Abstract
The immunological response in HTLV-1 infected individuals is characterized by a prominent Type-1 cytokine response with high production of IFN-γ and TNF-α. In contrast, helminthic infections and in particular chronic schistosomiasis are associated with a predominant production of IL-4, IL-5, IL-10 and IL-13. Liver fibrosis is the main pathological finding in schistosomiasis that occurs after many years of infection. This pathology is T cell dependent but the immune response mechanisms are not completely understood. The North-east region of Brazil is endemic for both HTLV-1 and schistosomiasis. In the present study the immune response, clinical severity, and therapeutic response to praziquantel of patients with schistosomiasis coinfected with HTLV-1 were compared with patients infected only with S. mansoni. Patients with HTLV-1 and S. mansoni had lower levels of IL-5 (P < 0·05) and higher levels of IFN-γ (P < 0·05) in cultures stimulated with S. mansoni antigen and decreased S. mansoni antigen specific IgE levels when compared with patients with schistosomiasis without HTLV-1 coinfection. Liver fibrosis was mild in all HTLV-1 coinfected patients and efficacy of praziquantel was lower in patients dually infected than in patients infected only with S. mansoni.
Keywords: HTLV-1, schistosomiasis, liver fibrosis
INTRODUCTION
Human T cell leukaemia virus type-1 (HTLV-1) infects an estimated 20 million people worldwide [1]. Adult T cell leukaemia and lymphoma (ATLL) and HTLV-1 associated myelopathy (HAM/TSP) are the main diseases caused by HTLV-1, but other diseases such us Sjögren syndrome, polyarthritis, uveitis, infective dermatitis and lymphocytic alveolitis are documented clinical manifestation of HTLV-1 infection. The immunologic response in HTLV-1 infection is characterized by an exaggerated T cell response with high production of IFN-γ and TNF-α [2,3]. The predominant IFN-γ production and lymphocyte proliferation occur even in unstimulated cultures [2,4]. In contrast, helminthic infections and in particular chronic schistosomiasis are associated with a predominant type-2 immune response [5,6]. The main pathological finding in schistosomiasis is liver fibrosis, and late severe fibrosis occurs in about 6% of chronically S. mansoni infected patients [7]. The hepatic pathology of schistosomiasis is T cell dependent but the immunologic mechanisms mediating liver damage are not completely understood.
The impact of HTLV-1 on helminthic infection has been reported in patients coinfected with Strongyloides stercoralis[8–10]. In such cases, coinfection with HTLV-1 decreases the predominant type-2 immune response observed in strongyloidiasis [11] as well as S. stercoralis specific and total IgE antibodies [12,13]. In addition, disseminated and recurrent strongyloidiasis are associated with HTLV-1 coinfection [14–16].
Salvador, the capital of the state of Bahia, located in the North-east of Brazil has the highest (1·35%) prevalence of HTLV-1 infection as reported in blood donors throughout Brazil [17]. The North-east region of Brazil is also endemic for schistosomiasis making it possible to evaluate the association of these two diseases. In the present study the immune response in patients with schistosomiasis coinfected with HTLV-1 was compared with that patients only infected with S. mansoni. Additionally, clinical and ultrassonography evaluation of liver fibrosis and therapeutic response to praziquantel were determined.
MATERIALS AND METHODS
Patients
Patients infected with HTLV-1 were recruited in the HTLV-1 multidisciplinary clinic, located in Hospital Universitário Prof Edgard Santos (HUPES) in Salvador, Bahia, Brazil. The clinic started in 2000 and follows more than 500 HTLV-1 infected individuals, most of them referrals from two blood banks in Salvador. All patients admitted in the clinic are asked to undergo 3 stool examinations. Of the 309 HTLV-1 individuals who had stool examination, S. mansoni eggs were found in 26 and 22 of them agreed to participate of this study (Group 1, schistosomiasis and HTLV-1 coinfected). These 22 patients had no clinical manifestations associated with HTLV-1 being considered HTLV-1 infected individuals. To determine the frequency of schistosomiasis, 331 HTLV-1-seronegative blood donors were screened by three stool examinations for schistosomiasis. To obtain patients with schistosomiasis who were HTLV-1-seronegative (n = 44, Group II) an existing cohort of patient (N = 164) in the S. mansoni endemic area of Caatinga do Moura, was used to select at a ratio of 2 to one by matching age and sex with Group I individuals (schistosomiasis and HTLV-1 coinfection). HTLV-1 positive and S. mansoni negative individuals (Group III) were selected from the HTLV-1 clinic matched by age and sex with Group II individuals (HTLV-1 and S. mansoni infection). Healthy University Hospital employees (n = 19) who were seronegative for HTLV-1 and absent helminthes by three stool examinations served as a control (group IV). The diagnosis of schistosomiasis was made by a positive fecal examination (Hoffman technique) and the Kato-Katz method was used to quantify the number of eggs per gram of stool [18]. After informed consent, a clinical history by standardized questionnaire was obtained and a complete physical examination and abdominal ultrasound were performed. The laboratory analysis included determination of cytokines (IFN-γ, IL-5, IL-10 and TNF-α) in supernatants of unstimulated cultures and in supernatant of soluble adult worm antigen preparation (SWAP) stimulated peripheral blood mononuclear cells (PBMC). After blood collection all patients with schistosomiasis were treated with praziquantel (50 mg/kg weight (n = 18) or oxamniquine 20 mg/kg weight (n = 2). The informed consent and experimental protocol were approved by the committee on human subjects of the Hospital Universitário Prof Edgard Santos, Salvador Bahia, Brazil that conforms to national guidelines.
