Abstract
Patients with poorly controlled diabetes are at high risk of acquiring bacterial infections. However, conflicting results have been reported on neutrophil function in diabetes. We periodically evaluated neutrophil dysfunction in multiple low-dose streptozotocin (STZ)-induced diabetic mice, and then evaluated the effects of troglitazone and other thiazolidinediones (TZDs) on the decline of neutrophil function. Zymosan was injected intraperitoneally and neutrophil infiltration and phagocytosis were evaluated. While phagocytosis of zymosan by peritoneal neutrophils was consistently reduced in diabetic mice, neutrophil infiltration was decreased on day 30, but increased on day 40 after STZ injection. The in vitro chemotactic and phagocytic activities of blood neutrophils in mice that did not receive zymosan were consistently reduced in diabetic mice. Phorbol myristate acetate (PMA)-stimulated superoxide production by zymosan-induced peritoneal neutrophils and the levels of zymosan-induced tumour necrosis factor (TNF)-α and interleukin (IL)-1β in peritoneal exudate fluids were also reduced in the diabetic mice. Treatment of the diabetic mice with troglitazone beginning 2 weeks after STZ injection did not improve hyperglycaemia but did prevent the decline of zymosan-induced neutrophil infiltration on day 30, and additionally promoted the increased infiltration on day 40. Troglitazone also promoted the chemotactic activity of blood neutrophils isolated from normal mice in vitro. Rosiglitazone but not pioglitazone induced a similar effect. Neutrophil phagocytosis was not enhanced by troglitazone either in vivo or in vitro. Taken together, neutrophil function is impaired by STZ-induced diabetes, but inflammatory infiltration does not always vary with the chemotactic disability or cytokine levels. Furthermore, troglitazone and rosiglitazone were suggested to improve at least neutrophil chemotactic activity in these animals.
Keywords: IL-1β, multiple low-dose streptozotocin, neutrophil infiltration, phagocytosis, TNF-α, troglitazone
INTRODUCTION
Long-term, poorly controlled diabetes mellitus is correlated strongly with increased susceptibility to infection and delayed wound healing [1,2]. Neutrophils are a primary immune defence against acute bacterial infection. Consequently, diabetic-induced neutrophil suppression is believed to lead to impaired immune defence. Diabetic-induced reduction in neutrophil chemotaxis, phagocytosis, bactericidal activity and superoxide production has been reported [3–12]. However, unchanged or even enhanced neutrophil function in diabetes has also been reported [4,5,8,9,13,14]. These discrepancies may have been due to differences in the status and duration of disease, extent of medical control and experimental variability, including the use of different strains or species of animals (genetic background) [4,5,10]. Thus, experimental parameters must be monitored periodically in order to evaluate accurately cellular changes in diabetes.
Although the precise cause of neutrophil dysfunction is still unclear, several diabetic-induced changes may interfere with neutrophil function. Neutrophils do not require insulin for glucose transport, and glucose is freely permeable across the cell membrane of diabetic as well as non-diabetic neutrophils [1,15,16]. Therefore, high concentrations of glucose may, at least partially, be responsible for the functional changes in diabetic neutrophils [3,17,18]. On the other hand, Pereira et al. [6] reported the presence of a substance that inhibits neutrophil chemotactic activity in the serum of alloxan-induced diabetic rats. The inhibitor was shown to be neither glucose nor insulin, but insulin was required to clear the substance. A correlation between plasma levels of glycated proteins and changes in neutrophil function has been also reported [7,9,19,20].
Troglitazone and structurally related compounds such as pioglitazone and rosiglitazone containing thiazolidinediones (TZDs) are a novel class of oral antidiabetic drugs which reduce insulin resistance, hyperglycaemia and hyperlipidaemia in obese and non-obese diabetic patients and in diabetic animal models [21,22]. Although their mechanism of action is not fully understood, at least some of the effects are mediated by the peroxisome proliferator-activated receptor (PPAR)-γ, a member of the nuclear hormone receptor superfamily, which is expressed at the highest levels in adipocytes [21–23]. Troglitazone and pioglitazone also reportedly activate another isotype of PPAR, PPAR-α [24,25]. PPAR-γ and PPAR-α are expressed not only in adipocytes but also in various types of cells such as macrophages, vascular smooth muscle cells and endothelial cells, and TZD activation of either PPAR-γ or PPAR-α in these cells modulates cell proliferation, differentiation and function [21,22,26–29]. Although neither PPAR-γ nor PPAR-α has been detected in neutrophils [30], there have been a few reports on direct action of troglitazone on neutrophils [31–33].
In this study, type I diabetes was induced in mice by the injection of multiple low-dose streptozotocin (MLDSTZ) [34–36] to examine: (1) the periodic changes in neutrophil infiltration, phagocytosis and superoxide production, as well as the local levels of the inflammatory cytokines tumour necrosis factor (TNF)-α and interleukin (IL)-1β following zymosan stimulation, and (2) therapeutic effects of troglitazone and other TZDs on neutrophil dysfunction, in vivo and in vitro.
MATERIALS AND METHODS
Animals and induction of diabetes with MLDSTZ
ICR mice (Japan SLC Inc., Shizuoka, Japan) were housed in our animal facility and given food and water ad libitum. Male mice (6–9 weeks of age) were injected intraperitoneally (i.p.) with streptozotocin (STZ; Wako Pure Chemical Industries, Osaka, Japan) at a dose of 40 mg/kg dissolved in 0·01 m citrate buffer for 5 consecutive days [34–36]. Plasma glucose levels (measured by the glucose oxidase method [37]) and white blood cell (WBC) numbers were examined in blood obtained from the retro-orbital sinus. Neutrophil population was estimated using Giemsa staining. Troglitazone (provided by Sankyo Co. Ltd, Tokyo, Japan) [36] (0·2%) was mixed with powdered chow and administered to mice beginning 2 weeks after STZ or vehicle injection. All protocols complied with institutional guidelines.
