Abstract
Viscum album agglutinin-I (VAA-I) is a plant lectin which possesses antitumoral properties. This lectin is also known for its immunostimulatory effects when used at low concentrations (1–100 ng/ml). We have demonstrated recently that VAA-I is a potent inducer of human neutrophil apoptosis in vitro when used at higher concentrations. The role of VAA-I on activated neutrophils has not so far been investigated and its potential proinflammatory properties in vivo are poorly documented. Herein, we demonstrated that VAA-I (1000 ng/ml) induces apoptosis in lipopolysaccharide (LPS)-treated human neutrophils in vitro as well as in murine neutrophils isolated from lipopolysaccharide (LPS)-induced neutrophil influx. Using this model, we found that administration of VAA-I (100 or 1000 ng/ml) did not induce an inflammatory response. However, when used at 1 or 10 ng/ml, VAA-I was found to significantly induce a transitory inflammatory response, based on an increased leucocyte infiltration (>98% neutrophils). Also, we found that VAA-I inhibits LPS-induced neutrophil influx when administered simultaneously with LPS. In such conditions, some characteristic apoptotic neutrophils were observed in the pouch. Unlike LPS, which increased the production of some cytokines, VAA-I (1 or 10 ng/ml) did not increase the production of tumour necrosis factor (TNF)-α, interleukin (IL)-1Ra, IL-1α, IL-β, IL-8, IL-10 or IL-12 (p70) in human neutrophils. We conclude that VAA-I possesses the ability to induce apoptosis of preactivated neutrophils at a concentration that does not induce a proinflammatory response. Moreover, we conclude that VAA-I can inhibit a LPS-induced proinflammatory response in vivo. These data may provide new clinical perspectives in future mistletoe therapy and favour its potential utilization based on anti-inflammatory activity that at first appears contradictory with its use as immunostimulant.
Keywords: apoptosis, inflammation, paxillin, vimentin, Viscum album
INTRODUCTION
The role of neutrophils in the inflammation process is well established. Because clearance of apoptotic neutrophils by cells such as macrophages can lead to the resolution of inflammation [1–4], it is important to develop therapeutic strategies based on the activation of neutrophil apoptosis in order to reverse or attenuate an inflammatory response. Several modulators of cell apoptosis have been discovered in the past few years. Among them, the mistletoe lectin Viscum album agglutinin-I (VAA-I) was found recently to be a potent inducer of apoptosis in different cells, including human neutrophils [5–7]. Extracts from mistletoe are used widely in the treatment of a variety of cancers and are known to modulate non-specific immune functions, such as the increase in number and activity of natural killer (NK) cells and induction of cytokine production [8–10].
VAA-I is a 63-kDa galactoside-specific plant lectin that belongs to the family of type II ribosome-inactivating proteins, including abrin, modeccin and ricin, which have been applied extensively in clinical fields as an anticancer adjuvant [11]. The VAA-I molecule consists of two distinct subunits, the A chain (29 kDa) and the B chain (34 kDa). The A chain confers the property of inhibition of protein synthesis to the VAA-I molecule by acting as a ribosome-inactivating agent. This is due to RNA-glycosidase activity that inhibits N-glycosylation of a single adenine within a universally conserved GAGA sequence on the 28S rRNA [4,5]. The B chain allows the VAA-I molecule to bind to terminal galactoside residues on membranes of various cells.
In accordance with its immunostimulating activity, VAA-I was found to induce cytokine expression at both gene and protein levels in human peripheral blood mononuclear cells when used at low concentrations (1–10 ng/ml). These cytokines include tumour necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-6, IL-10, interferon (IFN)-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) [8,9,12]. In addition, serum levels of IL-12 (p40 and p70) were increased after treatment with V. album extract as assessed by enzyme-linked immunosorbent assay (ELISA) [13]. The production of IL-2 and IFN-γ, but not IL-4, by peripheral blood mononuclear cells was increased after treatment [13]. It has been reported that an inflammatory reaction usually occurs at the site of injection of whole-plant mistletoe extract, but examination of skin biopsies from all subjects showed normal epidermis [14]. The dermal and subcutaneous regions were found to contain a dense perivascular lymphocyte infiltrate and an increased number of monocytes. In the blood, number of neutrophils and monocytes were increased 24 h after administration of 2·5 mg Iscador [14]. We have documented recently that high concentrations of VAA-I (>250 ng/ml) induce human neutrophil apoptosis [6]. In particular, we have demonstrated that this lectin induces the degradation of cytoskeletal proteins, including gelsolin, paxillin, vimentin but not vinculin, and both α- and β- tubulin, via caspases [7].
