Abstract
The mechanisms of virus-induced airway hyperresponsiveness in asthma and allergy and the failure of host defence in patients suffering from secondary airway infections are still largely unknown. The aim of this study was to examine whether the presence of allergic rhinitis or susceptibility to recurrent sinusitis affects the structural and cellular changes in nasal mucosa during natural colds and convalescence. We compared the mucosal changes in biopsy samples during acute natural colds (days 2–4 of illness) and convalescence (3 weeks later) in patients with allergic rhinitis (n = 9), patients with susceptibility to sinusitis (n = 19) and healthy controls (n = 20). We saw similarly increased numbers of mucosal T and B lymphocytes and mast cells and increased vascular density during the acute colds compared to convalescence in all the three groups. The allergic subjects had elevated levels of eosinophils in the acute phase (P = 0·03), and the allergic and sinusitis-prone subjects had elevated levels of epithelial T cells (P = 0·04) and low levels of mast cells (P = 0·005) in convalescence compared to the control group. The sinusitis-prone subjects lacked intraepithelial cytotoxic cells in convalescence. In the allergic subjects, the reticular basement membrane was thicker in the acute phase compared to the convalescence (P = 0·05). These results suggest that various cells of the airways, including inflammatory and structural cells, are involved during viral respiratory infections in subjects with allergic rhinitis. The small numbers of mast cells and cytotoxic lymphocytes in the sinusitis-prone subjects may be related to their susceptibility to bacterial complications.
Keywords: cellular immunity, common cold, epithelium, virus
INTRODUCTION
Viral respiratory infections involve significant morbidity and mortality in all age groups. In some specific groups of patients, including those with respiratory allergies or a predisposition to bacterial sinusitis, viral respiratory infections are of greater clinical significance. In allergic subjects, the baseline allergic inflammation may modify the course of infection and even cause airway obstruction in asthmatic children and adults [1,2]. The airway inflammation during viral infections in human subjects has been studied mainly in the nasal mucosa and secretions due to their good accessibility. Histopathological and cytological studies have shown that an acute respiratory viral infection is associated with an inflammatory cell reaction consisting mainly of neutrophil infiltration in both mucosa and mucosal secretions [3–5], although mucosal infiltration has not been demonstrated in all studies [6]. In addition, in natural colds there appears to be a T cell and B cell response and some evidence of a mast cell reaction [7], which have not been seen in experimental virus infections [6,8].
The mechanisms of virus-induced airway hyperresponsiveness in asthma and allergy and the failure of host defence in patients suffering from secondary airway infections are still largely unknown. Most of our knowledge of the virus-induced reactions is based on in vitro and animal studies [9], and more data on the connection between the viral immune response and airway inflammation in human subjects are needed. We hypothesized that the presence of allergic rhinitis or susceptibility to recurrent sinusitis could affect the quantity of inflammatory and immune cells (T and B cells, cytotoxic T cells, mast cells, neutrophils and eosinophils) and the quality of the structural characteristics (epithelial and reticular basement membrane thickness and vascular density) of nasal mucosa during natural colds and convalescence. To clarify this, we compared the cellular response and structural changes in nasal biopsy samples during acute natural colds (days 2–4 of illness) and convalescence (3 weeks later) in patients with allergic rhinitis, patients with susceptibility to sinusitis and healthy controls. The viral aetiology was studied by means of virus isolation, antigen detection and rhino-polymerase chain reaction (PCR) methods.
METHODS
Subjects
Forty-eight subjects (18–64 years of age; median 35 years, 36 female) with acute community-acquired cold were enrolled during two periods between 1 February and 15 May 1996 and 15 August and 31 December 1996. The subjects were to have had symptoms of acute cold for 2–4 days, nasal symptoms, no prior sinus surgery or nasal polyps and no respiratory infections or antibiotic treatment during the preceding month.
