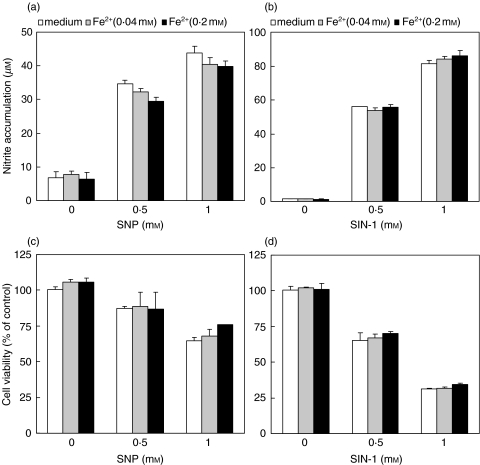
Finally, we examined the possibility that iron might partly interfere with NO-mediated anti-tumour action by scavenging NO radical or neutralizing its cytotoxic effect. However, such an assumption was excluded by finding that FeSO4 was unable to block either NO generation or the killing of C6 cells mediated by NO-releasing chemicals SNP and SIN-1 (Fig. 4a–d). Similar results were obtained with L929 fibrosarcoma cells as targets (data not shown). Therefore, iron seems to protect tumour cells by reducing both macrophage and tumour cell-derived NO release, rather than scavenging NO or blocking its toxicity towards tumour cells.
Fig. 4.
Iron does not affect tumoricidal action of exogenous NO. C6 cells were incubated with various doses of NO donors SNP (a,c) and SIN-1 (b,d), in the presence or absence of different concentrations of FeSO4. Nitrite concentration (a,b) and cellular respiration (c,d) were determined after 48 h of incubation.
DISCUSSION
In the present study we demonstrate that iron can down-regulate anti-tumour activity of macrophages by blocking their production of cytotoxic free radical NO. In accordance with our observation, it was described previously that iron reduced the transcription of iNOS in macrophages by inhibiting the binding affinity of transcription factors NF-IL-6 and hypoxia inducible factor-1 (HIF-1) to the iNOS promoter [10,11]. Besides macrophages, some tumour cells, including L929 fibrosarcoma and C6 astrocytoma cells, are capable of activating iNOS-dependent NO release in response to proinflammatory cytokines and/or LPS [18–22]. Our data obtained with L929 fibrosarcoma cells indicate for the first time that iron could also suppress NO production in tumour cells. Somewhat surprisingly, C6 astrocytoma cells were resistant to the inhibitory action of iron, thus indirectly revealing that intracellular signals responsible for iNOS induction in C6 cells might differ from those operative in macrophages and L929 fibroblasts. This is consistent with a number of reports suggesting that signal transduction pathways controlling iNOS expression indeed might differ in distinct cell types [19,21–23].
While the interference with macrophage anti-tumour activity has been previously proposed as partly responsible for tumour-promoting action of iron [24], a number of evidence from the present study indicate that this effect mainly depends on the interference of iron with tumoricidal NO release. There was a strong negative correlation between macrophage NO production and tumour cell viability in macrophage-tumour cell cocultures treated with iron, and iron failed to further improve tumour cell viability if macrophage iNOS activity was already blocked. Moreover, iron-mediated inhibition of NO release protected L929 fibrosarcoma cells from autocrine/paracrine action of NO, and the absence of the similar effect in C6 cells coincided with iron inability to affect their NO production. The latter result, in addition to data obtained with chemically generated NO, suggests that iron exerted its tumour-protecting action solely by inhibiting NO release, rather than scavenging NO radical or blocking its tumoricidal action. However, in another study with tumour cell lines and NO donors different from those used in our experiments, iron efficiently protected tumour cells from NO-mediated apoptosis [25], indicating that this effect might depend on cell type and/or NO source. It should be also noted that at the experimental conditions employed in our study, iron did not significantly affect tumour cell viability in the absence of IFN-γ stimulation and macrophages. While this seems to contradict previously reported ability of iron to augment tumour cell proliferation in vitro[26], this discrepancy might be easily explained by tumour cell-specificity of the growth-promoting effect of iron [27,28].
