Abstract
CD3−CD56+dim natural killer (NK) cells, which are cytotoxic against virally infected cells, may be important in hepatitis C virus (HCV)-infected patients who are successfully treated with pegylated interferon (PEG-IFN)-α. We used flow cytometry to enumerate activated (CD69+) and apoptotic (annexin-V+) dim (CD3−CD56+dim) and bright (CD3−CD56+bright) NK cells obtained from HCV-infected patients before treatment (n = 16) and healthy controls (n = 15) in the absence and presence of pegylated interferon (PEG-IFN)-α-2b. A subset of HCV-infected patients, subsequently treated with PEG-IFN-α-2b in vivo, was determined to have a sustained virological response (SVR, n = 6) or to not respond (NR) to treatment (n = 5). In the absence of IFN, activated dim (CD3−CD56+dim CD69+) NK cells were significantly decreased (P = 0·04) while activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells tended to be increased (P = 0·07) in SVR patients compared with NR patients. Activated bright (CD3−CD56+brightCD69+) and activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells were significantly correlated (P = 0·02 and P = 0·01, respectively) with increasing hepatic inflammation. These findings suggest that in the absence of PEG-IFN, activated dim (CD3−CD56+dimCD69+) NK cell turnover may be enhanced in SVR compared with NR patients and that activated bright (CD3−CD56+brightCD69+) NK cells may play a role in liver inflammation.
Keywords: natural killer cells, hepatitis C virus infection, pegylated interferon-α
INTRODUCTION
CD56+ natural killer (NK) cells, which comprise approximately 10% of human peripheral blood mononuclear cells (PBMCs) and up to 30% of intrahepatic mononuclear cells [1], are critical for innate antiviral host defence [2] since these cells have the capacity to limit viremia before adaptive T and B cell antigen-specific responses emerge. NK cells can be divided into two subsets, dim and bright, according to CD56 surface density expression [3,4]. Dim (CD3−CD56+dim CD16+) NK cells are cytolytic and comprise more than 90% of CD3−CD56+ NK cells, whereas bright (CD3−CD56+bright CD16−) NK cells are immunoregulatory principally through cytokine production. Bright (CD3−CD56+bright) NK cells, which lack perforin granules, display homing receptors required for migration to secondary lymph nodes [5].
In vivo, intrahepatic NK cell numbers and peripheral blood NK cells expressing perforin are decreased suggesting that cytotoxic function may be altered in patients with chronic hepatitis C virus (HCV) infection compared with healthy controls [6,7]. In chronic HCV-infected patients who achieve a successful treatment response with interferon (IFN), post-treatment intrahepatic CD3−CD56+ cells are increased compared with nonresponder patients [8]. The number of pretreatment NK cells is also increased 3-fold in HIV/HCV coinfected patients who achieve an early viral response with IFN-α compared with NR patients [9]. In vivo, IFN-α increases NK cell activation [10], augments NK cell cytotoxicity by increasing perforin/granzyme release [11,12] and promotes cytokine secretion (e.g. IFN-γ, IL-10, IL-13, TNF-β, GM-CSF) [13]. Recently, pegylated (PEG)-IFN-α, IFN-α conjugated to polyethylene glycol, has replaced nonpegylated IFN-α as standard treatment for HCV [14,15]. To our knowledge, the effects of PEG-IFN-α on NK cell subsets in HCV-infected patients have not been evaluated.
Since dim (CD3−CD56+dim) NK cells are primarily cytotoxic, we hypothesized that these cells might be important mediators of a therapeutic response to treatment with PEG-IFN-α. Additionally, since IFN-α augments NK cell cytotoxicity, we evaluated whether PEG-IFN-α would alter NK cell subsets in vitro or alter expression of activation (CD69) or apoptotic (annexin-V) markers. In a preliminary study, we used PEG-IFN-α-2b in vitro to stimulate pretreatment peripheral blood mononuclear cells (PBMCs) in a short-term culture system and flow cytometry to enumerate activated and apoptotic dim (CD3−CD56+dim) and bright (CD3−CD56+bright) NK cells. NK cell markers were evaluated in pretreatment PBMCs from patients with chronic HCV infection and in a subgroup of these patients who were subsequently treated with PEG-IFN-α. Treated patients could be characterized as responders or nonresponders in vivo after treatment as described below. Pretreatment NK cell percentages, the percentage of NK cell subsets and the percentage of activated and apoptotic NK cells from responders and nonresponders were compared to the larger group of patients with chronic HCV infection and to that of normal controls.