Nine subjects were suffering from allergic IgE-mediated rhinitis [10]. All had symptoms and a positive finding of immediate type hypersensitivity in allergen skin-prick tests (birch and alder in six cases, cat or dog hair in five, grass in four, dust mites in two and Artemisia vulgaris plant in one) (Prick-Lancett, Ewo Care AB, Gislaved, Sweden) [11]. The subjects had not used oral or nasal corticosteroids, nasal nedocromil and antihistamines during the preceding month. Four of these subjects had had a few sinusitis episodes in the past.
Nineteen subjects suffered from recurrent bacterial sinusitis defined as at least 2-yearly episodes during the preceding 2 years. The recurrent episodes had been verified by repeated maxillary punctures in all cases.
Twenty normal healthy volunteers were used as a control group. They were non-allergic (negative findings in skin-prick tests) and had never had clinical sinusitis.
All the subjects were examined at enrolment (acute phase) and again 21 days later (convalescent phase). Background variables were collected, and the subjects’ ratings of 10 symptoms (runny nose, nasal stuffiness, sneezing, sore throat, facial pain, cough, fatigue or lethargy, muscle aches, chills and headache) for 3 days with a scale from 0 (not present) to 10 (very severe) were summed to obtain the total symptom score.
The viral aetiology of the cold was evaluated by antigen detection (adeno, respiratory syncytial (RS), influenza A and B, parainfluenza 1–3 viruses) [12] from nasal mucus and virus isolations (adeno, RS, influenza A and B, parainfluenza 1–3 and enteroviruses) [12] and rhino-PCR from nasopharyngeal swabs [13–15]. Several methods were used to maximize virus detection.
The research was carried out with the approval of the Ethics Committee of Oulu University Hospital and the written informed consent of the subjects.
Nasal biopsies
Nasal mucosal biopsies were taken in the acute and convalescent phases from the inferior turbinate following local application of 4% lidocain [16]. The specimens were fixed in formalin and embedded in paraffin, and 4-µm-thick sections were stained with haematoxylin-eosin (eosinophils and neutrophils) and immunohistochemical methods (mast cells, T and B lymphocytes, F VIII, TIA1) [17]. In brief, for mast cell staining, the sections were treated with trypsin (1 g/l; 10 min at 37°C) and incubated with the mouse monoclonal antibody against mast cell tryptase (Clone AA1; Dako, Glostrup, Denmark). For T lymphocytes (CD3), rabbit polyclonal antiserum (Code A 0452; Dako) was used after boiling the sections in 10 ml/l citrate buffer for 20 min. For B lymphocytes (CD20) detection, the sections were incubated with monoclonal antibodies (Clone L26; Dako). For Factor VIII, rabbit polyclonal antiserum (Code A 0082; Dako) was used after boiling in Tris-EDTA (dilution 1 : 5000; 15 min) and incubated for 60 min at 37°C. Monoclonal antibody against human TIA1 (Coulter Corporation, Miami, FL, USA) was used for the recognition of cytotoxic lymphocytes, as described previously [17,18]. Bound antibodies were detected with the avidin–biotin method (Dako) and using DAB as a chromogen. For negative control stainings, the primary antibody was replaced with phosphate-buffered saline.
All histological analyses were performed by an investigator who was blinded to all the other information. Cells in the epithelium and in the mucosa underlying the reticular basement membrane of the epithelium were counted three or more consecutive fields and expressed as cells/mm2. The number of vessels in the subepithelial layer was calculated similarly, and expressed as vessels/mm2.
Haematoxylin and eosin-stained sections and an ocular micrometer were used to measure the thickness of the epithelium and the basement membrane. Due to the curling of the specimen during sampling and fixation, most sections showed random selection of perpendicular and non-perpendicular parts. A representative area perpendicular to the surface was to be included in the section use for the evaluation of these parameters, to avoid overestimating the thickness. The nasal mucosa included no structures with regularly perpendicular orientation to the surface that could have been utilized. Therefore, the region in the section where the basement membrane was thinnest (this region is theoretically closest to parallel orientation) was used for the measurement. The thickness was measured as the distance between the basal cell membrane of the basal epithelial cells and the lower border of the homogenous, eosinophilic collagen layer of the basement membrane zone. For the assessment of epithelial thickness, full thickness of the epithelium was to be included in the section in the same area. At least three measurements at random points with a minimum distance of 20 µm between the points were made in the appropriate area, and the mean was recorded for comparison. To assess the repeatability of the reticular basement membrane thickness measurement, 10 randomly selected cases were counted twice blindly, and a good correlation between the assays was obtained (correlation coefficient 0·9). Similarly, a good correlation was obtained between two independent cell counts (correlation coefficient 0·8).