Ultrassonography
Ultrassonography examination was performed with the Quantum 2000 Siemens ultrasound with a convex transductor of 3·5 Mhz, according to a previously published technique [19]. Grading of hepatic fibrosis was determined according with WHO criteria established in 1993 and previously revalidated [7]. Patients were classified in four different degrees according to the mean thickness of four portal tracts after the first division from the right and left branches of portal vein: degree 0: <3 mm thickness, degree I: 3–5 mm, degree II: 5–7 mm, degree III thickness: >7 mm.
Immunological studies
HTLV-1 serology.
A commercial HTLV-1 ELISA test (Cambridge Biotech, Cambridge, MA, USA) was used and positive tests were confirmed by commercial Western blot (HTLV Blot 2·4, Genelabs, Singapore).
Cytokine determination.
IFN-γ, IL-5, IL-10 and TNF-α in supernatants of mononuclear cells were measured by commercial ELISA. Briefly, peripheral blood monoclonal cells were obtained by density gradient centrifugation using lymphocyte separation media (LSM; Organon Teknika Coorporation, Durham, NC, USA) immediately the blood have been drawn. After washing in saline, the cells were adjusted to 3 × 106/ml in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% AB sera containing 100 U Penicillin G and 10 µg/ml of streptomycin. The cells were either unstimulated or stimulated with 2 µg/ml soluble adult Schistosoma mansoni worm antigen (SWAP). All cultures were incubated at 37°C in 5% CO2 for 72 h. Supernatant fluids were collected and stored at −20°C. IFN-γ (Genzyme Corp., Cambridge, MA, USA), IL-5, IL-10 and TNF-α (PharMingen, San Diego, CA, USA) levels were measured by sandwich ELISA and the results were expressed as pg/ml using a standard curve generated using recombinant cytokines. Although the expression of cytokines in vitro may not necessarily reflects what occurs in vivo, this is not the case in the infection caused by HTLV-1 or S. mansoni. TNF-α, IL-1 and IFN-γ are increased in perivascular infiltrating cells of HAM/TSP patients [20] and high levels of IFN-γ in serum and in cerebrospinal fluid are observed in HAM/TSP patients [21]. Similarly, in schistosomiasis high expression of type-2 cytokines are found in the liver [22] and in peripheral blood cells [5]. Reported values represent the difference between the stimulated cultures minus unstimulated cultures. Since we found that IFN-γ levels in subjects infected with HTLV-1 were similar in unstimulated or stimulated cultures, the IFN-γ data presented were the values observed in unstimulated cultures.
IgE specific to S. mansoni antigen.
Analysis of IgE specific to SWAP was performed by ELISA. Briefly, plates were coated with SWAP antigen at 40 µg/ml and left overnight at 4°C. In order to eliminate competition from IgG antibodies, immunoassay were performed with 100 µl of serum diluted 1 : 10 in phosphate-buffered saline with 0·05% Tween (PBS-T) containing 25% Sepharose–Protein G (Pharmacia, Uppsala, Sweden) following manufacturer's instructions. After incubation at room temperature for 15 min samples (tests and controls) were centrifuged at 650× g for 5 min. The IgG preabsorbed serum samples were used at final dilution of 1 : 4. The coated plates were blocked with PBS-5% bovine serum albumin and 100 µl of serum samples were incubated overnight to develop primary reactions. Secondary reactions were carried out with 100 µl of affinity-purified antibody phosphatase labelled goat anti-human IgE (Kirkegaard & Perry Laboratories, MD, USA). The ELISA was developed with 100 µl of p-nitrophenyl phosphate, and the absorbance changes (optical density [OD]) were measured by a spectrophotometer at 405 nm. The cut-offs of the immunoassay were determined using the mean plus 3 SD of the absorbance obtained with serum from 15 healthy unexposed individuals.