Despite the above observations, the potential proinflammatory effect of VAA-I has not been studied in depth. In fact, there has been no study reporting the effect of VAA-I on neutrophil cytokine production nor any study evaluating its pro- or anti-inflammatory effects in vivo. In the present study, we were interested in (i) answering whether or not VAA-I induces apoptosis in preactivated human neutrophils; (ii) verifying if VAA-I induces apoptosis in murine neutrophils; (iii) evaluating the potential pro- or anti-inflammatory effects of VAA-I in vivo; and (vi) studying human neutrophil cytokine production in response to VAA-I. We found that VAA-I induces apoptosis in lipopolysaccharide (LPS)-induced human neutrophils as well as in neutrophils isolated from LPS-treated murine air pouches. In addition, we report herein that VAA-I can inhibit a neutrophilic inflammation in vivo and that low concentrations of VAA-I do not increase the production of TNF-α, IL-1Ra, IL-1α, IL-β, IL-4, IL-8, IL-10 or IL-12(p70) cytokines in human neutrophils.
MATERIALS AND METHODS
Isolation of human neutrophils
Cells were isolated from venous blood of healthy volunteers by dextran sedimentation followed by centrifugation over Ficoll-Paque (Pharmacia Biotech Inc., Quebec, Canada), as described previously [6,7]. Blood donations were obtained from informed and consenting individuals according to our institutionally approved procedures. Cell viability (>98%) was monitored systematically by trypan blue exclusion in all experiments and the purity (>98%) was verified by cytology from cytocentrifuged preparations coloured by the Hema 3 Stain Set (Biochemical Sciences Inc., Swedesboro, NJ, USA).
The murine air pouch model
The murine air pouch model was used for two purposes; (i) to study the role of VAA-I on neutrophil apoptosis isolated from an inflammatory environment, the pouch and (ii) for evaluating the potential pro- or anti-inflammatory effects of VAA-I.
(i) Six to 8-week-old CD1 mice were obtained from Charles River, Canada. Animals (≥four/group) were anaesthetized with isofurane (Abott Laboratories Limited, Saint-Laurent, Quebec, Canada). Three ml of sterilized air (filtered through a 0·2 µm Millipore filter) were injected subcutaneously into the back using a 26-gauge needle to make an air pouch on day 0 and day 3. For studying the role of VAA-I on neutrophil apoptosis collected from an inflammatory site, 1 ml of LPS (1 µg/ml) or HBSS was injected at day 6 into the air pouches of mice 6 h before being killed by CO2 asphyxiation[15]. The air pouches were washed once with 1 ml, then twice with 2 ml of Hanks's balanced salt solution (HBSS) containing 5 mm EDTA. The exudates were centrifuged at 100 g for 10 min at room temperature. The cells were suspended in one ml of HBSS and then 400 000 cells were cytospun onto microscope slides using a Cyto-tek® centrifuge (Miles Scientific, IN, USA) and stained with Hema 3 Stain Set to allow quantification of granulocytic and other cell populations [6,7]. In our hands, we obtained greater than 99% of neutrophils following LPS treatment.
(ii) For evaluating the potential pro- or anti-inflammatory effect of VAA-I we proceeded as above, except that different concentrations of VAA-I (1, 10, 100 or 1000 ng/ml) were injected into the pouch. LPS was used as a positive control [15]. In other experiences, VAA-I was administered simultaneously with LPS in order to investigate its capability to alter LPS-induced neutrophil influx.
Apoptosis
For assessment of apoptosis by cytology, cytocentrifuged preparations of neutrophils (∼200 µl) were performed as described above. Cells were examined by light microscopy at 400× final magnification and apoptotic neutrophils were defined as cells containing one or more characteristic darkly stained pyknotic nuclei. An ocular containing a 10 × 10 square grill was used in order to count at least five different fields (>100 cells) for the assessment of apoptotic cells. Results were expressed as percentage of apoptotic cells.