Of the 48 subjects, 45 had biopsies evaluable by microscopy in the acute phase and 45 in the convalescent phase.
Statistical analysis
Summary statistics are expressed as medians and ranges. The data were analysed using Wilcoxon's ranked-pairs analysis for paired data. Unpaired data were analysed using χ2 analysis for categorical variables and the Mann–Whitney U-test for continuous variables with two groups and the Kruskal–Wallis analysis of variance with more groups.
RESULTS
Viral infection
Viral aetiology was verified in 32 (67%) of the subjects, the most frequent virus being the rhinovirus. The viral aetiologies and subject characteristics were similar in the three groups, except that the allergic subjects were younger and smoked more often than the subjects in the other groups (Table 1). The subjects with recurrent sinusitis had more severe symptoms compared to the other subjects. Up to the convalescent visit, 13 subjects (eight sinusitis-prone, four allergic and one control subject) had received antimicrobial treatment based on the clinical and radiological findings. At the convalescent phase, only three subjects (6%) had persistent symptoms.
Table 1.
Subject characteristics of 48 adults with natural colds according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis1
| Allergic rhinitis (n = 9) | Recurrent sinusitis (n = 19) | Healthy controls (n = 20) | P2 | |
|---|---|---|---|---|
| Mean (s.d.) age in years | 27 (6) | 32 (8) | 40 (12) | <0.01 |
| Women | 6 (67) | 14 (74) | 16 (80) | 0.73 |
| Current smokers | 5 (56) | 3 (16) | 8 (40) | 0.08 |
| Median (range) symptom scores | 140 (31–259) | 177 (29–375) | 121 (61–319) | 0.05 |
| Mean (s.d.) no. of sinusitisepisodes in the past year | 2.4 (3) | 2.7 (1) | 0 | <0.01 |
| Viral aetiology3 | 0.85 | |||
| No virus detected | 3 (33) | 5 (26) | 8 (40) | |
| Rhinovirus | 3 (33) | 5 (26) | 3 (15) | |
| Other | 3 (33) | 9 (48) | 9 (45) |
Numbers of subjects and percentages unless otherwise stated.
Tested with the χ2 test for categorical variables and the Kruskal–Wallis test for continuous variables.
Detected by means of viral culture, antigen detection and rhino-polymerase chain reaction.
Cellular response
Similarly in all of the three groups, the number of T lymphocytes (P < 0·001) and mast cells (P = 0·004) was increased in the nasal epithelium and that of T and B lymphocytes (P = 0·001 and P < 0·001, respectively) and mast cells (P < 0·001) in the subepithelial layer in the acute phase compared to the convalescent phase (Table 2). No significant changes could be seen in the neutrophil counts between the acute and convalescent phases. The only background characteristic that affected the cell counts was smoking, which resulted in lower subepithelial B lymphocyte counts than non-smoking at convalescence (P = 0·03). None of the cellular counts in the acute phase predicted the subsequent need for antimicrobial treatment.
Table 2.