Treatment of S. mansoni infection
The treatment of schistosomiasis was performed with praziquantel (50 mg/kg weight) divided in two doses and given in the same day or oxamniquine (20 mg/kg weight) in a single dose. The use of one or another drug was dependent on their availability, since the medications were provided by the Healthy Secretary's office. Cured was defined as three negative stool examinations by the Hoffman technique 60 days after therapy [22,23], because patients continue to release viable S. mansoni eggs shortly after treatment. The possibility of re-infection was ruled out based on epidemiological and parasitological data. The patients were not living in endemic areas of S. mansoni at the time of the study and all of them denied exposure to contaminated water after therapy.
Statistical analysis
The Rank Sum Test was used to compare the means. Fisher's exact test was used to compare the proportions. The Chi-square test was used to compare the prevalence of S. mansoni infection.
RESULTS
The frequency of S. mansoni infection was 4·7 fold higher (26/309, 8·4%) in individuals infected with HTLV-1 than a comparable group of HTLV-1-seronegative individuals (6/331, 1·8%) (P < 0·05). The clinical characteristics and the ultrasound findings of 22 schistosomiasis patients coinfected with HTLV-1 and schistosomiasis HTLV-1-seronegative controls are shown on Table 1. The control group had a slightly lower mean age than the HTLV-1 coinfected patients because the majority of individuals over 40 years of age living in the endemic area are free of schistosomiasis. With the exception of three schistosomiasis and HTLV-1 coinfected patients, the remaining patients were symptom-free. One coinfected patient complained of abdominal pain and diarrhea. A second coinfected patient had only abdominal pain and the third had only diarrhoea. None of the 22 patients had splenomegaly, an index of severe hepatic fibrosis. Only one case (4·5%) had mild hepatomegaly. In contrast, age- and sex-matched control schistosomiasis and HTLV-1-seronegatvie cases showed a significantly higher frequency of clinical parameters for hepatic fibrosis. Splenomegaly was observed in 4·5% and hepatomegaly in 25% of the HTLV-1-seronegative schistosomiasis controls from Caatinga do Moura. Ultrassonography studies were used to further quantify the observed clinical findings. An absence of or a mild degree of fibrosis was noted in 21 schistosomiasis patients coinfected with HTLV-1 studied by ultrasound (Table 1). In contrast, 34% of the HTLV-1-seronegative schistosomiasis control group had degree II evidence of liver fibrosis that was significantly different (P < 0·05) between the two groups. We also quantified current Schistosoma infection burden by measuring Schistosoma eggs in the stool. The number of eggs/gram of stool was significantly lower in HTLV-1 coinfected schistosomiasis patients than in HTLV-1 seronegative Schistosoma infected controls (Table 1).
Table 1.
Clinical and ultrasound evaluation in S. mansoni and HTLV-1 Co-infected patients and patients only infected by S. mansoni
| Schistosomiasis groups | |||
|---|---|---|---|
| Patient characteristics | HTLV-1 coinfected (n = 22) | HTLV-1-noninfected (n = 44) | P-value |
| Age (mean ± SD) | 40 ± 10 | 32 ± 12 | >0·05 |
| Gender (male/female) | 13/9 | 26/18 | >0·05 |
| Hepatomegaly | 1/22 (4·5%) | 11/44 (25%) | = 0·05 |
| Splenomegaly | 0/22 (0%) | 2/44 (4·5%) | >0·05 |
| Ultrasound degree I | 21/22 (95·5%) | 29/44 (66%) | <0·05 |
| Ultrasound degree II | 1/22 (4·5%) | 15/44 (34%) | <0·05 |
| Kato Katz (X) | 24 eggs/g stool gram | 399 eggs/g stool | <0·05 |
Grade III fibrosis was not observed. In this study only 8 of 66 patients evaluated had other parasites (S. stercoralis, A. lumbricoides and T. trichiura) that was distributed in each group. These patients were treated for other helminthes prior to the immunological evaluation.