CD16 expression is known to be down-regulated in apoptotic neutrophils and the level of CD16 expression was measured in order to evaluate the apoptotic rate according to previous procedures [16]. After incubation cells were washed, suspended at a concentration of 1·5 × 106 cells/ml and preincubated for 30 min (4°C, light protected) with 20% autologous serum to prevent non-specific binding via Fc receptors. Cells were then washed and incubated with 2 µl of FITC-mouse antihuman CD16 MoAb (PharMingen, Canada) for 30 min at 4°C, in the dark, before FACS analysis.
Degradation of cytoskeletal proteins
Freshly isolated human neutrophils (1 × 106 cells/ml in 24-well plates) treated for 6 h with 1 µg/ml LPS or murine neutrophils (1 × 106 cells/ml), harvested from the pouch in which LPS was or was not administered, were incubated in the presence or absence of VAA-I (1000 ng/ml) for the periods of time indicated and then harvested for the preparation of cell lysates in Laemmli's sample buffer. Aliquots corresponding to 225 000 cells were loaded and run on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred from gel to PVDF membranes. Non-specific sites were blocked with 1% bovine serum albumin (BSA) in TBS-Tween (25 mm Tris-HCl, pH 7·8, 190 mm NaCl, 0·15% Tween-20) overnight at 4°C. Membranes were incubated with monoclonal antihuman cytoskeletal antibodies for 1 h at room temperature followed by washes, and incubated with the appropriated horseradish peroxidase-labelled secondary antibody for 1 h at room temperature in fresh blocking solution, as documented previously [6,7]. Membranes were washed three times with TBS-Tween, and bands were revealed with the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Pharmacia Biotech Inc., Montreal, Canada). Protein loading was verified by staining the membranes with Coomassie blue at the end of the experiments.
Cytokine production
Freshly isolated human neutrophils (10 × 106 cells/ml in RPMI-1640 supplemented with 5% fetal calf serum) were stimulated with buffer, 1 µg/ml LPS, 1 or 10 ng/ml VAA-I for 24 h, as described previously [16,17]. After incubation, both cells and supernatants were harvested and centrifuged. Supernatants were then transferred into corresponding tubes and pellets were washed twice. Fractions (intracellular and extracellular) were conserved at −80°C. Cell pellets were gently sonicated just prior to quantification. Both intracellular and extracellular concentrations of IL-1α, IL-1β, IL-1Ra, IL-4, IL-8, IL-12p70 and TNF-α were measured using commercially available ELISA kits according to the manufacturer's instructions within 2 weeks after harvesting. The specific human IL-1Ra (sensitivity of 4 pg/ml), IL-1α (1 pg/ml), IL-β (<1 pg/ml), IL-8 (<5 pg/ml), IL-12p70 (0·5 pg/ml) and TNF-α (<1·7 pg/ml) ELISA kits were purchased from Medicorp (Montreal, Canada).
Statistical analysis
Statistical analysis was performed with SigmaStat for Windows version 2·0 using Student's t-test. Statistical significance was established at P < 0·05.
RESULTS
VAA-I induces apoptosis in activated neutrophils
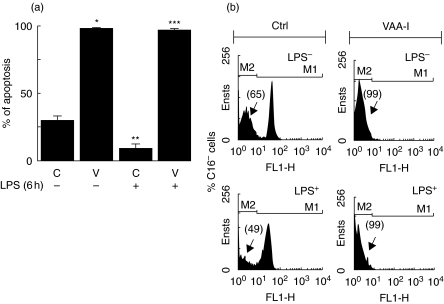
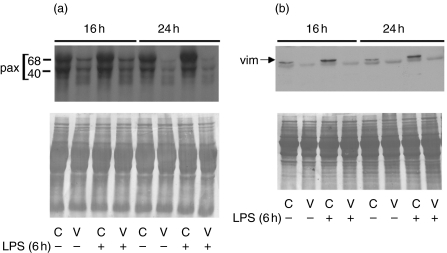
We have documented previously that VAA-I, at concentrations greater than 250 ng/ml, acts as a potent inducer of human neutrophil apoptosis [6,7]. Herein, we were interested in studying the effect of VAA-I on preactivated neutrophils, a situation that is likely to occur during inflammation. Freshly isolated human neutrophils were isolated and then treated for 6 h with 1 µg/ml LPS (similar conditions to the murine air pouch model) prior to addition of 1000 ng/ml VAA-I for 16 h. As expected, VAA-I induced apoptosis in unprimed neutrophils (Fig. 1a). When neutrophils were preactivated with LPS, VAA-I was still able to induce apoptosis. As expected, apoptosis was delayed when cells were incubated in the presence of LPS alone [18,19]. As illustrated in Fig. 1b, the number of apoptotic neutrophils (percentage of CD16-negative cells) induced by VAA-I was 99%, whether or not cells were preactivated with LPS. Apoptosis was confirmed further by monitoring the degradation of paxillin and vimentin [7]. As expected, the expression of paxillin (Fig. 2a) and vimentin (Fig. 2b) was barely detectable after VAA-I treatment whether or not cells were preactivated with LPS. When used alone LPS was found to prevent the degradation of these proteins, confirming its anti-apoptotic activity [18,19].