MATERIALS AND METHODS
Study subjects
PBMCs were obtained and isolated from 16 treatment-naïve, chronic HCV-infected patients and 15 healthy controls in accordance with a Weill Medical College of Cornell University Institutional Review Board approved protocol. HCV-infected patients were recruited from the clinical practice of one of the investigators (IMJ). Inclusion criteria specified that all patients be naïve to anti-HCV treatment. PBMCs were obtained prior to initiation of PEG-IFN-α-2b therapy and cryopreserved as previously described [16]. Patients infected with HCV genotype 1 were subsequently treated with PEG-IFN-α-2b and ribavirin for one year, while those infected with HCV genotypes 2 or 3 were treated for six months. Patients who had undetectable HCV RNA for at least six months post treatment were classified as sustained viral responders (SVR, n = 6) and those who had detectable HCV RNA were classified as nonresponders (NR, n = 5). Five of the 16 HCV-infected patients remained treatment naïve. Study entry also required that patients have a pretreatment liver biopsy. Necroinflammatory grade and fibrosis stage were assessed using the Metavir system [17].
PBMC thawing and incubation
Aliquots of 10 million PBMCs per patient were rapidly thawed at 37°C, counted by haemocytometer using trypan blue dye exclusion, diluted to 1·5 million lymphocytes/ml, aliquoted at one ml per tube and cultured for 24 h at 37°C in 5% CO2 without or with PEG-IFN-α-2b 2·7 µg/ml (Schering-Plough, Kenilworth, NJ, USA), chosen through dose-titration, PBMCs were incubated in RPMI-1640 (Seromed, Fakola, Basel, Switzerland), 10% fetal bovine serum (Cellgro, Herndon, VA, USA), 2 mM l-glutamine (Gibco, Invitrogen, Carlsbad, CA, USA), 17·8 mm NaHCO3, 1 mm Na pyruvate (Cellgro), 1% penicillin/streptomycin (Gibco), 25 mM d-glucose, 10 mm Hepes buffer (Sigma, St. Louis, MO, USA).
Surface staining and flow cytometry
Following incubation without or with PEG-IFN-α-2b, PBMCs were removed, washed, and resuspended for staining. For each experiment, cells were incubated with the appropriate antibodies for four-colour flow cytometry: CD3-energy-coupled dye (ECD) (clone UCHT1), CD56-phycoerythrin-cyanin 5·1 (PC5) (clone N901), CD161-phycoerythrin (PE) (clone 191B8), CD4-PC5 (clone 13B8·2), and CD69-PE (clone TP1·55·3) (Immunotech, Marseille, France) for 30 min at 4°C. Cells were washed three times with D-PBS, 0·5% BSA with 10 mm NaN3 and resuspended in annexin binding buffer (1·4 m NaCl, 0·025 m CaCl2, 0·1 m HEPES). To determine the level of apoptosis, PBMCs were incubated with annexin-V conjugated to fluorescein isothiocyanate (FITC) (Molecular Probes, Eugene, OR, USA) for 15 min in the dark at room temperature. After staining, all samples were resuspended in annexin binding buffer and acquired on an EPICS XL four-colour flow cytometer (Beckman-Coulter, Hialeah, FL, USA). Isotype-matched negative control antibodies were included in all experiments.
Analysis of flow cytometric data
A minimum of 10 000 events was acquired in the gating region and analysed using WinMDI (Scripps Research Institute, La Jolla, CA, USA). Total lymphocytes were identified by forward versus side scatter. T-cells were identified by their CD3 bright side-scatter-low profile. NK cells (CD56+CD3−) were determined by gating on CD56+ cells and excluding CD3+ cells. CD56+bright and CD56+dim cells were distinguished by CD56 fluorescence density, CD56+bright cells had a mean fluorescence channel of ≥613 and CD56+dim cells had a mean fluorescence channel of 612–303. Activated NK cells were defined as CD3−CD56+CD69+ and activated apoptotic NK cells were defined as CD3−CD56+CD69+annexin-V+. Gating was determined on the basis of isotypic negative controls.