Isolation of zymosan-induced peritoneal exudate cells (PECs)
Mice were injected i.p. with 1 ml of zymosan solution (5 mg/ml; Sigma-Aldrich Japan K.K., Osaka, Japan) or 10% casein (Wako Pure Chemical Industries) in phosphate buffered saline (PBS). As described previously [38,39], PECs were collected by washing the peritoneum with modified Eagle's medium (MEM) (Gibco BRL, Life Technologies Inc., Tokyo, Japan) containing 1% heparin under pentobarbital sodium anaesthesia (0·1 mg/100 g body weight). PEC numbers were counted and the neutrophil population was estimated using Giemsa staining. The percentage of zymosan-ingesting neutrophils was also assessed from a total of 200 neutrophils stained with Giemsa. For the superoxide production assay, neutrophils were purified from the zymosan-induced PECs using discontinuous Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden) density gradients [38].
Isolation of peripheral blood neutrophils
Heparinized peripheral blood was obtained by cardiac puncture under pentobarbital sodium anaesthesia (0·1 mg/100 g body weight). Neutrophils were purified by a previously described method [39]. Briefly, blood was mixed with an equal volume of plasma gel, prewarmed at 37°C. The plasma gel was prepared by dissolving 3 g gelatin, 0·7 g NaCl and 0·2 g CaCl2 in 100 ml distilled water at 60°C and stored at 4°C until used. The mixture of blood and plasma gel was allowed to stand until erythrocytes settled. The leucocyte-rich supernatant was transferred to another tube and washed with MEM. The pellet was suspended in 5 ml MEM, and overlaid onto the same volume of Ficoll-Paque (sp. gr. 1·077; Amersham Pharmacia Biotech AB), and then centrifuged at 800 g for 10 min at 4°C. Erythrocytes in the pellet containing neutrophils and erythrocytes were lysed with hypotonic NaCl and washed twice with MEM. The average purity (± s.e.m.) of neutrophils was 87 ± 1·7%, with the remaining cells primarily lymphocytes and eosinophils.
Assay for chemotaxis
Peripheral blood neutrophils were suspended in MEM containing 5% newborn calf serum (Gibco BRL) at a density of 1 × 105 cells/100 µl. A chemotactic chamber with a 3 µm pore-sized cellulose filter (Chemotaxicell; Kurabo Industries Ltd, Osaka, Japan) was placed in each well of a 24-well culture plate (Corning Glass Works, Corning, NY, USA), and 1 × 105 neutrophils were loaded into the top chamber. In the lower chamber, 300 µl MEM containing 5% newborn calf serum with or without 3% casein was added [40]. After a 4-h incubation of the chamber at 37°C, medium in the upper chamber was removed. Cells transferred to the lower side of the membrane were dropped by washing with PBS, and the number of neutrophils in the lower chamber was counted. Neutrophil movement to casein was calculated based on the absence of casein and expressed as transferal rate (-fold).
In some experiments, neutrophils were pre-incubated with various doses of troglitazone or another TZD (pioglitazone or rosiglitazone) for 30 min at 37°C. Cells were then washed twice, and the chemotactic assay was performed as described above.
In vitro assay for phagocytosis
Phagocytosis was assessed by a modification of a technique described previously [3,38,39,41]. Peripheral blood neutrophils (1 × 105 cells) were incubated with 1·5 × 106 zymosan particles for 30 min at 37°C. Cytocentrifuge preparations were made and stained with Giemsa. The percentage of neutrophils phagocytosing more than one zymosan particle was estimated from a total of 200 neutrophils under light microscopy (×400).
In some experiments, neutrophils were pre-incubated with various doses of troglitazone for 30 min at 37°C. Cells were washed twice, and the phagocytosis assay was performed as described above.
Assay for superoxide production
Phorbol myristate acetate (PMA) (Sigma Chemical Co.) was suspended at a concentration of 2 mg/ml in dimethylsulphoxide (DMSO) (Sigma Chemical Co.) and was stored at −80°C until use. The stock solution was diluted at a concentration of 0·5 µg/ml with PBS. Superoxide production by the zymosan-induced peritoneal neutrophils was assessed by measuring superoxide dismutase (SOD)-inhibitable cytochrome C reduction, as described previously [38]. Peritoneal neutrophils collected 6 h after zymosan injection were suspended in 2 mg/ml d-glucose-containing Hanks’ balanced salt solution (HBSS) (with Ca2+Gibco BRL) at a density of 1 × 106 cells/700 µl. Ten µl SOD (40 µg/ml; Sigma Chemical Co.) or H2O was added to the suspension, which was incubated for 2 min at 37°C, following addition of 50 µl cytochrome C (80 µmol in PBS; Sigma Chemical Co.) and 750 µl PMA. Further incubation was performed at 37°C, and the reaction was stopped by incubation on ice. Cytochrome C reduction was measured at 540 nm.
Determination of cytokine levels in zymosan-injected peritoneal exudate fluids
Peritoneal exudate fluids (PEFs) were collected 1, 3 and 6 h after zymosan injection into vehicle-injected or STZ-injected mice. Levels of TNF-α and IL-1β in PEFs were determined using enzyme-linked immunosorbent assay (ELISA) kits for murine TNF-α and IL-1β (Biosorce International, Camalliro, CA, USA).
Statistical analysis
anova followed by Fisher's post hoc test (PLSD) was performed for multiple comparisons. For comparisons of two measurements, the Student's t-test was used.
RESULTS
Changes in plasma glucose levels during development of MLDSTZ-induced diabetes
At the start of the five daily injections, plasma glucose levels were 157 ± 3·6 mg/dl in STZ-injected mice (STZ mice) and 163 ± 4·2 mg/dl in vehicle-injected mice (control mice). In control mice, glucose levels remained virtually constant until 60 days later (148 ± 11·9 mg/dl on day 60). Alternatively, glucose levels in STZ mice were significantly higher than levels in control mice on day 10 (250 ± 14·1 mg/dl), and increased rapidly on day 15 (511 ± 20·0 mg/dl). Glucose levels kept rising and reached a plateau level in 30 days (660 ± 27·6 mg/dl on day 30).