Fig. 1.
VAA-I induces apoptosis in activated human neutrophils. Freshly isolated human neutrophils were isolated and incubated (1 × 106 cells/ml) in the presence (+) or absence (–) of 1 µg/ml LPS for 6 h and then incubated with (V) or without (C) 1000 ng/ml VAA-I for 16 h. Apoptosis was evaluated by cytology (a) or monitoring CD16 expression by flow cytometry (b) as described in Materials and methods. (a) Results are means ± s.e.m. (n = 4) and (b) results are from one representative experiment of four. Ctrl, control cells incubated with the buffer. *P < 0·05 (versus c); **P < 0·05 (versus c) and ***P < 0·05 (versus c in presence of LPS) by Student's t-test.
Fig. 2.
VAA-I induces the degradation of the cytoskeletal proteins paxillin and vimentin in preactivated human neutrophils. Freshly isolated human neutrophils were isolated and incubated (1 × 106 cells/ml) in the presence (+) or absence (–) of 1 µg/ml LPS and then incubated with (V) or without (C) 1000 ng/ml VAA-I for the indicated periods of time. Degradation of paxillin (a) and vimentin (b) were monitored by Western blot as described in Materials and methods. Results are from one representative experiment of four. The position of the two paxillin (pax) fragments known to be recognized by the antibody [7] is illustrated, as well as the position of vimentin (vim). Bottom panels, membrane were stained with Coomassie blue at the end of the experiment in order to verify equivalent loading.
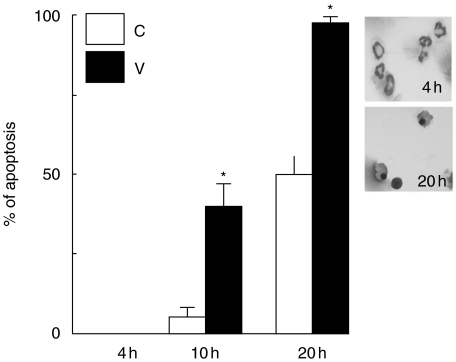
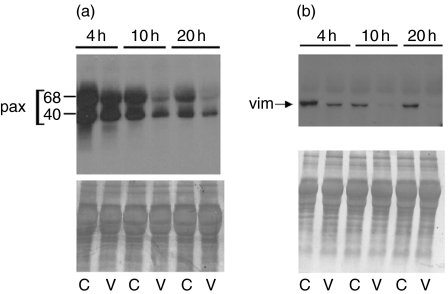
In order to investigate further how VAA-I acts on primed neutrophils in a condition mimicking an inflammatory response, we used the murine air pouch model and harvested LPS-attracted neutrophils. When these cells were incubated in vitro with VAA-I for 10 or 20 h we confirmed that the lectin was still able to induce neutrophil apoptosis, as assessed by cytology (Fig. 3). As expected, no apoptosis was observed (by cytology) after 4 h of incubation with VAA-I [6,7]. The apoptotic state of the cells was confirmed by studying the degradation of paxillin and vimentin (Fig. 4). However, degradation of the cytoskeletal proteins, especially vimentin, was observed after 4 h of incubation with VAA-I, indicating that these events occur prior to the morphological changes observed by cytology.
Fig. 3.
VAA-I induces apoptosis in neutrophils isolated from LPS-induced murine air pouch. Neutrophils were harvested from LPS-induced murine air pouch and incubated in vitro with (V) or without (C) 1000 ng/ml VAA-I for the indicated period of time, and apoptosis was evaluated by cytology as described under Materials and Methods. *, P < 0·05 (versus corresponding c) by Student's t-test. Inset, photomicrograph of representative results obtained after 4 h (no apoptosis) and 20 h (≥95% apoptosis). Results are means ± s.e.m. (n = 6).