HCV genotyping and HCV RNA quantification
HCV genotypes were determined by sequencing the NS5B region [18] and classified according to the method of Simmonds et al. [19,20]. HCV RNA levels were determined by the Roche Amplicor assay (Version 2·0, Roche Diagnostics, Branchberg, NJ, USA) with linear ranges from 103 to 107 HCV copies/ml. Samples above 1 million copies were not diluted.
Statistical analysis
Results are expressed as mean ± standard error (SE). The effect of PEG-IFN-α-2b on the percentage of cells within individual strata and differences in the percentage of cells between patients in different categories were assessed using the Wilcoxon signed-rank and rank-sum tests, respectively (Version 4, STATA Corporation, College Station, TX, USA). The chi-squared test was used to evaluate for significant associations between categorical variables. The association between necroinflammatory grade and the percentage of CD3−CD56+bright cells was assessed using the Spearman correlation coefficient and linear regression. All comparisons were two-tailed and P≤ 0·05 was considered significant.
RESULTS
Patient characteristics
No differences were observed between the naïve, nonresponder, and sustained responder patient groups with regard to age, gender, race, genotype, HCV RNA, fibrosis stage, necroinflammatory grade, serum alanine aminotransferase (ALT) levels, and exposure risk (Table 1). In general, patients who were sustained responders were more likely to be infected with HCV genotype non-1 compared with the naïve group as a whole and the nonresponder group (P = 0·06).
Table 1.
Patient clinical and virological characteristics
| Controls (n = 15)* | Naive (n = 5) | Nonresponders (n = 5) | Sustained responders (n = 6) | P-value† | |
|---|---|---|---|---|---|
| Age (years; mean ± SE) | 39·9 ± 2·8 | 51·4 ± 3·0 | 44·0 ± 6·0 | 51·7 ± 3·6 | 0·41 |
| Gender | |||||
| Male | 5/11 (45%) | 2/5 (40%) | 2/5 (40%) | 5/6 (83%) | 0·24 |
| Female | 6/11 (55%) | 3/5 (60%) | 3/5 (60%) | 1/6 (17%) | |
| Race | |||||
| Caucasian | 2/11 (18%) | 5/5 (100%) | 5/5 (100%) | 5/6 (83%) | 0·41 |
| African American | 1/11 (9%) | ||||
| Hispanic | 4/11 (36%) | ||||
| Asian | 3/11 (27%) | 1/6 (17%) | |||
| Genotype | |||||
| Genotype 1 | N/A | 5/5 (100%) | 5/5 (100%) | 2/6 (33%) | 0·06 |
| Genotype 2,3 | 4/6 (67%) | ||||
| HCV RNA (cps/ml) | |||||
| ≥1 million | N/A | 3/5 (60%) | 5/5 (100%) | 3/6 (50%) | 0·18 |
| <1 million | 2/5 (40%) | 3/6 (50%) | |||
| Fibrosis stage (Metavir) | |||||
| Stage 0–2 | N/A | 5/5 (100%) | 4/5 (80%) | 3/6 (50%) | 0·18 |
| Stage 3–4 | 1/5 (20%) | 3/6 (50%) | |||
| Necroinflammatory grade (Metavir) | |||||
| Grade 0–2 | N/A | 4/5 (80%) | 4/5 (80%) | 6/6 (100%) | 0·23 |
| Grade 3–4 | 1/5 (20%) | 1/5 (20%) | |||
| Mean serum ALT (U/l ±SE) | N/A | 56·4 ± 7 | 110·6 ± 35 | 171·7 ± 52 | 0·47 |
| Exposure risk | |||||
| Blood transfusion | N/A | 2/5 (40%) | 2/5 (40%) | 1/6 (17%) | 0·50 |
| IDU | 2/5 (40%) | 1/6 (17%) | |||
| Intranasal cocaine | 1/6 (17%) | ||||
| Tattoos | 1/5 (20%) | ||||
| Unknown | 1/5 (20%) | 2/5 (40%) | 3/6 (50%) | ||
Clinical and demographic information available in only 11 controls.
χ2 analysis was used between three HCV (+) groups: treatment naïve, nonresponders, and sustained responders. SE, standard error; IDU, injection drug use; ALT, alanine aminotransferase; cps, copies; U/l, units/l.