Changes in zymosan-induced neutrophil influx and phagocytosis in MLDSTZ-induced diabetic mice
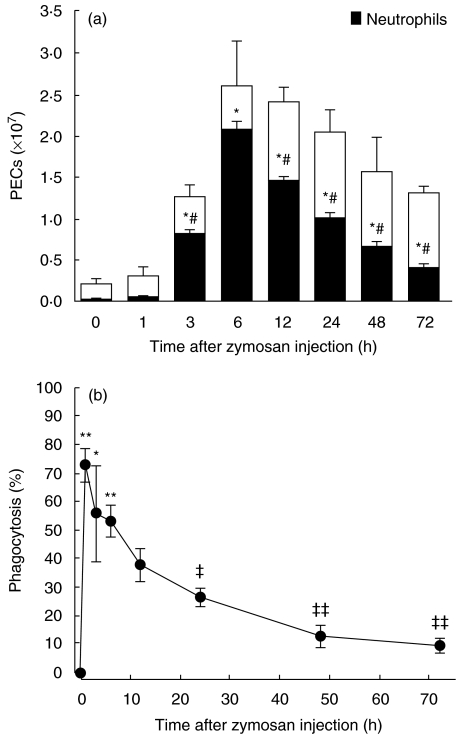
We first examined the time-course of neutrophil influx into the peritoneum (Fig. 1a) and neutrophil ingestion of zymosan (Fig. 1b) after i.p. zymosan injection in normal mice. At time 0 (just after the injection), the neutrophil number was (0·01 ± 0·001) × 107 cells, which represented 5 ± 0·1% of the total PEC population (the other cells being primarily macrophages). After 3 h, the number of neutrophils increased drastically to (0·8 ± 0·05) × 107 cells (65 ± 5·5% of the PEC population), and peaked at 6 h with a count of (2·1 ± 0·14) × 107 cells (80 ± 5·5% of the total PECs). Starting at 12 h, neutrophil influx decreased gradually and monocyte influx subsequently increased.
Fig. 1.
Changes in zymosan-induced peritoneal exudate cell (PEC) numbers (a) and zymosan-ingesting neutrophils (b) in mice injected neither with STZ or citrate buffer. Mice were injected i.p. with 5 mg zymosan. After the indicated time, PECs were collected and the cell numbers were counted. PECs were cytocentrifuged and stained with Giemsa. The percentages of neutrophils (a) and zymosan-ingested neutrophils (b) were evaluated. Each value represents the mean ± s.e. (n = 4–11). *P < 0·05, **P < 0·01 compared to 0 h, #P < 0·01 compared to 6 h, and ‡P < 0·05, ‡‡P < 0·01 compared to 1 h.
The percentage of zymosan-ingesting neutrophils was maximal at 1 h (73·2 ± 6·06% of total peritoneal neutrophils), and decreased gradually from 12 h (38·2 ± 5·49% at 12 h).
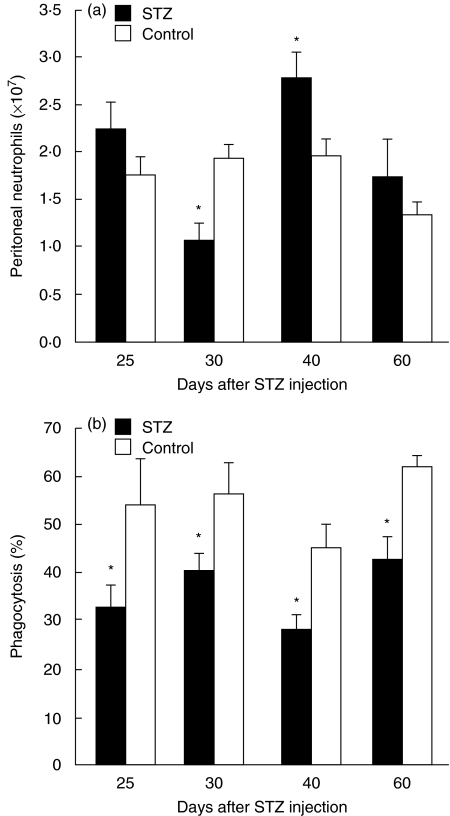
Because neutrophil influx was maximal at 6 h and, at the same time, more than half of the neutrophils were found to have ingested zymosan, we decided to examine the effects of MLDSTZ-induced diabetes on neutrophil influx and phagocytosis 6 h after the zymosan injection in the following experiments. As shown in Fig. 2a, the numbers of zymosan-induced peritoneal neutrophils varied from day to day after STZ injection, whereas they were constant in control mice. On day 25, neutrophil numbers did not differ significantly between control and STZ mice, but were significantly lower in STZ mice on day 30 [(1·1 ± 0·18) × 107 cells versus (1·9 ± 0·29) × 107 cells]. Interestingly, the number of neutrophils was higher in STZ mice than in control mice on day 40 [(2·8 ± 0·25) × 107versus (2·0 ± 0·21) × 107 cells], while their numbers were again insignificantly different from control mice on day 60. Unlike the numbers of zymosan-induced peritoneal neutrophils, those of circulating blood neutrophils (counted prior to zymosan injection) did not show date-sensitive variation in STZ mice, and there were no significant differences in those numbers between control and STZ mice until day 60 (Table 1).
Fig. 2.
Changes in zymosan-induced peritoneal neutrophil numbers (a) and zymosan-ingesting neutrophils (b) following MLDSTZ injection. Mice were injected i.p. with 5 mg zymosan on the indicated days after STZ or citrate buffer injection (control). After 6 h, PECs were collected and the cell numbers were counted. PECs were cytocentrifuged and stained with Giemsa, then the percentage of neutrophils with or without zymosan particles was evaluated. Each value represents the mean ± s.e. (n = 5–10). *P < 0·05 compared to control.
Table 1.
Number of peripheral blood neutrophils in control and MLDSTZ-injected mice
| Day 25 | Day 30 | Day 40 | Day 60 | |
|---|---|---|---|---|
| Control | 1414·3 ± 145·94 | 1182·5 ± 329·71 | 1226·7 ± 128·12 | 1506·5 ± 288·85 |
| STZ | 1407·4 ± 108·46 | 1454·8 ± 162·62 | 1215·6 ± 194·28 | 1452·5 ± 255·26 |
Mice were injected with STZ or citrate buffer as a control. After the indicated time, peripheral blood was obtained from the retro-orbital sinus, and WBC counting was performed. Neutrophil numbers were estimated from the total WBC numbers and neutrophil population, and the means ± s.e. were calculated (n = 3–8).
Moreover, unlike the variable changes in neutrophil infiltration in STZ mice, the percentage of zymosan-ingesting peritoneal neutrophils was consistently lower in STZ mice than in control mice (Fig. 2b).