Fig. 4.
VAA-I induces degradation of paxillin and vimentin in neutrophils isolated from LPS-induced murine air pouch. Neutrophils were harvested from LPS-induced murine air pouch as described in legend of Fig. 3 and incubated in vitro with (V) or without (C) 1000 ng/ml VAA-I for the indicated period of time. Protein expression was assessed by Western blotting as described in Materials and methods. Results are from one representative experiment of four. The positions of paxillin (pax) and vimentin (vim) are indicated. Bottom panels: membranes were stained with Coomassie blue at the end of the experiment in order to verify equivalent loading.
VAA-I inhibits the inflammatory response induced by LPS in vivo
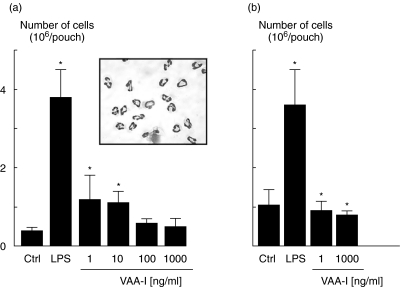
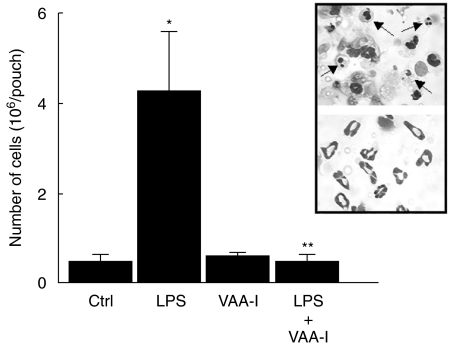
We investigated in vivo the potential proinflammatory effect of VAA-I using the murine air pouch model [17]. Concentration-dependent experiments (1, 10, 100 and 1000 ng/ml), were performed, and VAA-I was found to induce a neutrophilic inflammation when used at 1 or 10 ng/ml, but not at 100 or 1000 ng/ml, after 6 h of treatment (Fig. 5a). Under these conditions, more than 99% of attracted cells are neutrophils (see inset). To demonstrate further that the high concentration of 1000 ng/ml VAA-I does not attract cells into the pouch, we performed similar experiments in which VAA-I was added for 12 h. As illustrated in Fig. 5b VAA-I, unlike LPS, did not promote cell attraction in vivo whether it was administered at 1 or 1000 ng/ml. This indicates that the proinflammatory effect of a low concentration of VAA-I persist for a shorter period of time than LPS. Figure 6 illustrates that VAA-I inhibits the proinflammatory effect of LPS when administered simultaneously with this potent proinflammatory agent. Interestingly, about 30% of cells were typical of apoptotic neutrophils as assessed by cytology (inset).
Fig. 5.
Effect of VAA-I on neutrophil influx in vivo. The murine air pouch model was used as described in Materials and methods and VAA-I was administered directly into the pouch at a concentration of 1, 10, 100 or 1000 ng/ml for 6 h (a) or 12 h (b), and cells were counted as described in Materials and methods. LPS (1 µg/ml) was used as a positive control [17]. *P < 0·05 (versus Ctrl) by Student's t-test. Inset, more than 98% of attracted cells are neutrophils.
Fig. 6.
VAA-I inhibits the inflammatory response induced by LPS in vivo. The murine air pouch model was used as described in Materials and methods and 1000 ng/ml VAA-I was administered simultaneously with LPS (1 µg/ml) directly into the pouch. *P < 0·05 (versus Ctrl); **P < 0·05 (versus LPS) by Student's t-test. Inset, more than 98% of attracted cells are neutrophils. Results are means ± s.e.m. (n = 5) and have been reproduced in at least another experiment.
Low concentrations of VAA-I does not induce the production and release of TNF-α, IL-1Ra, IL-1α, IL-β, IL-4, IL-8, IL-10 and IL-12. Knowing that only low concentrations of VAA-I can induce a neutrophilic inflammation, whereas high concentrations induce apoptosis, and that human neutrophil cytokine production in response to VAA-I is lacking in the literature, we next investigated the production and release of several cytokines in response to 1 and 10 ng/ml VAA-I. As illustrated in Table 1, in contrast to LPS that increase the production of TNF-α, IL-8, IL-1α and IL-1β, VAA-I did not increase the production of TNF-α, IL-12, IL-10, IL-8, IL-1Ra, IL-1α and IL-1β by human neutrophils.