It is well known that macrophages, besides using NO, can kill tumour cells by other mechanisms, including secretion of TNF or reactive oxygen species [29,30]. Conflicting results, describing both positive and negative modulation, were obtained as to the iron ability to influence TNF synthesis in macrophages [31,32]. Nevertheless, the similar extent of iron-mediated protection in TNF-sensitive L929 cells [33] and TNF-resistant C6 cells [34] indicates that this effect of iron in our study was mainly independent of its putative interference with macrophage TNF release. Besides TNF, superoxide anion could also contribute to macrophage-mediated tumour cell killing, since it combines with NO to form extremely potent tumoricidal molecule peroxynitrite (ONOO–) [30]. However, neutralization of superoxide with superoxide dismutase did not affect tumoricidal activity of IFN-γ + LPS-stimulated macrophages (unpublished observation), indicating that superoxide was not involved in tumour cell killing in our experimental system. Thus, although Loegering et al. [35] reported an inhibitory effect of iron on macrophage oxidative burst, this probably did not contribute to iron-mediated tumour cell protection described in the present report. On the other hand, having in mind the limitations of the reductionist in vitro approach, it is possible that iron might protect tumour cells in vivo by simultaneously reducing macrophage NO and superoxide release.
Although NO was initially regarded as mainly tumoricidal, it has become increasingly clear in recent years that NO produced by tumour cells could also stimulate tumour growth by promoting vasodilatation and angiogenesis [36]. In contrast to its mainly pro-apoptotic action, NO has also been shown to prevent tumour cell apoptosis induced by some other apoptotic stimuli [37,38]. Thus, contrary to general view that iron primarily acts to protect tumour cells, which is supported by our present findings, it is also possible that iron-mediated inhibition of tumour cell NO release could interfere with angiogenesis and tumour growth in certain conditions. Indeed, it would be interesting to test this hypothesis in cancer models in which iron surprisingly protected animals from tumour growth [39]. While an extensive search for new iron chelators with better anti-tumour activity and improved pharmacokinetics is under way, elucidating the precise role of iron-mediated modulation of NO release in tumour growth remains of great importance for iron-based cancer therapy.
Acknowledgments
This work was supported by the Ministry of Science and Technology, Republic of Serbia (Grants no. 1664 and no. 2020). The authors wish to thank Dr Mihajlo Spasic (Institute for Biological Research, Belgrade, Serbia) for helpful discussion and for providing FeSO4 and o-phenanthroline.
REFERENCES
- 1.Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 2.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553–66. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 3.Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047–52. doi: 10.1056/NEJM198810203191603. [DOI] [PubMed] [Google Scholar]
- 4.Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer. 1994;56:364–9. doi: 10.1002/ijc.2910560312. [DOI] [PubMed] [Google Scholar]
- 5.Richardson DR. Iron chelators as therapeutic agents for the treatment of cancer. Crit Rev Oncol Hematol. 2002;42:267–81. doi: 10.1016/s1040-8428(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Meth Enzymol. 1996;269:134–48. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- 7.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 8.Drapier JC, Hirling H, Wietzerbin J, Kaldy P, Kuhn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12:3643–9. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss G, Goossen B, Doppler W, et al. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993;12:3651–7. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dlaska M, Weiss G. Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J Immunol. 1999;162:6171–7. [PubMed] [Google Scholar]
- 11.Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L. Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem. 1997;272:12236–43. doi: 10.1074/jbc.272.18.12236. [DOI] [PubMed] [Google Scholar]
- 12.Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–76. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Meth. 1984;68:167–75. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 16.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 17.Brune B, von Knethen A, Sandau KB. Nitric oxide (NO). an effector of apoptosis. Cell Death Differ. 1999;6:969–75. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 18.Trajkovic V, Markovic M, Samardzic T, Miljkovic DJ, Popadic D, Mostarica Stojkovic M. Amphotericin B potentiates the activation of inducible nitric oxide synthase and causes nitric oxide-dependent mitochondrial dysfunction in cytokine-treated rodent astrocytes. Glia. 2001;35:180–8. doi: 10.1002/glia.1083. [DOI] [PubMed] [Google Scholar]