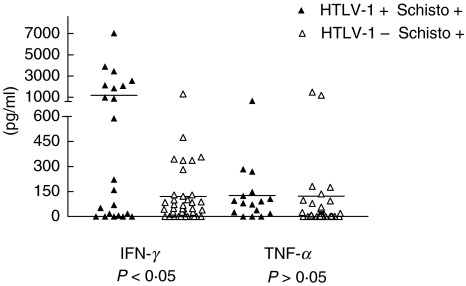
We next evaluated whether HTLV-1 infection modified the immune response in patients with S. mansoni by measuring the cytokine profile. Participated as controls patients with schistosomiasis without HTLV-1, individuals only infected with HTLV-1 and healthy subjects without HTLV-1 and S. mansoni. The levels of IFN-γ, TNF-α determined in supernatants from adult SWAP stimulated lymphocytes cultures are shown in Fig. 1. The mean ± SD of IFN-γ levels in 22 patients coinfected with S. mansoni and HTLV-1 was 1182 ± 1785 pg/ml ranging from 0 to 7040 pg/ml. This was higher (P < 0·05) than the mean of IFN-γ of 40 patients only infected with S. mansoni (120 ± 229 pg/ml ranging from 0 to 1317 pg/ml). In supernatant of unstimulated cultures of subjects only infected with HTLV-1 without schistosomiasis, IFN-γ levels were 1540 ± 1628 pg/ml with variation of 0–6995 pg/ml (data not shown). The mean levels of TNF-α in supernatants of lymphocyte cultures of 16 patients with S. mansoni and HTLV-1 (127 ± 168 pg/ml) ranging from 0 to 667 were similar than that observed in 30 patients who were only infected with S. mansoni (122 ± 339 pg/ml).
Fig. 1.
IFN-γ and TNF-α production in PBMC stimulated with SWAP in S. mansoni and HTLV-1 coinfected patients (▴) and in chronic schistosomiasis patients (Δ) from an endemic area.
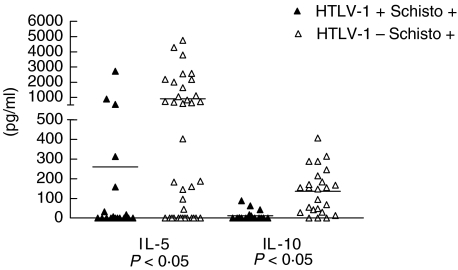
Cytokines, IL-5, IL-10 and IL-4, are thought to reflect the Th2 immune response of schistosomiasis. We therefore, directly measured IL-5 and IL-10 production and indirectly evaluated IL-4 by assessing S. mansoni specific IgE production. The mean ± SD of IL-5 levels in 18 patients coinfected with S. mansoni and HTLV-1 was 260 ± 659 pg/ml with variation of 0–2716 pg/ml. This value was lower than (P < 0·05) that observed in 37 patients only infected with S. mansoni (907 ± 1289 pg/ml with ranging of 0–4747 pg/ml (Fig. 2). IL-5 levels in subjects without HTLV-1 and without S. mansoni infection were 2 ± 2 pg/ml (data not shown). Moreover the IL-10 levels in 18 patients coinfected with S. mansoni and HTLV-1 (12 ± 25 pg/ml with variation of 0–88 pg/ml) was lower (P < 0·05) than that observed in 24 patients only infected with S. mansoni (136 ± 114 pg/ml with variation of 0–407 pg/ml).
Fig. 2.
IL-5 and IL-10 production in PBMC stimulated with SWAP in S. mansoni and HTLV-1 coinfected (▴) and in chronic schistosomiasis patients (Δ) from an endemic area.
Considering that the degree of S. mansoni infection is associated with liver fibrosis and that egg load may alter the immunological response, the frequency of liver fibrosis determined by ultrasound and the cytokine levels in the HTLV-1 S mansoni coinfected patients were compared with that observed in a subgroup of patients who had only schistosomiasis and had low degree of S. mansoni egg excretion. In 17 of the 44 patients with only schistosomiasis the degree of egg was lower than 200 eggs/g/stool with mean of 57 ± 59 eggs/g/stool. The frequency of grade II of liver fibrosis in these patients was 50% compared to 4·5% in patients coinfected with HTLV-1 and S. mansoni. Furthermore levels of IFN-γ (1182 ± 1785 pg/ml) was significantly higher and of IL-5 (260 ± 659 pg/ml) significantly lower in the patients coinfected with HTLV-1 and S. mansoni than in the 17 patients with only schistosomiasis and similar egg load.
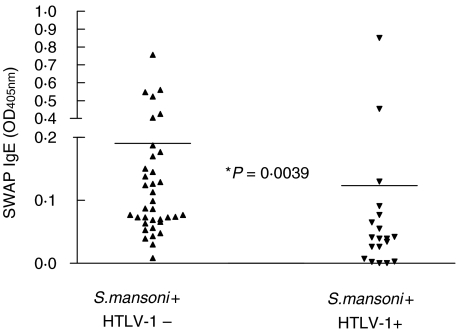
The distribution of IgE, expressed in OD in patients with schistosomiasis without HTLV-1 infection, and in those coinfected with HTLV-1 is shown on Fig. 3. The mean IgE in patients without HTLV-1 infection was 0·190 ± 0·170 compared to 0·123 ± 0·191 in patients with schistosomiasis associated with HTLV-1 infection (P < 0·05).