.Nasal inflammatory cells/mm2 (median and ranges) in 48 adults with natural colds according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis1
| Allergic rhinitis (n = 9) | Recurrent sinusitis (n = 19) | Healthy controls (n = 20) | ||||
|---|---|---|---|---|---|---|
| Acute | Conv | Acute | Conv | Acute | Conv | |
| T lymphocytes | ||||||
| Epithelial | 508 (253·787) | 297 (106·711)2 | 592 (128·1168) | 309 (0·600)2 | 457 (114·1714) | 81 (0·487)2 |
| Subepithelial | 215 (82·760) | 79 (56·286) | 288 (102·939) | 128 (41·378) | 232 (31·735) | 102 (5·204) |
| B lymphocytes | ||||||
| Epithelial | 0 (0·82) | 0 (0·27) | 0 (0·20) | 0 (0·20) | 0 (0·20) | 0 (0·27) |
| Subepithelial | 133 (0·495) | 56 (5·367) | 138 (26·388) | 68 (0·156) | 99 (5·255) | 51 (0·184) |
| Mast cells | ||||||
| Epithelial | 0 (0·84) | 0 (0·119) | 0 (0·300) | 0 (0·31) | 26 (0·196) | 0 (0·107) |
| Subepithelial | 88 (82·245) | 34 (7·95)3 | 109 (61·245) | 51 (10·102)3 | 149 (61·408) | 82 (20·136)3 |
| Neutrophils | ||||||
| Epithelial | 0 (0·79) | 0 (0·0) | 0 (0·128) | 0 (0·267) | 0 (0·38) | 0 (0·80) |
| Subepithelial | 0 (0·20) | 0 (0·0) | 0 (0·41) | 0 (0·41) | 0 (0·41) | 0 (0·20) |
| Eosinophils | ||||||
| Epithelial | 0 (0·0) | 0 (0·0) | 0 (0·64) | 0 (0·0) | 0 (0·38) | 0 (0·62) |
| Subepithelial | 20 (0·82) | 0 (0·10) | 10 (0·61) | 0 (0·41) | 0 (0·20) | 0 (0·20) |
Acute = acute infection, Conv = convalescent phase 21 days later.
Figures are medians and ranges unless otherwise stated.
Difference between allergic, recurrent sinusitis and control subjects; P = 0.03 (Kruskal–Wallis analysis).
Difference between allergic, recurrent sinusitis and control subjects; P = 0.005 (Kruskal–Wallis analysis).
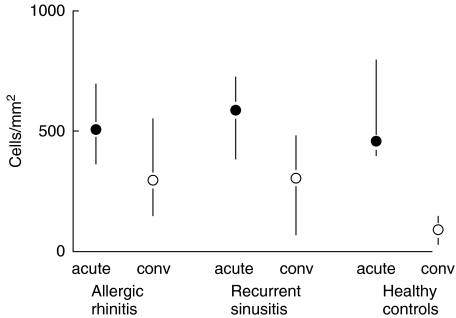
In the allergic and sinusitis-prone subjects, the epithelial T lymphocyte counts in the convalescent phase remained significantly higher compared to the control group (Fig. 1, Table 2). The allergic subjects had the largest amounts of TIA1-positive intraepithelial lymphoid cells, particularly during the convalescent phase (Table 3). The quantity of TIA1-positive cells in the acute and convalescent phases was different in the sinusitis-prone subjects only (P = 0·01), who had no TIA1 cells at the convalescent phase.
Fig. 1.
Difference in epithelial T lymphocyte counts (median and interquartile range) between the acute and convalescent phases of natural colds (acute = acute infection, conv = convalescent phase 21 days later) according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis (P = 0·04 for the difference in the convalescent counts between the groups, Kruskal–Wallis analysis).
Table 3.
Cytotoxic (TIA1) T cells in 48 adults with natural colds according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis
| Allergic rhinitis (n = 9) | Recurrent sinusitis (n = 19) | Healthy controls (n = 20) | ||||
|---|---|---|---|---|---|---|
| Acute | Conv | Acute | Conv | Acute | Conv | |
| Epithelial cytotoxic (TIA1) T lymphocytes | ||||||
| Median (range) number of cells/mm2 | 73 (0·472) | 50 (0·237)2 | 43 (0·400) | 0 (0·97)2 | 14 (0·382) | 21 (0·142)2 |
| Median percentage from epithelial T cells1 | 10 % | 15 %3 | 7 % | 0 %3 | 2 % | 5 %3 |
Acute = acute infection, Conv = convalescent phase 21 days later.
Calculated by dividing the quantity of TIA1 positive T cells by the total amount of epithelial T cells in each subjects and taking the median from these values.