Table 1.
Human neutrophil cytokine production in response to VAA-Ia
| Cytokine | Fraction | Ctrl | LPS | VAA-I (1 ng/ml) | VAA-I (10 ng/ml) |
|---|---|---|---|---|---|
| TNF-α | intra | 3 ± 1 | 8 ± 4 | 8 ± 5 | 4 ± 1 |
| extra | 0 | 226 ± 67* | 0 | 0 | |
| IL-12 | intra | 1 ± 0·1 | 1 ± 0·1 | 1 ± 0·1 | 1 ± 0·1 |
| extra | 0 | 0 | 0 | 0 | |
| IL-10 | intra | 3 ± 0·2 | 3 ± 0·2 | 3 ± 0·3 | 3 ± 0·3 |
| extra | 0 | 2 ± 1 | 0 | 0 | |
| IL-8 | intra | 3119 ± 248 | 8226 ± 527* | 3034 ± 292 | 3315 ± 252 |
| extra | 182 ± 62 | 1184 ± 220* | 124 ± 17 | 134 ± 18 | |
| IL-1Ra | intra | 2267 ± 96 | 2329 ± 154 | 2032 ± 64 | 2454 ± 62 |
| extra | 1260 ± 173 | 3000 ± 55 | 1283 ± 159 | 1305 ± 161 | |
| IL-1α | intra | < | 164 ± 36* | < | < |
| extra | < | 12 ± 3* | < | < | |
| IL-1β | intra | 0·6 ± 0·6 | 12 ± 3* | 0 | 1 ± 0·9 |
| extra | 1 ± 1 | 39 ± 9* | 0 | 0 |
Freshly isolated human neutrophils (10 × 106 cells/ml) were incubated in the presence of buffer (Ctrl), 1 µg/ml LPS or the indicated concentration of VAA-I for 24 h. Cytokine concentration was evaluated by ELISA from the intracellular (intra) and extracellular (extra) fractions as described in Materials and methods. Results (means ± s.e.m. from at least five separate experiments) are expressed in pg/ml; <, under the sensitivity limit of the essay.
P < 0·05 (versus Ctrl) by Student's t-test.
DISCUSSION
The effect of VAA-I on non-stimulated cells has been studied widely [5–8,8,9,9–12]. No doubt VAA-I is a potent inducer of apoptosis in a variety of cells, including neutrophils [6,7]. The effect of VAA-I in combination with other molecules or in preactivated cells is, however, poorly documented. Recently, cytotoxic effects of VAA-I in human lung carcinoma A549 cells were enhanced in combination with chemotherapeutic drugs, including doxorubicin, cisplatin and taxol [20]. We have documented previously that the ability of GM-CSF and IL-15 to suppress human neutrophil apoptosis was reversed when cells were stimulated simultaneously with VAA-I + GM-CSF or VAA-I + IL-15 [21]. In fact, under these conditions the effect of VAA-I was not altered, as virtually all neutrophils were in apoptosis.
In this study we demonstrated that the plant lectin VAA-I induces apoptosis in LPS-activated human neutrophils with the same potency as in naive or resting neutrophils. This was confirmed by cytology, flow cytometry (CD16 shedding) and degradation of the cytoskeletal proteins vimentin and paxillin. Such information concerning the apoptotic effect of VAA-I on activated human neutrophils was not available prior to the present study. We decided to stimulate neutrophils with LPS prior to incubation with VAA-I because of its well-recognized proinflammatory properties and also because of its ability to attract cells in the murine air pouch model [17]. We also report herein that VAA-I induced apoptosis in neutrophils harvested from LPS-treated air pouches, as well as inducing the degradation of vimentin and paxillin proteins in LPS-attracted murine neutrophils. Collectively, our results indicate that VAA-I induces apoptosis with the same potency in non-stimulated [6,7] or preactivated human neutrophils (this study) as in LPS-attracted murine neutrophils (this study).
A variety of agents, including cytokines, have been detected in large amounts at sites of inflammation. Interestingly, adenosine and proinflammatory cytokines present in synovial fluids from patients with rheumatoid arthritis were found to inhibit neutrophil apoptosis [22]. Thus, one can imagine that presence of cytokines at inflamed sites favours inflammation. Knowing that elimination of neutrophils from an inflamed site can lead to the resolution of inflammation [1–4], a potential therapeutic strategy would be to administer locally a potent inducer of neutrophil apoptosis that does not induce production of cytokines by these cells.