Effect of PEG-IFN-α on NK cells and NK cell subsets
CD3−CD56± NK cells.
CD3−CD56+ NK cells as a percentage of lymphocytes did not differ significantly between healthy controls, HCV-infected, and NR patients in the absence of IFN (Table 2). SVR patients showed a lower percentage of CD3−CD56+ NK cells. After IFN stimulation, a slight but significant decrease in CD3−CD56+ NK cells was observed in healthy control PBMCs (5·3 ± 0·5–4·7 ± 0·5, P = 0·002), but not in the HCV-infected patients.
Table 2.
Mean percentage of total and activated NK cells without and with PEG-IFN stimulation in four patient groups
| CD3− CD56+ cells | Bright (CD3− CD56+bright) NK cells | Dim (CD3− CD56+dim) NK cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Without PEG-IFN | With PEG-IFN | P-value | Without PEG-IFN | With PEG-IFN | P-value | Without PEG-IFN | With PEG-IFN | P-value | |
| Control | |||||||||
| % Total | 5·3 ± 0·5* | 4·7 ± 0·5* | 0·002 | 8·4 ± 1·1‡ | 12·0 ± 1·8‡ | 0·002 | 91·6 ± 1·1‡ | 88·0 ± 1·8‡ | 0·002 |
| % CD69+ | 60·6 ± 3·8† | 91·2 ± 1·3† | 0·001 | 42·1 ± 5·1§ | 83·5 ± 3·5§ | 0·001 | 62·7 ± 4·6§ | 92·6 ± 1·2§ | 0·001 |
| HCV-infected | |||||||||
| % Total | 4·5 ± 0·5* | 4·6 ± 0·5* | 0·562 | 20·1 ± 3·5‡ | 25·2 ± 3·8‡ | 0·001 | 79·9 ± 3·5‡ | 74·8 ± 3·8‡ | 0·001 |
| % CD69+ | 45·9 ± 3·3† | 82·6 ± 2·5† | <0·001 | 29·7 ± 3·1§ | 77·7 ± 3·6§ | <0·001 | 49·6 ± 3·7§ | 83·4 ± 2·6§ | <0·001 |
| NR | |||||||||
| % Total | 4·1 ± 0·5* | 3·9 ± 0·5* | 0·414 | 24·0 ± 6·3‡ | 29·4 ± 6·2‡ | 0·043 | 76·0 ± 6·3‡ | 70·6 ± 6·2‡ | 0·043 |
| % CD69+ | 47·5 ± 3·0† | 78·3 ± 3·7† | 0·043 | 33·4 ± 7·1§ | 78·8 ± 5·1§ | 0·043 | 51·8 ± 2·5§ | 78·8 ± 4·8§ | 0·043 |
| SVR | |||||||||
| % Total | 3·0 ± 0·4* | 3·6 ± 0·4* | 0·126 | 18·4 ± 6·6‡ | 24·6 ± 7·2‡ | 0·028 | 81·7 ± 6·6‡ | 75·4 ± 7·2‡ | 0·028 |
| % CD69+ | 35·4 ± 4·1† | 78·7 ± 4·2† | 0·028 | 27·3 ± 2·3§ | 68·1 ± 6·2§ | 0·028 | 37·8 ± 4·9§ | 80·7 ± 4·0§ | 0·028 |
Values refer to total CD3−CD56+ NK cells as a percentage of all lymphocytes.
Values refer to activated (CD3−CD56+CD69+) NK cells as a percentage of total CD3−CD56+ NK cells.
Values refer to CD3−CD56+brght/dim cells as a percentage of total CD3−CD56+ NK cells.
Values refer to activated dim/bright (CD3−CD56+bright/dimCD69+) NK cells as percentage of CD3−CD56+ bright or dim cells. All values represent mean± standard error and were compared using the nonparametric signed-rank test. PEG-IFN, pegylated interferon; HCV, hepatitis C virus; NR, nonresponder; SVR, sustained virological responder
CD3−CD56+bright and CD3−CD56+dim subsets.