Difference between allergic, recurrent sinusitis and control subjects; P = 0.01 (Kruskal–Wallis analysis).
Difference between allergic, recurrent sinusitis and control subjects; P = 0.006 (Kruskal–Wallis analysis).
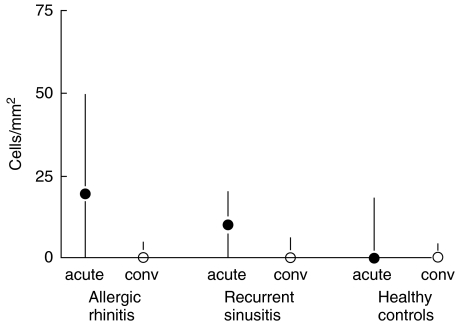
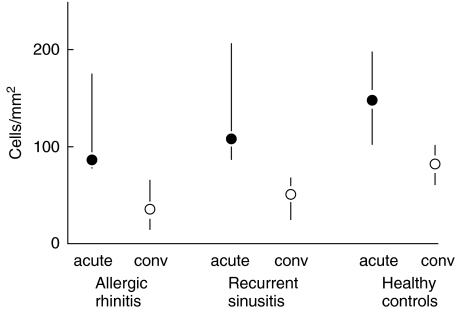
Among the allergic subjects, the number of subepithelial eosinophils was increased significantly in the acute phase compared to the convalescent phase (P = 0·03, Fig. 2, Table 2). The number of subepithelial mast cells in convalescence was significantly lower in the allergic and sinusitis-prone subjects than in the control subjects (Fig. 3, Table 2).
Fig. 2.
Difference in subepithelial eosinophil counts (median and interquartile range) between the acute and convalescent phases of natural colds (acute = acute infection, conv = convalescent phase 21 days later) according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis (P = 0·05 for the difference in the acute counts between the allergic and control subjects, Mann–Whitney U-test).
Fig. 3.
Difference in subepithelial mast cell counts (median and interquartile range) between the acute and convalescent phases of natural colds (acute = acute infection, conv = convalescent phase 21 days later) according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis (P = 0·005 for the difference in the convalescent counts between the groups, Kruskal–Wallis analysis).
Structural changes
Similarly, in all the three groups, the vascular density was significantly higher in the acute phase than in the convalescent phase (P < 0·001, Table 4). None of the background characteristics associated with the structural variables.
Table 4.
Nasal mucosal structure in 48 adults with natural colds according to the presence of allergic rhinitis and susceptibility to recurrent sinusitis1
| Allergic rhinitis (n = 9) | Recurrent sinusitis (n = 19) | Healthy controls (n = 20) | ||||
|---|---|---|---|---|---|---|
| Acute | Conv | Acute | Conv | Acute | Conv | |
| Epithelial shedding (n, %) | 2 (22) | 0 | 6 (31) | 2 (10) | 4 (20) | 4 (20) |
| Epithelial thickness (µm) | 50 (17·113) | – | 42 (5·88) | – | 42 (10·105) | – |
| Reticular basement membrane thickness (µm) | 6 (2·10)2 | 3 (2·8)2 | 4 (2·9) | 4 (1·10) | 5 (2·10) | 5 (2·7) |
| Vascular density (FVIII) | 184 (122·245)3 | 119 (82·163) | 166 (102·252)3 | 122 (82·156) | 204 (136·245)3 | 109 (51·177) |
Acute = acute infection, Conv = convalescent phase 21 days later.
Figures are medians and ranges unless otherwise stated.
Difference between the acute and convalescent phases; P = 0.05 (Wilcoxon's ranked-pairs analysis).
Difference between allergic, recurrent sinusitis and control subjects; P = 0.07 (Kruskal–Wallis analysis).
The allergic subjects had a significantly thicker reticular basement membrane in the acute phase compared to the convalescent phase (P = 0·05), whereas the thickness remained the same in the other groups (Table 3). The thickness of the reticular basement membrane was related inversely to the symptom scores in the sinusitis-prone subjects (rs = −0·64, P = 0·005), but not in the other two groups.