Fig. 3.
Soluble (adult) worm antigen (SWAP)-specific IgE from patients with S. mansoni infection and S. mansoni coinfected with HTLV-1.
Twenty-two HTLV-1 and S. mansoni coinfected patients were treated (20 with praziquantel and 2 with oxamniquine) but repeated stool results were available in only 20 cases. Therapeutic failure, defined as the presence of S. mansoni eggs two months after therapy was documented in 4 of 20 (20%) HTLV-1 and S. mansoni coinfected patients; all were treated with praziquantel. The 20% of therapeutic failure in HTLV-1 and S. mansoni coinfected patients was significantly higher than the 2·3% rate (1 of 44) observed in HTLV-1-seronegative S. mansoni-infected controls (P < 0·05) (Table 2). Two patients with HTLV-1 and S. mansoni coinfection required three courses of treatment before becoming egg-free (two with praziquantel and once with oxamniquine). A third patient remained S. mansoni-infected, despite two courses of therapy with praziquantel and oxamniquine. The fourth patient has persistence of eggs of S. mansoni in the stool despite 8 courses of treatment with both drugs. Neither patient had re-exposure to contaminated water.
Table 2.
Reduced therapeutic response to anti-schistosomal drugs in S. mansoni and HTLV-1 co-infected patients
| S. mansoni infected groups | Numbers cured (%) |
|---|---|
| HTLV-1-noninfected | 43/44 (98%)* |
| HTLV-1 coinfected | 16/20 (80%)* |
P < 0·05, Fisher's exact test.
DISCUSSION
Although HTLV-1 and schistosomiasis occurs in areas where both diseases are endemic, no previous study has evaluated the impact of HTLV-1 on schistosomiasis. Here, we showed that the prevalence of S. mansoni is higher in HTLV-1 infected subjects than in HTLV-1-seronegative controls. Importantly, HTLV-1 modified the clinical outcome of schistosomiasis and altered the immune response to parasitic antigens. Additionally, HTLV-1 coinfection reduced the efficacy of antischistosomal drugs and at least one patient failed to eradicate S. mansoni infection after 8 courses of treatment.
Schistosomiasis is one of most important helminthic diseases, affecting two hundred millions of individuals mainly in Africa, Asia and South America. The majority of patients with schistosomiasis have an intestinal or hepatointestinal form of the disease characterized by diarrhea and/or constipation and abdominal pain. Liver fibrosis, the main pathology is observed in 6% of patients with long-standing chronic schistosomiasis [7]. In this severe form of the disease, patients develop hepatosplenomegaly, portal hypertension and died as a result of uncontrollable gastrointestinal bleeding. Several factors have been associated to liver fibrosis including the genetic background, the degree of infestation and host immunological response. Initial studies in experimental model of schistosomiasis suggested that type-1 cytokines were associated with granuloma formation [24]. However, more recent accumulated data point to the importance of IL-4 and IL-13 in inducing fibrosis [25–27]and the ability of IL-12 and IFN-γ to decrease the fibrosis in experimental model of schistosomiasis [28,29]. The changes in the immune response to S. mansoni antigen observed in patients coinfected with HTLV-1 may have modified the clinical manifestation of schistosomiasis in these patients. In such case it is possible that the high IFN-γ production by itself or by decreasing the Th2 type of immune response is preventing the development of fibrosis, and would be the clinical correlate to the experimental findings [28,29].
Patients coinfected with HTLV-1 and schistosomiasis did show a lower S. mansoni eggs burden in the stool. We cannot exclude a low infection/exposure rate as a contribution to lessen clinical disease. However, in such clinical study where the exposure/infection burden could not be accurately determined, it can also be argued that the lower egg burden may also be directly due to HTLV-1 infection. In such case the high Th1 response could facilitate a Th1-associated antischistosome response and contribute for parasite killing and reduced egg excretion. The low levels of S. mansoni egg excretion observed in our patients may also be due the fact that they live in urban areas were re-exposure of S. mansoni infection is uncommon. Alternatively, excretion of fewer eggs may be due to a decrease in S. mansoni type-2 cytokine production, since egg excretion is directly correlated with type-2 immune response [30]. This supposition can be tested in future studies by evaluating whether immunological perturbation by HTLV-1 interferes with the passage of eggs from the portal system to the intestinal lumen. Anyway the low egg excretion observed in the HTLV-1 coinfected patients does not seem to be the reason for the differences in the immunological and ultrasound findings observed between the two groups. When the ultrasound and immunological findings of the HTLV-1 S mansoni coinfected patients were compared with a subgroup of patients having only schistosomiasis but with similar degree of infection than those dually infected with S. mansoni and HTLV-1, this last group had significantly lower liver fibrosis, increasing levels of IFN-γ and decreasing in IL-5 than patients with only schistosomiasis.