A similar pattern of neutrophil influx was observed when mice were injected i.p. with casein. The number of casein-induced peritoneal neutrophils was significantly lower in STZ on day 30, but was reversed on day 40 (Table 2).
Table 2.
Number of casein-induced peritoneal neutrophils in control and MLDSTZ-injected mice
| Day 30 (×107 cells) | Day 40 (×107 cells) | |
|---|---|---|
| Control | 1·28 ± 0·09 | 1·57 ± 0·24 |
| STZ | 1·06 ± 0·04* | 1·94 ± 0·15* |
Mice were injected i.p. with 10% casein or PBS (control). After 6 h, peritoneal exudates cells (PECs) were collected, then the number of neutrophils was assayed, and the means ± s.e. were calculated (n = 5).
P < 0·05 compared to control.
In vitro chemotaxis and phagocytosis in peripheral blood neutrophils
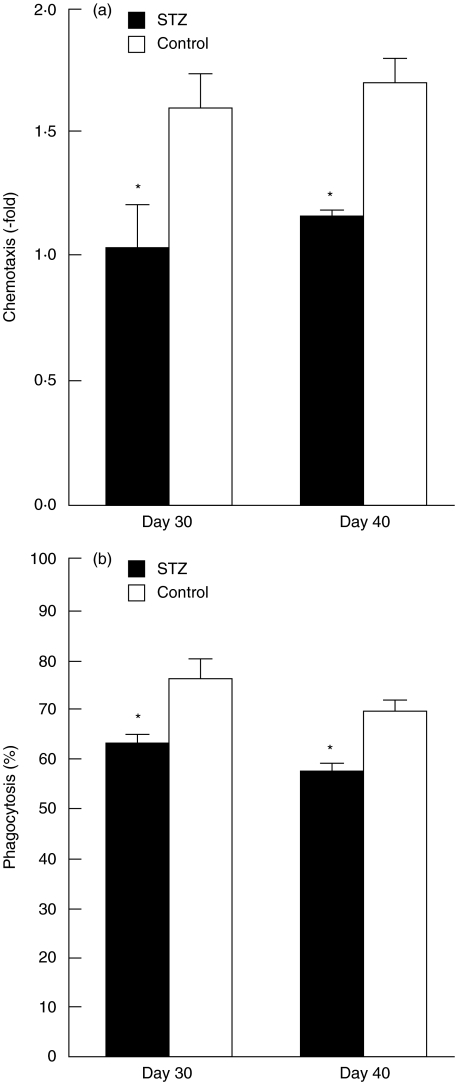
To determine if MLDSTZ-induced diabetes alter migrative and phagocytic abilities of peripheral blood neutrophils, we isolated neutrophils from circulating blood of control and STZ mice on days 30 and 40, and performed in vitro chemotaxis and phagocytosis assays. In the absence of casein, the number of cells transferred was not altered between control and STZ mice [(3·7 ± 0·34) × 103 cells versus (3·3 ± 0·49) × 103 cells on day 30 and (3·4 ± 0·25) × 103 cells versus (3·8 ± 0·22) × 103 cells on day 40]. In the presence of 3% casein in a lower compartment, the neutrophil transferral rates to filter from an upper compartment of a chemotactic chamber toward the lower chamber increased 1·6-fold (day 30) and 1·7-fold (day 40) in control mice (Fig. 3a). However, the transferral rates were significantly lower in STZ mice than control mice on both days 30 (1·0-fold) and 40 (1·2-fold).
Fig. 3.
Changes in chemotactic (a) and phagocytic (b) abilities of neutrophils following MLDSTZ injection. Circulating blood neutrophils were isolated 30 and 40 days after STZ or citrate buffer injection (control). Chemotactic chambers were incubated for 4 h, and the number of neutrophils that passed into lower chambers was counted (n = 3–5, in duplicate). The vertical axis in (a) represents the increase in the rate of neutrophil accumulation following the addition of 3% casein to the lower chambers. The phagocytosis assay was performed by incubating neutrophils with zymosan for 30 min, after which the percentage of zymosan-ingesting neutrophils was evaluated (b; n = 5–6). Each value represents the mean ± s.e. *P < 0·05 compared to control.
In a similar fashion, phagocytosis during a 30-min incubation of neutrophils with zymosan was significantly lower in STZ mice than in control mice (Fig. 3b).
PMA-stimulated superoxide production by peritoneal neutrophils
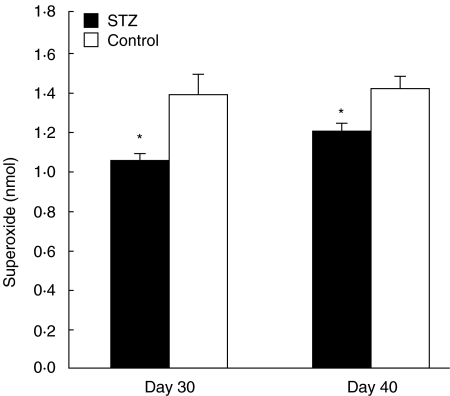
We examined the time course of PMA-stimulated superoxide production of peritoneal neutrophils collected at 6 h after zymosan injection in normal mice. Superoxide was first detected 3 min after PMA stimulation [(0·2 ± 0·04) nmol/106 cells], and its levels increased gradually and peaked after 10 min [(1·4 ± 0·12) nmol/106 cells]. We then examined the effects of MLDSTZ-induced diabetes on superoxide production by measuring its levels at the 10-min time-point. As shown in Fig. 4, the amount of superoxide produced was significantly less in STZ mice compared to control mice, on both days 30 and 40.
Fig. 4.
PMA-stimulated superoxide production by peritoneal neutrophils in normal and diabetic mice. Mice were injected i.p. with 5 mg zymosan on the indicated days after STZ or citrate buffer injection (control). After 6 h, PECs were collected and neutrophils were purified. Neutrophils (1 × 106 cells) were incubated in the presence of SOD, cytochrome C and PMA, and cytochrome C reduction was monitored at 540 nm for 10 min at 37°C. Each value represents the mean ± s.e. (n = 15–24). *P < 0·05 compared to control.