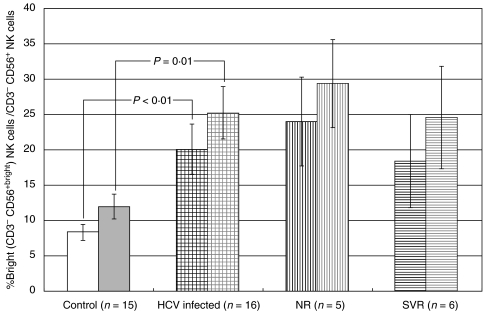
We then evaluated the relative differences in the percentage of bright (CD3−CD56+bright) and dim (CD3−CD56+dim) NK cells as a percentage of all NK cells in all groups. In the HCV-infected patients, the percentage of bright (CD3−CD56+bright) NK cells was significantly greater than in healthy controls both in the absence (20·1 ± 3·5 versus 8·4 ± 1·1, P = 0·004) and in the presence (25·2 ± 3·8 versus 12·0 ± 1·8, P = 0·006) of IFN stimulation (Fig. 1). CD56 geometric mean fluorescence was also increased in HCV-infected patients (data not shown). IFN-α significantly increased bright (CD3−CD56+bright) NK cells in all patient groups and controls (Table 2). Dim (CD3−CD56+dim) NK cells, which comprise the dominant subset in all groups, were reduced in HCV-infected patients compared to controls both in the absence (79·9 ± 3·5 versus 91·6 ± 1·1, P = 0·004) and in the presence (74·8 ± 3·8 versus 88·0 ± 1·8, P = 0·006) of IFN. IFN treatment reduced the mean percentage of dim (CD3−CD56+dim) NK cells in all groups proportionately. Thus, the overall effect of PEG-IFN-α-2b treatment in HCV-infected patients was to increase the percentage of bright (CD3−CD56+bright) NK cells (P = 0·001), which was associated with a small decrease in the percentage of dim (CD3−CD56+dim) NK cells (P = 0·001).
Fig. 1.
Bright (CD3−CD56+bright) NK cells without and with PEG-IFN-α. IFN-α induced increases in bright (CD3−CD56+bright) NK cells as a percentage of total CD3−CD56+ NK cells in healthy controls and HCV-infected patients. HCV-infected patients had significantly greater bright (CD3−CD56+bright) NK cells than healthy controls both without (P < 0·01) and with (P = 0·01) IFN stimulation. Error bars represent standard error. Legend: Without IFN stimulation- healthy controls (white bar), HCV-infected (black hatched), nonresponder (NR) patients (black vertical lines), sustained viral responder (SVR) patients (black horizontal lines). With IFN stimulation – healthy controls (grey bar), HCV-infected patients (grey hatched), NR patients (grey vertical lines), SVR patients (grey horizontal lines).
Differential effect of PEG-IFN on CD69 expression
HCV infection and healthy controls.
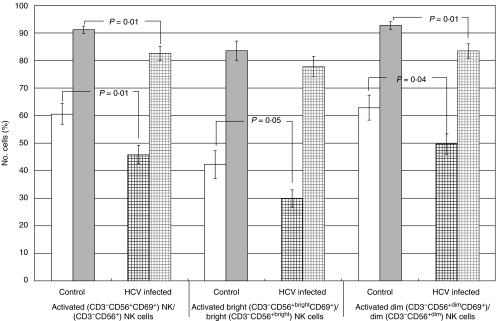
As a measure of NK cell activation, we assessed the change in CD69 surface expression on CD3−CD56+ NK cells. Compared with healthy controls, HCV-infected patients had fewer activated (CD3−CD56+CD69+) NK cells as a percentage of all NK cells in the absence (45·9 ± 3·3 versus 60·6 ± 3·8, P = 0·009) and in the presence (82·6 ± 2·5 versus 91·2 ± 1·3, P = 0·008) of IFN (Fig. 2). We then evaluated the percentage of activated bright and dim (CD3−CD56+CD69+) NK cells within each subset. HCV-infected patients had fewer activated bright (CD3−CD56+brightCD69+) NK cells within the bright subset in the absence of IFN (29·7 ± 3·1 versus 42·1 ± 3·1, P = 0·048). After IFN stimulation, the level of activated bright (CD3−CD56+brightCD69+) NK cells in both groups was equivalent (Fig. 2). HCV-infected patients had a significantly smaller percentage of activated dim (CD3−CD56+dimCD69+) NK cells as a percentage of dim NK cells both in the absence (49·6 ± 3·7 versus 62·7 ± 4·6, P = 0·040) and in the presence (83·4 ± 2·6 versus 92·6 ± 1·2, P = 0·006) of IFN stimulation. Therefore, activated (CD3−CD56+CD69+) NK cells, whether bright or dim, were reduced in their respective subsets in the HCV-infected group compared with controls. We also correlated NK cell subsets with the severity of liver disease. Increasing necroinflammatory grade, assessed using a five-point scale [17], correlated with increasing percentage of activated bright (CD3−CD56+brightCD69+) NK cells (r = 0·6, P = 0·02) (Table 3). The percentage of activated bright (CD3−CD56+brightCD69+) NK cells increased by 11·6 ± 2·5% per increase in grade (P = 0·001).