- 19.Miljkovic D, Markovic M, Bogdanovic N, Mostarica Stojkovic M, Trajkovic V. Necrotic tumor cells oppositely affect nitric oxide production in tumor cell lines and macrophages. Cell Immunol. 2002;215:72–7. doi: 10.1016/s0008-8749(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 20.Feinstein DL, Galea E, Roberts S, Berquist H, Wang H, Reis DJ. Induction of nitric oxide synthase in rat C6 glioma cells. J Neurochem. 1994;62:315–21. doi: 10.1046/j.1471-4159.1994.62010315.x. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic V, Samardzic T, Stosic-Grujicic S, Popadic D, Trajkovic V. Cell-specific inhibition of inducible nitric oxide synthase activation by leflunomide. Cell Immunol. 2000;199:73–80. doi: 10.1006/cimm.1999.1600. [DOI] [PubMed] [Google Scholar]
- 22.Miljkovic D, Samardzic T, Mostarica Stojkovic M, Stosic-Grujicic S, Popadic D, Trajkovic V. Leflunomide inhibits activation of inducible nitric oxide synthase in rat astrocytes. Brain Res. 2001;889:331–8. doi: 10.1016/s0006-8993(00)03181-4. [DOI] [PubMed] [Google Scholar]
- 23.Rao KM. Molecular mechanisms regulating iNOS expression in various cell types. J Toxicol Environ Health B Crit Rev. 2000;3:27–58. doi: 10.1080/109374000281131. [DOI] [PubMed] [Google Scholar]
- 24.Huot AE, Gundel RM, Hacker MP. Effect of erythrocytes on alveolar macrophage cytostatic activity induced by bleomycin lung damage in rats. Cancer Res. 1990;50:2351–5. [PubMed] [Google Scholar]
- 25.Ferry-Dumazet H, Mamani-Matsuda M, Dupouy M, et al. Nitric oxide induces the apoptosis of human BCR-ABL-positive myeloid leukemia cells: evidence for the chelation of intracellular iron. Leukemia. 2002;16:708–15. doi: 10.1038/sj.leu.2402404. [DOI] [PubMed] [Google Scholar]
- 26.Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 27.Neumannova V, Richardson DR, Kriegerbeckova K, Kovar J. Growth of human tumor cell lines in transferrin-free, low-iron medium. In Vitro Cell Dev Biol Anim. 1995;31:625–32. doi: 10.1007/BF02634316. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheim EW, Nasrallah IM, Mastri MG, Stover PJ. Mimosine is a cell-specific antagonist of folate metabolism. J Biol Chem. 2000;275:19268–74. doi: 10.1074/jbc.M001610200. [DOI] [PubMed] [Google Scholar]
- 29.Wanebo HJ. Tumor necrosis factors. Semin Surg Oncol. 1989;5:402–13. doi: 10.1002/ssu.2980050606. [DOI] [PubMed] [Google Scholar]
- 30.Bauer G. Reactive oxygen and nitrogen species. efficient, selective, and interactive signals during intercellular induction of apoptosis. Anticancer Res. 2000;20:4115–39. [PubMed] [Google Scholar]
- 31.Tsukamoto H. Iron regulation of hepatic macrophage TNF-α expression. Free Radic Biol Med. 2002;32:309–13. doi: 10.1016/s0891-5849(01)00772-9. [DOI] [PubMed] [Google Scholar]
- 32.Olynyk JK, Clarke SL. Iron overload impairs pro-inflammatory cytokine responses by Kupffer cells. J Gastroenterol Hepatol. 2001;16:438–44. doi: 10.1046/j.1440-1746.2001.02456.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto M, Yip YK, Vilcek J. Tumor necrosis factor. specific binding and internalization in sensitive and resistant cells. Proc Natl Acad Sci USA. 1985;82:7626–30. doi: 10.1073/pnas.82.22.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George P, Louisot P, Levrat C. A possible involvement of endogenous polyamines in the TNF-alpha cellular sensitivity. FEBS Lett. 1998;425:371–5. doi: 10.1016/s0014-5793(98)00268-3. [DOI] [PubMed] [Google Scholar]
- 35.Loegering DJ, Raley MJ, Reho TA, Eaton JW. Macrophage dysfunction following the phagocytosis of IgG-coated erythrocytes: production of lipid peroxidation products. J Leukoc Biol. 1996;59:357–62. doi: 10.1002/jlb.59.3.357. [DOI] [PubMed] [Google Scholar]
- 36.Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol. 2000;50:139–48. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay U. Tumour immunotherapy: developments and strategies. Immunol Today. 1999;20:480–2. doi: 10.1016/s0167-5699(99)01526-1. [DOI] [PubMed] [Google Scholar]
- 38.Melino G, Bernassola F, Catani MV, et al. Nitric oxide inhibits apoptosis via AP-1-dependent CD95L transactivation. Cancer Res. 2000;60:2377–83. [PubMed] [Google Scholar]
- 39.Omara FO, Blakley BR. Influence of low dietary iron and iron overload on urethan-induced lung tumors in mice. Can J Vet Res. 1993;57:209–11. [PMC free article] [PubMed] [Google Scholar]