Zymosan-stimulated cytokine release in STZ mice
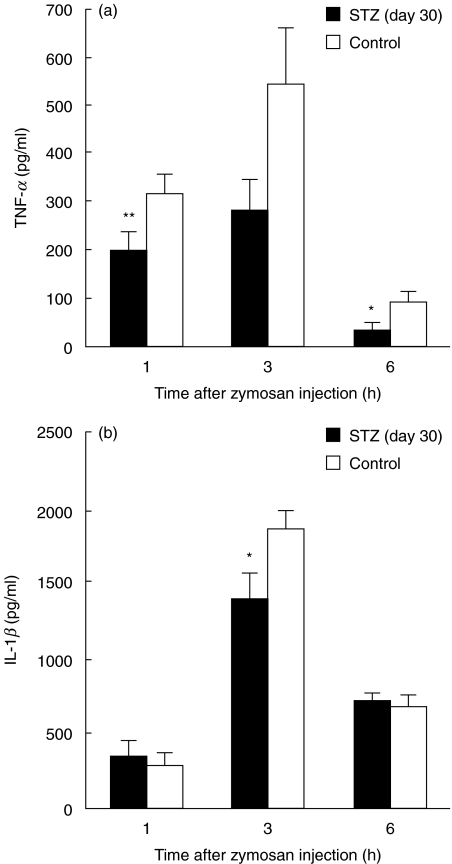
To investigate the diabetic influence on the release of inflammatory cytokines, PEFs were collected 1, 3 and 6 h after zymosan injection into STZ (day 30) and control mice. Results showed that TNF-α levels peaked at 3 h in both groups, but they were significantly lower in STZ mice at 1 and 6 h (Fig. 5a). At 3 h, the TNF-α levels were 281·3 ± 67·13 pg/ml and 543·0 ± 116·73 pg/ml in STZ and control mice, respectively (P < 0·07). IL-1β levels also peaked at 3 h in both groups, but were significantly lower in STZ mice compared to control mice (Fig. 5b). The levels of TNF-α (at 1 h) and IL-1β (at 3 h) were also examined 40 days after STZ injection. Although the overall concentrations of the cytokines seemed to be lower in STZ mice, no significant differences were obtained between STZ and control mice (200·3 ± 50·31 pg/ml versus 257·6 ± 49·77 pg/ml on TNF-α and 1160·3 ± 106·99 pg/ml versus 1346·3 ± 82·64 pg/ml on IL-1β, n = 5–8).
Fig. 5.
Zymosan-induced peritoneal TNF-α(a) and IL-1β(b) levels in normal and diabetic mice. Mice were injected i.p. with 5 mg zymosan 30 days after STZ or citrate buffer injection (control). After the indicated time, PEFs were collected and each cytokine level was assessed using the appropriate ELISA kit. Each value represents the mean ± s.e. (n = 3–16, in duplicate). *P < 0·05 and **P < 0·01 compared to control.
In vivo and in vitro effects of TZDs on neutrophil function
Troglitazone was mixed with powered chow and administered to control and STZ mice starting day 14 after the injection, when hyperglycaemia had developed in STZ mice. Troglitazone treatment did not reverse hyperglycaemia in STZ mice 30 days after STZ injection (600·8 ± 59·76 mg/dl). However, reduction in zymosan-induced neutrophil influx was not observed in troglitazone-treated STZ mice on day 30 (Table 3). On day 40, the neutrophil influx increased in troglitazone-treated STZ mice by more than twofold compared to troglitazone-treated control mice. On the other hand, neutrophils from troglitazone-treated STZ mice showed reduced phagocytosis on both days 30 and 40 (Table 3).
Table 3.
Effects of troglitazone on peritoneal neutrophil influx and phagocytosis in control and MLDSTZ-injected mice
| Peritoneal neutrophils (×107 cells) | Phagocytosis (%) | |
|---|---|---|
| Day 30 | ||
| Control | 1·8 ± 0·42 | 62 ± 2·5 |
| STZ | 1·6 ± 0·26 | 55 ± 2·5* |
| Day 40 | ||
| Control | 2·1 ± 0·35 | 52 ± 6·3 |
| STZ | 4·5 ± 0·80* | 38 ± 4·4* |
Mice were injected with STZ or citrate buffer as a control. From day 14, troglitazone mixed with chow (0·2%) was administered to mice. After 30 and 40 days, mice were injected with zymosan i.p. and PECs were collected 6 h later. Neutrophil numbers and phagocytosis were assayed, and the means ± s.e. were calculated (n = 5–8).
P < 0·05 compared to control.
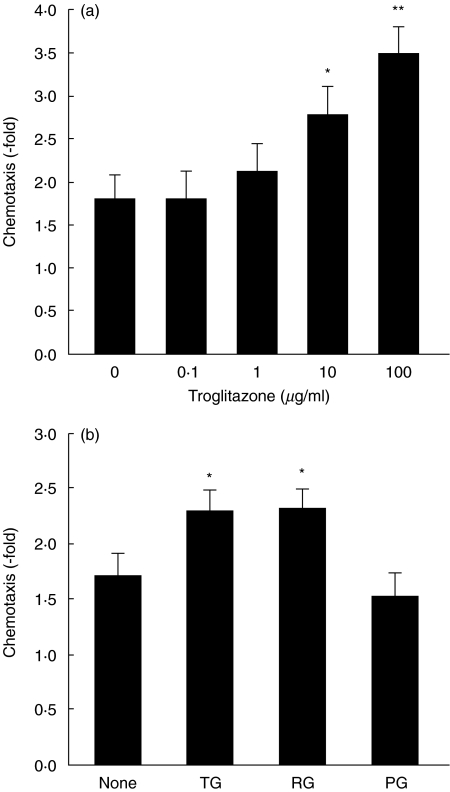
To clarify whether troglitazone acts directly on neutrophils, circulating blood neutrophils isolated from normal, untreated mice were pre-incubated with various doses of troglitazone for 30 min after which their chemotactic and phagocytic abilities were assessed in vitro. As shown in Fig. 6a, troglitazone pre-incubation augmented neutrophil migration towards casein significantly in a dose-dependent manner at 10 and 100 µg/ml. In the absence of casein, transferred cell numbers did not increase following any doses of troglitazone pretreatment. Alternatively, phagocytosis was not affected by troglitazone (54 ± 3·9% without troglitazone pretreatment and 51 ± 4·7% following pretreatment with 100 µg/ml of troglitazone).
Fig. 6.