VAA-I or different extracts of V. album were found to induce cytokine production in keratinocytes and peripheral blood mononuclear cells in vitro, and high concentrations of cytokines were also detected in VAA-I treated-patients [10,23,24]. Although several of these cytokines are proinflammatory (including IL-1, IL-6 and TNF-α), the effect of VAA-I on human neutrophil cytokine production has never been reported before this present study. Our results demonstrate that in vitro treatment of human neutrophils with low concentrations of VAA-I (1 and 10 ng/ml) did not induce the production of IL-1α, IL-1β, IL-1Ra, IL-4, IL-8, IL-12p70 and TNF-α. Neutrophils were, however, responsive because, as expected, LPS was found to increase levels of TNF-α, IL-8, IL-1β and IL-1R [25,26]. We are aware that we have not tested all known cytokines, but the fact that VAA-I does not induce TNF-α, IL-1 and IL-8 suggests that neutrophils are not high producers of these cytokines in response to VAA-I, even though an increase was observed in vivo in patients’ serum [10,24]. There is still much work to conduct in this area, including kinetics and concentration–response experiments, in order to detect several cytokines and chemokines. This remains to be performed both in vitro and in vivo. Our results suggest that the initiation of the transitory proinflammatory response induced by a low concentration of VAA-I differs from that induced by LPS by two parameters: (i) the distinct cytokine profile observed in human neutrophils and (ii) the duration of the proinflammatory response in vivo.
The present study provides the first evidence that activated human neutrophils, or neutrophils isolated from an inflamed site such as the LPS-induced murine air pouch, are easily driven to undergo apoptosis after a treatment of 1000 ng/ml VAA-I. In addition, this concentration used to induce this apoptosis is not by itself proinflammatory. Moreover, this concentration inhibits LPS-induced inflammation. Collectively, these data may provide new clinical perspectives in future mistletoe therapy and support the administration of local inducers of apoptosis that do not induce an inflammatory response.
Acknowledgments
We thank Mary Gregory for reading this manuscript. This study was supported partly by Canadian Institutes of Health Research (CIHR) and Fonds de la Recherche en Santé du Québec (FRSQ). V. L. and C. R. hold a PhD CIHR award and D. G. is a Scholar from FRSQ.
REFERENCES
- 1.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–93. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 2.Ward I, Dransfield I, Chilvers ER, Haslett I, Rossi AG. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol Sci. 1999;20:503–9. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- 3.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 4.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Diff. 1998;5:563–8. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 5.Hostanska K, Hajto T, Weber K, et al. A natural immunity-activating plant lectin, Viscum album agglutinin-I, induces apoptosis in human lymphocytes, monocytes, monocytic THP-1 cells and murine thymocytes. Nat Immun. 1996–97;15:295–311. [PubMed] [Google Scholar]
- 6.Savoie A, Lavastre V, Pelletier M, Hajto T, Hostanska K, Girard D. Activation of human neutrophils by the plant lectin Viscum album agglutinin-I. modulation of de novo protein synthesis and evidence that caspases are involved in induction of apoptosis. J Leukoc Biol. 2000;68:845–53. [PubMed] [Google Scholar]
- 7.Lavastre V, Pelletier M, Saller R, Hostanska K, Girard D. Mechanisms involved in spontaneous and Viscum album agglutinin-I-induced human neutrophil apoptosis: Viscum album agglutinin-I accelerates the loss of antiapoptotic Mcl-1 expression and the degradation of cytoskeletal paxillin and vimentin proteins via caspases. J Immunol. 2002;168:1419–27. doi: 10.4049/jimmunol.168.3.1419. [DOI] [PubMed] [Google Scholar]
- 8.Hostanska K, Hajto T, Spagnoli GC, Fischer J, Lentzen H, Herrmann R. A plant lectin derived from Viscum album induces cytokine gene expression and protein production in cultures of human peripheral blood mononuclear cells. Nat Immun. 1995;14:295–304. [PubMed] [Google Scholar]