Another structural difference was that the vascular density during the acute infection was significantly lower in the allergic and recurrent sinusitis groups than in the control group (Table 4).
DISCUSSION
We compared the cellular and structural changes in the nasal mucosa during natural colds in subjects with allergic rhinitis and susceptibility to recurrent sinusitis to the responses seen in healthy controls. What we saw was similarly increased numbers of mucosal T and B lymphocytes and mast cells and increased vascular density during the acute cold compared to convalescence in all of the three groups. However, some differences also emerged between the groups. The allergic subjects had elevated levels of eosinophils in the acute phase, and the allergic and sinusitis-prone subjects had elevated levels of epithelial T cells and low levels of mast cells in convalescence compared to the control group, even though almost all of the subjects were asymptomatic at convalescence. In convalescence, the allergic subjects also had the highest numbers of intraepithelial cytotoxic lymphocytes (TIA1-positive cells), while such cells were absent in the sinusitis-prone subjects. Furthermore, among the allergic subjects the reticular basement membrane was thicker in the acute phase compared to the convalescent phase.
Natural colds instead of experimentally induced viral colds were studied. Although this meant that true baseline levels could not be obtained because the subjects were recruited based on the presence of symptoms, it was thought important to study natural colds, as previous findings have suggested differences between experimental and natural respiratory infections, with experimentally induced infections producing fewer symptoms [19]. However, all subjects had symptoms compatible with a respiratory infection that either disappeared or was alleviated during follow-up, and we were able to verify the viral aetiology in 67% of the cases, in line with other studies dealing with natural colds [20]. There were no significant differences in the clinical characteristics or symptom scores between the subjects with and without viruses identified. The subjects were not recruited during the period of seasonal allergies (from the end of May until the beginning of August), to avoid allergy symptoms that could have confounded the cold symptoms. Taken together, these factors seem to suggest that the subjects truly had a respiratory viral infection.
Studies conducted on a murine model have shown a clear T lymphocyte response in the nasal mucosa during a viral infection, with persistence of virus-specific T cells [21]. In contrast, the findings on human subjects with viral respiratory infection are contradictory. During experimental rhinovirus colds, increased T lymphocyte counts in nasal secretions together with unaltered mucosal lymphocyte counts in nasal biopsies have been reported [4,6,8]. Fraenkel et al. had 10 atopic subjects in their nasal biopsy study, but there were also no changes in the inflammatory cells during the colds of these subjects [6]. Here, we were able to demonstrate a clear and rapid accumulation of T and B lymphocytes in the nasal mucosa during the acute phase of a natural cold, which confirmed our earlier observations [7]. It may be that a symptomatic natural cold results in a more marked immune response than an experimental viral infection.
The responses in the allergic and sinusitis-prone subjects were similar to those seen in the control subjects, except that a delayed accumulation of intraepithelial T cells was seen, indicating a prolonged inflammatory reaction in spite of the fact that almost all subjects were asymptomatic. Theoretically, this late response suggests that these were virus-specific T cells, but we cannot be conclusive because only the total number of T cells was counted. We do not know whether this prolonged T cell accumulation in the allergic and sinusitis-prone subjects would have disappeared slowly over time or if it was a permanent feature, as we did not take any further biopsies. However, Igarashi et al. did not find any significant differences in T and B cells in the nasal mucosa between allergic and nonallergic subjects, even though the biopsies were taken in their allergy seasons [16].
TIA1 is a cytotoxic granule-associated protein expressed in T cells and natural killer cells [18]. Increased expression is considered to indicate activation of T cells [22]. This response may be linked with the termination of the inflammatory response, such as elimination of virally infected epithelial cells [23]. During the acute phase, we saw no significant differences in TIA1-expressing cells between the groups. In convalescence, the allergic subjects had the highest numbers of TIA1-positive cells, while such cells were absent in the sinusitis-prone subjects. We have shown previously that subjects with allergic rhinitis have more severe mucosal changes in their paranasal sinuses than non-allergic subjects during viral colds [24]. The higher level of T-cell cytotoxicity found here might be one explanation for the more severe mucosal reaction. Our finding of the absence of TIA1-positive cells in the sinusitis-prone subjects in convalescence may be linked with their susceptibility to suffer from bacterial infections. TIA1 regulates tumour necrosis factor (TNF)-α expression, and in mutant mice lacking TIA1, TNF-α expression was increased and the response to bacterial lipopolysaccharide amplified [25].