Direct effects of troglitazone and other TZDs on neutrophil chemotaxis. Circulating blood neutrophils isolated from mice without STZ or citrate buffer injection were incubated with the indicated doses of troglitazone for 30 min at 37°C, and then chemotaxis was assessed in vitro(a). Neutrophils were also pre-incubated with 10 µg/ml troglitazone (TG), pioglitazone (PG) or rosiglitazone (RG), followed by chemotactic assay (b). Each value represents the mean ± s.e. (n = 8–13). *P < 0·05 and **P < 0·01 compared to the absence of TZDs.
To examine the effects of other TZDs on neutrophil chemotaxis, circulating blood neutrophils were pre-incubated with 10 µg/ml pioglitazone, rosiglitazone or troglitazone. As shown in Fig. 6b, rosiglitazone, but not pioglitazone, increased neutrophil migration towards casein, similar to troglitazone.
DISCUSSION
We studied the sequential changes in neutrophil infiltration into the peritoneum and phagocytosis following zymosan injection in mice developing type I diabetes induced by MLDSTZ injection. Low doses of STZ are believed to either induce antigens on pancreatic islet cells or allow T cells to gain access to islet antigens [34–36]. Therefore, lymphocyte infiltration to islets precedes the massive loss of pancreatic β cells and insulin deficiency in MLDSTZ-induced diabetes. These diabetic processes induced by MLDSTZ resemble the development process of human type I diabetes in many aspects. Glucose levels in this study, comparable to previously published levels [35,36], rose 10 days after the initial STZ injection and settled at high levels in 30 days.
Neutrophils were the first cell type to infiltrate the zymosan-injected peritoneum of normal mice, increasing rapidly by 3 h and reaching their highest numbers at 6 h after injection, concomitantly with phagocytosis of zymosan particles (Fig. 1). This confirms a previous report [42]. Zymosan-induced peritoneal neutrophil numbers varied from day to day after STZ injection (Fig. 2a), with numbers reaching their lowest point 30 days after STZ injection but rebounding impressively by 40 days. These irregular infiltrative alterations were not the zymosan-specific phenomena, because they were also observed when STZ mice were injected i.p. with casein (Table 2). Because the number of neutrophils in the peripheral blood of STZ and control mice prior to zymosan or casein injection was normal and remained constant (Table 1), it was considered unlikely that neutrophils were affected by infection. In order to clarify whether the infiltrative alterations are caused by a neutrophil chemotactic abnormality, chemotaxis of non-activated neutrophils isolated from peripheral blood was examined in vitro. Casein-induced chemotaxis was reduced significantly in STZ mice on both days 30 and 40 (Fig. 3a). There have been conflicting reports concerning the effects of diabetes on neutrophil migration [5,13,14]. Our current in vivo and in vitro results suggest that neutrophil infiltration into inflammatory sites can be altered periodically, even though neutrophil chemotactic activity is consistently reduced, throughout the progress of type I diabetes.
On the other hand, the concentration of zymosan-ingesting neutrophils was consistently lower in STZ mice than control mice, both in vivo and in vitro (Figs 2b and 3b). Moreover, we found that the PMA-stimulated superoxide production by zymosan-induced peritoneal neutrophils was reduced in STZ mice (Fig. 4). Oxygen free radicals are produced during a burst of oxidative metabolism which occurs in conjunction with phagocytosis or in response to several soluble stimuli such as PMA, a protein kinase C activator [7,10,43]. Therefore, the continuous impairment of neutrophil phagocytosis was confirmed in STZ mice, a finding that is in general agreement with previously published reports concerning diabetic subjects [3–5,7–12].
In response to inflammatory stimuli, local macrophages and mast cells secrete several early response cytokines; TNF-α and IL-1β act as potent primers of neutrophils by inducing, for example, neutrophil chemoattractant production [44–48]. Reduced in vitro cytokine production by macrophages was reported in rodent models of both type I and type II diabetes [49,50]. In our current study, the levels of zymosan-induced TNF-α and IL-1β were lower in STZ mice on day 30 (Fig. 5), suggesting that reduced secretion of cytokines by an inflammatory microenvironment, in addition to a direct neutrophil impairment, may be responsible for the reduction in neutrophil function that we observed in STZ mice. On day 40, we obtained no significant differences in the levels of these cytokines between STZ and control mice. Consequently, no correlation was recognized between the enhanced neutrophil infiltration and the peritoneal cytokine levels in STZ mice at that time. The reason for the enhanced neutrophil infiltration remains to be elucidated. However, several reports have demonstrated an increased level of endothelial–neutrophil cell adhesion molecules (ex. CD11b/CD18, ICAM-1, P-selectin) in diabetes [13,14,51,52]. Further studies are required to investigate the periodical changes of these molecules in MLDSTZ mice.
TZDs are therapeutic agents known to increase sensitivity to insulin, and they are novel treatment modalities for type II diabetes [21,22]. TZDs have been also shown to have anti-inflammatory and antioxidant effects. Ogawa et al. reported that troglitazone administration at 0·2% in chow to DBA/2 mice for 4 week from the start of STZ injection prevented hyperglycaemia and the development of type I diabetes by suppressing insulitis and the subsequent β cell destruction induced by inflammatory cytokines in MLDSTZ mice [36]. In our study, troglitazone was administered to STZ mice after diabetes had already developed; nevertheless, troglitazone-treated STZ mice showed no decrease in zymosan-induced neutrophil infiltration on day 30 and additionally promoted the increased infiltration on day 40 (Table 3). Furthermore, pre-incubation of blood neutrophils isolated from normal mice with troglitazone dose-dependently promoted neutrophil migration towards casein (Fig. 6a), a finding that was also seen with rosiglitazone, but not pioglitazone (Fig. 6b). On the other hand, the reduction in zymosan phagocytosis by neutrophils from STZ mice was not eliminated by troglitazone treatment (Table 3). From these in vivo and in vitro data, troglitazone probably promoted neutrophil infiltration but not phagocytosis, at least in part by directly inducing chemotaxis in neutrophils. Troglitazone and pioglitazone are thought to exert their anti-diabetic effect via PPAR-γ or PPAR-α[21–25]. However, neither PPAR-γ nor PPAR-α have yet been detected in neutrophils [30]. None the less, there have been a few reports that troglitazone acted directly on human and rat neutrophils to inhibit their reactive oxygen species generation [31–33]. In light of these and our findings, it is possible that troglitazone and rosiglitazone may act on neutrophil function in a PPAR activation-independent manner.