Immunological response in patients with chronic schistosomiasis is characterized by decreased IFN-γ production and enhancement in IL-4, IL-5 and IL-10 levels. This predominant type-2 immune response is independent of the degree of infection measured by eggs/stool gram and occurs in all clinical forms of schistosomiasis [5,31]. It is induced by parasite antigen and has been related to host defense mechanism against S. mansoni[23,32,33]. HTLV-1 infection is associated with an exaggerated T cell response with spontaneous lymphocyte proliferation and production of high levels of IFN-γ and TNF-α[2,4]. We have previously shown that HTLV-1 decreases antigen specific type-2 immune response in patients with strongyloidiasis [12,13] another important helminthic infection in tropical countries. In such case HTLV-1 down regulate defense mechanisms against S. stercoralis leading to the development of disseminated and recurrent S. stercoralis infection [14–16,34,35]. In this study we demonstrated that IFN-γ secretion was up-regulated in patients with schistosomiasis and HTLV-1. In contrast IL-5, a typical Th2 cytokine highly secreted in schistosomiasis was down regulated in patients coinfected with HTLV-1 and S. mansoni. Since there was a tendency for an inverse correlation between IFN-γ and IL-5 levels, it is possible that the down regulation of IL-5 was related to the enhancement of IFN-γ observed during HTLV-1 infection. IgE is elevated in parasitic infection and S. mansoni parasite specific IgE [36] and high IgE:IgG4 ratio have been associated with resistance to re-infection with S. mansoni[37]. Herein, we showed that infection with HTLV-1 significantly reduces the levels of parasite-specific IgE for schistosomiasis in patients infected with this parasite.
Oxamniquine and praziquantel are the standard treatment for schistosomiasis and there is no report of resistance to these drugs in Brazil. In this study, we observed a high rate of therapeutic failure to these drugs. A high rate of therapeutic failure against another helminthes has also been observed in patients coinfected with HTLV-1 and S. stercoralis [38,39]. Although the mechanism of therapeutic failure observed in strongyloidiasis and herein in schistosomiasis is not clear, it is possible that the cure of this helminthic infection is also dependent of the presence of an appropriate immune response present.
HTLV-1 infection induces activation of T cells with a predominant and exaggerated production of IFN-γ. This study showed that HTLV-1 and S. mansoni coinfection changes the immunological response to S. mansoni, reduced clinical disease and fibrosis resulting from schistosomiasis and lowered the therapeutic response to antischistosomal drugs.
Acknowledgments
We thank Andréa Magalhães for technical assistance and Elbe Silva for preparation of the manuscript. This work was supported by the Brazilian Research Council (CNPq) and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB). EMC is a senior investigator of the CNPq.
REFERENCES
- 1.Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I) J Emerg Med. 2000;18:109–19. doi: 10.1016/s0736-4679(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho EM, Bacellar O, Porto MAF, Santos SB, Galvão-Castro B, Neva FA. Cytokine Profile and Immunomodulation In Asymptomatic HTLV-1 Infected Blood Donors. J Acquir Immune Defic Syndr Hum Retrov. 2001;26:1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Goon PKC, Igakura T, Hanon E, Mosley AJ, Gould BAKG. High Circulating Frequencies of Tumor Necrosis Factor Alpha-and Interleukin-2-Secreting Human T-lymphotropic Virus Type 1-Specific CD4+ T cells in Patients with HTLV-1-Associated Neurological Disease. J Virol. 2003;77:9716–22. doi: 10.1128/JVI.77.17.9716-9722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer A, Jacobson S, Reuben JF. Spontaneous lymphocyte proliferation is elevated in asymptomatic HTLV-1 positive Jamaicans. Lancet. 1989;8668:923–4. doi: 10.1016/s0140-6736(89)91591-2. [DOI] [PubMed] [Google Scholar]