- 9.Hajto T, Hostanska K, Frei K, Rordorf C, Gabius HJ. Increased secretion of tumor necrosis factors alpha, interleukin 1, and interleukin 6 by human mononuclear cells exposed to beta-galactoside-specific lectin from clinically applied mistletoe extract. Cancer Res. 1990;50:3322–6. [PubMed] [Google Scholar]
- 10.Hajto T, Hostanska K, Weber K, et al. Effect of a recombinant lectin, Viscum album agglutinin on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat Immun. 1998;16:34–46. doi: 10.1159/000069428. [DOI] [PubMed] [Google Scholar]
- 11.Olsnes S, Stirpe F, Sandvig K, Pihl A. Isolation and characterization of viscumin, a toxic lectin from Viscum album L. (mistletoe) J Biol Chem. 1982;257:13263–70. [PubMed] [Google Scholar]
- 12.Hajto T, Hostanska K, Fischer J, Saller R. Immunomodulatory effects of Viscum album agglutinin-I on natural immunity. Anticancer Drugs. 1997;8:S43–6. doi: 10.1097/00001813-199704001-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs E. Serum levels of IL-12 and the production of IFN-gamma, IL-2 and IL-4 by peripheral blood mononuclear cells (PBMC) in cancer patients treated with Viscum album extract. Biomed Pharmacother. 2000;54:305–10. doi: 10.1016/S0753-3322(00)80052-9. [DOI] [PubMed] [Google Scholar]
- 14.Gorter RW, van Wely M, Stoss M, Wollina U. Subcutaneous infiltrates induced by injection of mistletoe extracts (Iscador) Am J Ther. 1998;5:181–7. doi: 10.1097/00045391-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Moulding DA, Hart CA, Edwards SW. Regulation of neutrophil FcgammaRIIIb (CD16) surface expression following delayed apoptosis in response to GM-CSF and sodium butyrate. J Leukoc Biol. 1999;65:875–82. doi: 10.1002/jlb.65.6.875. [DOI] [PubMed] [Google Scholar]
- 16.Ratthé C, Pelletier M, Roberge CJ, Girard D. Activation of human neutrophils by the pollutant sodium sulfite: effect on cytokine production, chemotaxis and cell surface expression of cell adhesion molecules. Clin Immunol. 2002;105:169–75. doi: 10.1006/clim.2002.5282. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier M, Roberge CJ, Gauthier M, Vandal K, Tessier PA, Girard D. Activation of human neutrophils in vitro and dieldrin-induced neutrophilic inflammation in vivo. J Leukoc Biol. 2001;70:367–73. [PubMed] [Google Scholar]
- 18.Klein JB, Buridi A, Coxon PY, et al. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell Signal. 2001;13:335–43. doi: 10.1016/s0898-6568(01)00151-6. [DOI] [PubMed] [Google Scholar]
- 19.Hachiya O, Takeda Y, Miyata H, Watanabe H, Yamashita T, Sendo F. Inhibition by bacterial lipopolysaccharide of spontaneous and TNF-alpha-induced human neutrophil apoptosis in vitro. Microbiol Immunol. 1995;39:715–23. doi: 10.1111/j.1348-0421.1995.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 20.Siegle I, Fritz P, McClellan M, Gutzeit S, Murdter TE. Combined cytotoxic action of Viscum album agglutinin-1 and anticancer agents against human A549 lung cancer cells. Anticancer Res. 2001;21:2687–91. [PubMed] [Google Scholar]
- 21.Pelletier M, Lavastre V, Savoie A, et al. Modulation of interleukin-15-induced human neutrophil responses by the plant lectrin Viscum album agglutinin-I. Clin Immunol. 2001;101:229–36. doi: 10.1006/clim.2001.5105. [DOI] [PubMed] [Google Scholar]
- 22.Ottonello L, Cutolo M, Frumento G, et al. Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology. 2002;41:1249–60. doi: 10.1093/rheumatology/41.11.1249. [DOI] [PubMed] [Google Scholar]
- 23.Gorter RW, Joller P, Stoss M. Cytokine release of a keratinocyte model after incubation with two different Viscum album L. extracts. Am J Ther. 2003;10:40–7. doi: 10.1097/00045391-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Ribereau-Gayon G, Dumont S, Muller C, Jung ML, Poindron P, Anton R. Mistletoe lectins I, II and III induce the production of cytokines by cultured human monocytes. Cancer Lett. 1996;109:33–8. doi: 10.1016/s0304-3835(96)04401-1. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 26.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]