In summary, the current in vivo and in vitro results indicate that: (1) neutrophil phagocytosis and chemotaxis, and inflammatory cytokine secretion were reduced in MLDSTZ-diabetic mice; (2) neutrophil infiltration into inflammatory sites did not parallel the reduced chemotactic activity; (3) troglitazone promoted the neutrophil infiltration even under hyperglycaemic conditions; and (4) troglitazone and rosiglitazone augmented chemotactic activity by direct action on neutrophils.
Acknowledgments
We would like to thank Drs Hiroyoshi Horikoshi and Toshihiko Fujiwara, Sankyo Co. Ltd., for their helpful discussion and financial and material assistance. We wish to express our sadness at the loss of our co-author, the late Professor Tsukasa Sugano, and dedicate this paper to him in memoriam. This work was supported by Grants-in-Aid for Scientific Research C (11660300) from the Ministry of Education, Science, Sports and Culture of Japan and by Sankyo Co. Ltd.
REFERENCES
- 1.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–50. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 2.Fahey TJ, III, Sadaty A, Jones WG, II, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res. 1991;50:308–13. doi: 10.1016/0022-4804(91)90196-s. [DOI] [PubMed] [Google Scholar]
- 3.Davidson NJ, Sowden JM, Fletcher J. Defective phagocytosis in insulin controlled diabetics: evidence for a reaction between glucose and opsonising proteins. J Clin Pathol. 1984;37:783–6. doi: 10.1136/jcp.37.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierusz-Wysocka B, Wysocki H, Wykretowicz A, Szczepanik A, Sadurska K. Phagocytosis, bactericidal capacity, and superoxide anion (O2−) production by polymorphonuclear neutrophils from patients with diabetes mellitus. Folia Haematol Int Mag Klin Morph Blutforsch. 1985;112:658–68. [PubMed] [Google Scholar]
- 5.Naghibi M, Smith RP, Baltch AL, et al. The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes. Diabetes Res Clin Pract. 1987;4:27–35. doi: 10.1016/s0168-8227(87)80030-x. [DOI] [PubMed] [Google Scholar]
- 6.Pereira MA, Sannomiya P, Leme JG. Inhibition of leukocyte chemotaxis by factor in alloxan-induced diabetic rat plasma. Diabetes. 1987;36:1307–14. doi: 10.2337/diab.36.11.1307. [DOI] [PubMed] [Google Scholar]
- 7.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–60. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 8.Serlenga E, Garofalo AR, De Pergola G, Ventura MT, Tortorella C, Antonaci S. Polymorphonuclear cell-mediated phagocytosis and superoxide anion release in insulin-dependent diabetes mellitus. Cytobios. 1993;74:189–95. [PubMed] [Google Scholar]
- 9.Wierusz-Wysocka B, Wykretowicz A, Byks H, Sadurska K, Wysocki H. Polymorphonuclear neutrophils adherence, superoxide anion (O2−) production and HBA1 level in diabetic patients. Diabetes Res Clin Pract. 1993;21:109–14. doi: 10.1016/0168-8227(93)90057-c. [DOI] [PubMed] [Google Scholar]
- 10.Abu el-Asrar AM, Soliman RT, al-Amro SA, al-Shammary FJ. Production of superoxide anion by polymorphonuclear leukocytes from diabetic patients with or without diabetic retinopathy. Doc Ophthalmol. 1996;91:243–54. doi: 10.1007/BF01204175. [DOI] [PubMed] [Google Scholar]
- 11.Zozulinska DA, Wierusz-Wysocka B, Wysocki H, Majchrzak AE, Wykretowicz A. The influence of insulin-dependent diabetes mellitus (IDDM) duration on superoxide anion and hydrogen peroxide production by polymorphonuclear neutrophils. Diabetes Res Clin Pract. 1996;33:139–44. doi: 10.1016/0168-8227(96)01289-2. [DOI] [PubMed] [Google Scholar]
- 12.Mazade MA, Edwards MS. Impairment of type III group B Streptococcus-stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol Genet Metab. 2001;73:259–67. doi: 10.1006/mgme.2001.3185. [DOI] [PubMed] [Google Scholar]
- 13.Barouch FC, Miyamoto K, Allport JR, et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–8. [PubMed] [Google Scholar]
- 14.Hokama JY, Ritter LS, Davis-Gorman G, Cimetta AD, Copeland JG, McDonagh PF. Diabetes enhances leukocyte accumulation in the coronary microcirculation early in reperfusion following ischemia. J Diabetes Complications. 2000;14:96–107. doi: 10.1016/s1056-8727(00)00068-4. [DOI] [PubMed] [Google Scholar]
- 15.Esmann V. The diabetic leukocyte. Enzyme. 1972;13:32–55. [PubMed] [Google Scholar]
- 16.Tan AS, Ahmed N, Berridge MV. Acute regulation of glucose transport after activation of human peripheral blood neutrophils by phorbol myristate acetate, fMLP, and granulocyte–macrophage colony-stimulating factor. Blood. 1998;91:649–55. [PubMed] [Google Scholar]
- 17.Tebbs SE, Lumbwe CM, Tesfaye S, Gonzalez AM, Wilson RM. The influence of aldose reductase on the oxidative burst in diabetic neutrophils. Diabetes Res Clin Pract. 1992;15:121–9. doi: 10.1016/0168-8227(92)90015-j. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Kashima K, Uehara Y, Ohtani K, Shimizu H, Mori M. Epalrestat, an aldose reductase inhibitor, improves an impaired generation of oxygen-derived free radicals by neutrophils from poorly controlled NIDDM patients. Diabetes Care. 1997;20:995–8. doi: 10.2337/diacare.20.6.995. [DOI] [PubMed] [Google Scholar]
- 19.Hostetter MK. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes. 1990;39:271–5. doi: 10.2337/diab.39.3.271. [DOI] [PubMed] [Google Scholar]
- 20.Sengoelge G, Fodinger M, Skoupy S, et al. Endothelial cell adhesion molecule and PMNL response to inflammatory stimuli and AGE-modified fibronectin. Kidney Int. 1998;54:1637–51. doi: 10.1046/j.1523-1755.1998.00157.x. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara T, Horikoshi H. Troglitazone and related compounds: therapeutic potential beyond diabetes. Life Sci. 2000;67:2405–16. doi: 10.1016/s0024-3205(00)00829-8. [DOI] [PubMed] [Google Scholar]