- 5.Araujo MI, de Jesus AR, Bacellar O, Sabin E, Pearce E, Carvalho EM. Evidence of a T helper type-2 activation in human schistosomiasis. Eur J Immunol. 1996;26:1399–403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD, Donohue TS, Goldhill J. Cytokine Regulation of Host Defense Against Parasitic Gastrointestinal Nematodes. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 7.De Jesus AR, Miranda DG, Miranda RG, Araujo I, Magalhaes A, Bacellar M, Carvalho EM. Morbidity associated with Schistosoma mansoni infection determined by ultrasound in an endemic area of Brazil, Caatinga do Moura. Am J Trop Med Hyg. 2000;63:1–4. doi: 10.4269/ajtmh.2000.63.1. [DOI] [PubMed] [Google Scholar]
- 8.Robinson RD, Lindo JF, Neva FA, Gam AA, Vogel P, Terry SI, Cooper ES. Immunoepidemiologic studies of strongyloides stercolalis and human T lymphtropic virus type I infections in Jamaica. J Inf Dis. 1994;169:692–6. doi: 10.1093/infdis/169.3.692. [DOI] [PubMed] [Google Scholar]
- 9.Plumelle Y, Edouard A. Strongyloides stercoralis in T-cell leukemia/lymphoma in adults and acquired immunodeficiency syndrome. Rev Med Interne. 1996;17:125–9. doi: 10.1016/0248-8663(96)82961-4. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi J, Kishihara Y, Yoshimura E, Furusyo N, Yamaji K, Kawakami T, Murakami H, Kashiwagi S. Correlation between human T cell lymphotropic virus type-1 and Strongyloides stercoralis infections and serum immunoglobulin E reponses in residents of Okinawa. Japan Am J Trop Med Hyg. 1997;56:71–5. doi: 10.4269/ajtmh.1997.56.71. [DOI] [PubMed] [Google Scholar]
- 11.Porto MAF, Neva FA, Lisboa W, Thompson R, Alcântara L, Carvalho EM. htlv-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol. 2001;23:503–7. doi: 10.1046/j.1365-3024.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 12.Neva FA, Filho JO, Gam AA, Thompson R, Freitas V, Carvalho EM. Interferon-γ and Interleukin-4 Responses in Relation to Serum IgE Levels in Persons Infected with Human T Lymphotropic Virus Type I and Strongyloides stercoralis. J Infect Dis. 1998;178:1856–9. doi: 10.1086/314507. [DOI] [PubMed] [Google Scholar]
- 13.Porto MAF, Oliveira Filho J, Neva FA, Orge G, Alcântara L, Gam A, Carvalho EM. Influence of HTLV-1 infection on the serological and Skin Test for strongyloidiasis. Am J Trop Med Hyg. 2001;65:610–3. doi: 10.4269/ajtmh.2001.65.610. [DOI] [PubMed] [Google Scholar]
- 14.Phelps KR, Ginsberg SS, Cinningham AW, Tschachler E, Dosik H. Case report: adult T-cell leukemia/ lymphoma associated with recurrent strongyloides hyperinfection. Am J Med Sci. 1991;302:224–8. doi: 10.1097/00000441-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Newton RC, Limpuangthip P, Greenberg S, Gam A, Neva FA. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. Am J Med. 1992;92:202–7. doi: 10.1016/0002-9343(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 16.Patey O, Gessain A, Breuil J, et al. Seven years of recurrent severe strongyloidiasis in an HTLV-I-infected man who developed adult T-cell leukaemia. AIDS. 1992;6:575–9. doi: 10.1097/00002030-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Galvão CB, Loures L, Rodrigues LGM. Geographic distribution of human T-lymphotropic virus type-I among blood donors: a Brazilian nation wide study. Transfusion. 1997;37:242. doi: 10.1046/j.1537-2995.1997.37297203532.x. [DOI] [PubMed] [Google Scholar]
- 18.Katz N, Chaia G. Cropological diagnosis of schistosomiasis. I. Evaluation of quantitative techniques. Rev Inst Med Trop São Paulo. 1968;10:295–8. [PubMed] [Google Scholar]
- 19.Abdel-Wahab MF, Esmat G, Farrag A, El-Boraey YA, Strickland GT. Grading of hepatic schistosomiasis by the use of ultrasonography. Am J Trop Med Hyg. 1992;46:403–8. doi: 10.4269/ajtmh.1992.46.403. [DOI] [PubMed] [Google Scholar]
- 20.Umehara F, Izumo S, Ronquillo AT, Matsumuro K, Sato E, Osame M. Cytokine expression in the spinal cord lesions in HTLV-1-associated myelopathy. J Neuropathol Exp Neurol. 1994;53:72–7. doi: 10.1097/00005072-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Furuya T, Nakamura T, Fujimoto T, et al. Elevated levels of interleukin-12 and interferon-gamma in patients with human T lymphotropic virus type I-associated myelopathy. J Neuroimmunol. 1999;95:185–9. doi: 10.1016/s0165-5728(98)00263-x. [DOI] [PubMed] [Google Scholar]