Our finding of eosinophilic inflammation and accumulation of mast cells in the nasal mucosa in the allergic subjects during a natural cold confirms the findings on rodents showing that viral infections stimulate allergic airway inflammation [26]. The eosinophilic inflammation was only temporary, as it had disappeared by the time of the follow-up assessment at convalescence. The decreased number of mast cells in the allergic and sinusitis-prone subjects compared to the control subjects during the period of convalescence was an unexpected finding, which may be due to increased mast cell degranulation. Another possible explanation is that the low number of mast cells in convalescent phase represents a constant abnormality in the sinusitis-prone subjects. As the mast cells may have an important role in the innate immune response [27,28], their scarcity may be associated with the increased risk of bacterial sinusitis.
We found that the reticular basement membrane in the allergic subjects was thicker during the acute viral infection compared to convalescence. Chanez et al. did not report basement membrane thickening in the nasal mucosa in asthmatic patients with allergic rhinitis during a steady state [29]. Basement membrane thickening is due to an increased number of subepithelial myofibroblasts that deposit interstitial collagens [30]. Our findings indicate that subjects with allergic rhinitis may have this feature similarly to asthmatic subjects and support the idea that basement membrane thickening is a dynamic process and not a permanent feature of airway inflammation [30].
In conclusion, we sought differences in virus-induced airway inflammation in patients with allergic rhinitis and recurrent sinusitis by examining the cellular and structural responses in the nasal mucosa during natural colds. We found various cells of the airways, including inflammatory and structural cells, to be involved during viral respiratory infections in allergic rhinitis. These cells included T and B cells, mast cells and eosinophils. The sinusitis-prone subjects had low numbers of mast cells and intraepithelial cytotoxic lymphocytes in convalescence, which may be linked with the susceptibility to bacterial complications. Both the sinusitis-prone and allergic subjects had a similar increase in the numbers of epithelial T cells in convalescence, but there was a difference in the subset of the cells, namely the cytotoxic T cells. This difference, as well as other dissimilarities in the mast cells and eosinophils, shows clearly that the two diseases have their own separate immunological pathogenesis.
Acknowledgments
We thank Hanna Tuokko PhD for analysing the viral samples. Ms Mirja Vahera and Erja Tomperi are acknowledged for technical help in immunohistochemical stainings. No external financial support was received.
REFERENCES
- 1.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br Med J. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winther B, Farr B, Turner RB, Hendley JO, Gwaltney JM, Jr, Mygind N. Histopathologic examination and enumeration of polymorphonuclear leukocytes in the nasal mucosa during experimental rhinovirus colds. Acta Otolaryngol. 1984;413(Suppl.):S19–24. doi: 10.3109/00016488409128537. [DOI] [PubMed] [Google Scholar]
- 4.Levandowski RA, Weaver CW, Jackson GG. Nasal-secretion leukocyte populations determined by flow cytometry during acute rhinovirus infection. J Med Virol. 1988;25:423–32. doi: 10.1002/jmv.1890250406. [DOI] [PubMed] [Google Scholar]
- 5.Naclerio RM, Proud D, Lichtenstein LM, et al. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988;157:133–42. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Immunohistochemical analysis of nasal biopsies during rhinovirus experimental colds. Am J Respir Crit Care Med. 1994;150:1130–6. doi: 10.1164/ajrccm.150.4.7921447. [DOI] [PubMed] [Google Scholar]
- 7.Alho OP, Karttunen TJ, Karttunen R, Tuokko H, Koskela M, Uhari M. Lymphocyte and mast cell counts are increased in the nasal mucosa in symptomatic natural colds. Clin Exp Immunol. 2003;131:138–42. doi: 10.1046/j.1365-2249.2003.02037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winther B, Innes DJ, Bratsch J. Lymphocyte subsets in the nasal mucosa and peripheral blood during experimental rhinovirus infection. Am J Rhinol. 1992;6:149–56. [Google Scholar]