- 22.Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34:217–24. [PubMed] [Google Scholar]
- 23.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto J, Kimura H, Moriyama S, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–11. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 25.Aljada A, Ghanim H, Friedman J, Garg R, Mohanty P, Dandona P. Troglitazone reduces the expression of PPARgamma while stimulating that of PPARalpha in mononuclear cells in obese subjects. J Clin Endocrinol Metab. 2001;86:3130–3. doi: 10.1210/jcem.86.7.7624. [DOI] [PubMed] [Google Scholar]
- 26.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte–endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda U, Shimpo M, Murakami Y, Shimada K. Peroxisome proliferator-activated receptor-gamma ligands inhibit nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 2000;35:1232–6. doi: 10.1161/01.hyp.35.6.1232. [DOI] [PubMed] [Google Scholar]
- 29.Chen NG, Han X. Dual function of troglitazone in ICAM-1 gene expression in human vascular endothelium. Biochem Biophys Res Commun. 2001;282:717–22. doi: 10.1006/bbrc.2001.4628. [DOI] [PubMed] [Google Scholar]
- 30.Greene ME, Blumberg B, McBride OW, et al. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr. 1995;4:281–99. [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue I, Katayama S, Takahashi K, et al. Troglitazone has a scavenging effect on reactive oxygen species. Biochem Biophys Res Commun. 1997;235:113–6. doi: 10.1006/bbrc.1997.6512. [DOI] [PubMed] [Google Scholar]
- 32.Garg R, Kumbkarni Y, Aljada A, et al. Troglitazone reduces reactive oxygen species generation by leukocytes and lipid peroxidation and improves flow-mediated vasodilatation in obese subjects. Hypertension. 2000;36:430–5. doi: 10.1161/01.hyp.36.3.430. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita M, Kushihara M, Hirasawa N, et al. Inhibition by troglitazone of the antigen-induced production of leukotrienes in immunoglobulin E-sensitized RBL-2H3 cells. Br J Pharmacol. 2000;129:367–73. doi: 10.1038/sj.bjp.0703044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci USA. 1977;74:2485–9. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herold KC, Vezys V, Sun Q, et al. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1996;156:3521–7. [PubMed] [Google Scholar]
- 36.Ogawa J, Takahashi S, Fujiwara T, et al. Troglitazone can prevent development of type 1 diabetes induced by multiple low-dose streptozotocin in mice. Life Sci. 1999;65:1287–96. doi: 10.1016/s0024-3205(99)00364-1. [DOI] [PubMed] [Google Scholar]
- 37.Kiang SW, Kuan JW, Kuan SS, Guilbault GG. Measurement of glucose in plasma, with use of immobilized glucose oxidase and peroxidase. Clin Chem. 1976;22:1378–82. [PubMed] [Google Scholar]
- 38.Kannan Y, Ushio H, Koyama H, et al. 2.5S nerve growth factor enhances survival, phagocytosis, and superoxide production of murine neutrophils. Blood. 1991;77:1320–5. [PubMed] [Google Scholar]
- 39.Mizuno T, Kannan Y, Tokunaga M, et al. Role of hypothermia induced by tumor necrosis factor on apoptosis and function of inflammatory neutrophils in mice. Am J Physiol Regul Integr Comp Physiol. 2000;278:R157–65. doi: 10.1152/ajpregu.2000.278.1.R157. [DOI] [PubMed] [Google Scholar]
- 40.Valerius NH. Chemotaxis, spreanding and oxidative metabolism of neutrophils: influence of albumin in vitro. Acta Pathol Microbiol Immunol Scand [C] 1983;91:43–9. [PubMed] [Google Scholar]
- 41.Czop JK, Puglisi AV, Miorandi DZ, Austen KF. Perturbation of beta-glucan receptors on human neutrophils initiates phagocytosis and leukotriene B4 production. J Immunol. 1988;141:3170–6. [PubMed] [Google Scholar]
- 42.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–82. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djaldetti M, Salman H, Bergman M, Djaldetti R, Bessler H. Phagocytosis − the mighty weapon of the silent warriors. Microsc Res Tech. 2002;57:421–31. doi: 10.1002/jemt.10096. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro RA, Vale ML, Thomazzi SM, et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–8. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 45.Perretti M, Solito E, Parente L. Evidence that endogenous interleukin-1 is involved in leukocyte migration in acute experimental inflammation in rats and mice. Agents Actions. 1992;35:71–8. doi: 10.1007/BF01990954. [DOI] [PubMed] [Google Scholar]
- 46.Torok K, Nemeth K, Erdo F, Aranyi P, Szekely JI. Measurement and drug induced modulation of interleukin-1 level during zymosan peritonitis in mice. Inflamm Res. 1995;44:248–52. doi: 10.1007/BF01782977. [DOI] [PubMed] [Google Scholar]
- 47.Fantuzzi G, Sacco S, Ghezzi P, Dinarello CA. Physiological and cytokine responses in IL-1 beta-deficient mice after zymosan-induced inflammation. Am J Physiol. 1997;273:R400–6. doi: 10.1152/ajpregu.1997.273.1.R400. [DOI] [PubMed] [Google Scholar]
- 48.Utsunomiya I, Ito M, Oh-ishi S. Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo. Cytokine. 1998;10:956–63. doi: 10.1006/cyto.1998.0376. [DOI] [PubMed] [Google Scholar]
- 49.Doxey DL, Nares S, Park B, Trieu C, Cutler CW, Iacopino AM. Diabetes-induced impairment of macrophage cytokine release in a rat model: potential role of serum lipids. Life Sci. 1998;63:1127–36. doi: 10.1016/s0024-3205(98)00374-9. [DOI] [PubMed] [Google Scholar]
- 50.Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes. 2000;49:1451–8. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]
- 51.Rao KM, Hatchell DL, Cohen HJ, De La Paz MA. Alterations in stimulus-induced integrin expression in peripheral blood neutrophils of patients with diabetic retinopathy. Am J Med Sci. 1997;313:131–7. doi: 10.1097/00000441-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 52.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]