- 22.Sleigh AC, Mott KE, Silva JT, et al. A three-year follow-up of chemotherapy with oxamniquine in Brazilian community with endemic schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1981;75:234–8. doi: 10.1016/0035-9203(81)90323-0. [DOI] [PubMed] [Google Scholar]
- 23.Dessein AJ, Begley M, Demeure C, et al. Human resistance to Schistosoma mansoni is associated with reactivity to a 37-kDa larval surface antigen. J Immunol. 1988;140:2727–36. [PubMed] [Google Scholar]
- 24.Leptak CL, McKerrow JH. Schistosome Egg Granulomas and Hepatic Expression of TNF-α Are Dependent on Immune Priming Parasite Maturation. J Immunol. 1997;158:301–7. [PubMed] [Google Scholar]
- 25.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–85. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Wynn T. IL-13 is a Key Regulatory Cytokine for Th2 Cell-Mediated Pulmonary Granuloma Formation and IgE Responses Induced by Schistosoma mansoni Eggs. J Immunol. 1999;162:920–30. [PubMed] [Google Scholar]
- 27.Jankovic D, Kulberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A. Schistosome-Infected IL-4 receptor Knockout (KO) Mice, in Contrast to IL-4 KO Mice, Fail to Develop Granulomatous Pathology While Maintainig the Same Lymphokine Expression Profile. J Immunol. 1999;163:337–42. [PubMed] [Google Scholar]
- 28.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin-12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogeneous IL-12 both inhibits and prophylatically immunizes against egg pathology. J Exp Med. 1994;179:1551–61. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezende SA, Oliveira VR, Silva AM, Alves JB, Góes-Reis LFL. Mice Lacking the Gamma Interferon Receptor Have an Impaired Granulomatous Reaction to Shistosoma mansoni Infection. Infect Immun. 1997;65:3457–61. doi: 10.1128/iai.65.8.3457-3461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya. I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56:515–21. doi: 10.4269/ajtmh.1997.56.515. [DOI] [PubMed] [Google Scholar]
- 31.Jesus AM, Almeida RP, Bacellar O, Araujo MI, Demeure C, Bina J, Dessein A, Carvalho EM. Correlation between cell-mediated immunity and degree of infection in subjects living in na endemic area of schistosomiasis. Eur J Immunol. 1993;23:152–8. doi: 10.1002/eji.1830230125. [DOI] [PubMed] [Google Scholar]
- 32.Correa-Oliveira REJ, Pearce GC, Oliveira DB, et al. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans R Soc Trop Med Hyg. 1989;83:798–804. doi: 10.1016/0035-9203(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro de Jesus A, Araujo I, Bacellar O, Magalhaes A, Pearce E, Harn D, Strand M, Carvalho EM. Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect Immun. 2000;68:2797–803. doi: 10.1128/iai.68.5.2797-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gotuzzo E, Terashima A, Alvarez H. Strongyloides Stercoralis Hyperinfection Associated with Human T Cell Lymphotropic Virus Type-1Infection in Peru. Am J Trop Med Hyg. 1999;60:146–9. doi: 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 35.Pagliuca A. Strongyloides hyperinfection in adult t-cell leukaemia/lymphoma. Br J Haematol. 1999;105:1. doi: 10.1111/j.1365-2141.1999.01401.x. [DOI] [PubMed] [Google Scholar]
- 36.Dunne DW, Butterworth AE, Fulford AJ, Ouma JH, Sturrock RF. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz. 1992;87:99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 37.Caldas IR, Correa-Oliveira R, Colosimo E, Carvalho OS, Massara CL, Colley DG, Gazzinelli G. Susceptibility and resistance to Schistosoma mansoni reinfection. parallel cellular and isotypic immunologic assessment. Am J Trop Med Hyg. 2000;62:57–64. doi: 10.4269/ajtmh.2000.62.57. [DOI] [PubMed] [Google Scholar]
- 38.Satoh M, Toma H, Sato Y, Takara M, Shiroma Y, Kiyuna S, Hirayama K. Reduced efficacy of treatment of strongyloidiasis in HTLV-1 carriers related to enhanced expresión of IFN-γ and TGF-β1. Clin Exp Immunol. 2002;127:354–9. doi: 10.1046/j.1365-2249.2002.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shikiya K, Zaha O, Niimura S, Uehara T, Ohshiro J, Kinjo F, Saito A, Asato R. Clinical study on ivermectin against 125 strongyloidiasis patients. Kansenshogaku Zasshi. 1994;68:13–20. doi: 10.11150/kansenshogakuzasshi1970.68.13. [DOI] [PubMed] [Google Scholar]