- 9.Holtzman MJ, Morton JD, Shornick LP, et al. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial × viral-allergic paradigm. Physiol Rev. 2002;82:19–46. doi: 10.1152/physrev.00020.2001. [DOI] [PubMed] [Google Scholar]
- 10.Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–24. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Skin tests used in type I allergy testing. Position paper. Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology Allergy. 1989;44(Suppl.):1–59. [PubMed] [Google Scholar]
- 12.Arstila P, Halonen P. Direct antigen detection. In: Lennette EH, Halonen P, Murphy FA, editors. Laboratory diagnosis of infectious diseases: principle and practice. New York: Springer-Verlag; 1988. pp. 60–75. [Google Scholar]
- 13.Al-Nakib W, Tyrrell DA. Picorna viridae: rhinoviruses-common cold viruses. In: Lennette EH, Halonen P, Murphy FA, editors. Laboratory diagnosis of infectious diseases: principle and practice. New York: Springer-Verlag; 1988. pp. 723–42. [Google Scholar]
- 14.Halonen P, Rocha E, Hierholzer J, et al. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–53. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyypia T, Auvinen P, Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989;70:3261–8. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi Y, Goldrich MS, Kaliner MA, Irani AM, Schwartz LB, White MV. Quantitation of inflammatory cells in the nasal mucosa of patients with allergic rhinitis and normal subjects. J Allergy Clin Immunol. 1995;95:716–25. doi: 10.1016/s0091-6749(95)70177-x. [DOI] [PubMed] [Google Scholar]
- 17.Augustin M, Karttunen TJ, Kokkonen J. TIA1 and mast cell tryptase in food allergy of children: increase of intraepithelial lymphocytes expressing TIA1 associates with allergy. J Pediatr Gastroenterol Nutr. 2001;32:11–8. doi: 10.1097/00005176-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Anderson P, Nagler-Anderson C, O'Brien C, et al. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990;144:574–82. [PubMed] [Google Scholar]
- 19.Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–86. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 20.Corne JM, Lau L, Scott SJ, Davies R, Johnston SL, Howarth PH. The relationship between atopic status and IL-10 nasal lavage levels in the acute and persistent inflammatory response to upper respiratory tract infection. Am J Respir Crit Care Med. 2001;163:1101–7. doi: 10.1164/ajrccm.163.5.9902047. [DOI] [PubMed] [Google Scholar]
- 21.Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8 (+) T cells persist in the upper respiratory tract following influenza virus infection. J Immunol. 2001;167:3293–9. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- 22.Cesano A, Visonneau S, Clark SC, Santoli D. Cellular and molecular mechanisms of activation of MHC nonrestricted cytotoxic cells by IL-12. J Immunol. 1993;151:2943–57. [PubMed] [Google Scholar]
- 23.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–17. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 24.Alho OP, Karttunen TJ, Karttunen R, et al. Subjects with allergic rhinitis show signs of more severely impaired paranasal sinus functioning during viral colds than nonallergic subjects. Allergy. 2003;58:767–71. doi: 10.1034/j.1398-9995.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 25.Piecyk M, Wax S, Beck AR, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–63. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barends M, Boelen A, de Rond L, et al. Influence of respiratory syncytial virus infection on cytokine and inflammatory responses in allergic mice. Clin Exp Allergy. 2002;32:463–71. doi: 10.1046/j.1365-2222.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- 27.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–7. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 28.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 29.Chanez P, Vignola AM, Vic P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med. 1999;159:588–95. doi: 10.1164/ajrccm.159.2.9801022. [DOI] [PubMed] [Google Scholar]
- 30.Vignola AM, Mirabella F, Costanzo G, et al. Airway remodeling in asthma. Chest. 2003;123(Suppl.):S417–22. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]