Fig. 2.
Activated total (CD3−CD56+CD69+), bright (CD3−CD56+brightCD69+), and dim (CD3−CD56+dimCD69+) NK cells without and with PEG-IFN-α in healthy controls versus HCV-infected patients. Activated (CD3−CD56+CD69+) NK cells as a percentage of total CD3−CD56+ NK cells are decreased in HCV-infected patients (hatched) compared to healthy controls (white bar) both without (P = 0·01) and with (P = 0·01) IFN-α stimulation. In the absence of PEG IFN, activated bright (CD3−CD56+brightCD69+) and dim (CD3−CD56+dimCD69+) NK cells as a percentage of bright (CD3−CD56+bright) and dim (CD3−CD56+dim) NK cells, respectively, are decreased in HCV-infected patients (black hatched) compared with healthy controls (open bar) (P = 0·05 and P = 0·04, respectively). Activated dim (CD3−CD56+dimCD69+) cells are reduced in HCV-infected patients (grey hatched) compared with healthy controls (grey bar) with PEG IFN (P = 0·01). Error bars represent standard error.
Table 3.
Correlation between activated NK cell subsets and activated apoptotic NK cell subsets and severity of liver disease*
| Activated (CD3− CD56+CD69+) NK cells | Activated bright (CD3− CD56+dimCD69+) NK cells | Activated dim(CD3− CD56+brightCD69+) NK cells | Activated apoptotic bright (CD3− CD56+brightCD69+annexin-V+) NK cells | Activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells | ||||
|---|---|---|---|---|---|---|---|---|
| Without PEG-IFN | With PEG-IFN | Without PEG-IFN | With PEG-IFN | Without PEG-IFN | With PEG-IFN | |||
| Fibrosis stage (rho, P) | − 0·0, 0·91 | − 0·2, 0·46 | 0·3, 0·25 | − 0·3, 0·36 | − 0·0, 0·91 | − 0·3, 0·35 | 0·4, 0·11 | 0·4, 0·09 |
| Necroinflammatory grade (rho, P) | 0·2, 0·39 | − 0·1, 0·71 | 0·6, 0·02 | 0·2, 0·37 | 0·3, 0·33 | − 0·2, 0·42 | 0·6, 0·01 | 0·4, 0·17 |
| Serum ALT (rho, P) | − 0·3, 0·31 | − 0·2, 0·42 | − 0·01, 0·97 | − 0·2, 0·57 | − 0·4, 0·20 | − 0·2, 0·48 | 0·2, 0·39 | 0·1, 0·82 |
Spearman correlation coeffiecient. NK, natural killer; IFN, interferon; ALT, alanine aminotransferase
NR and SVR patients.
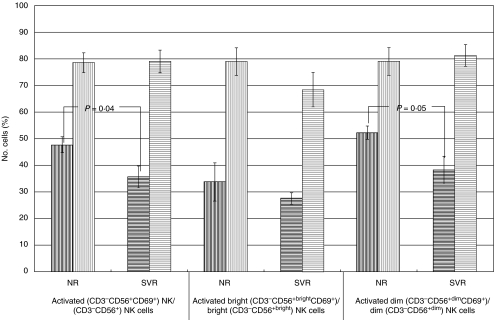
Activated (CD3−CD56+CD69+) NK cells as a percentage of all NK cells and activated dim (CD3−CD56+dimCD69+) NK cells as a percentage of all dim cells were significantly increased in the NR compared with the SVR group without IFN stimulation (47·5 ± 3·0 versus 35·4 ± 4·1, P = 0·035) and (51·8 ± 2·5 versus 37·8 ± 4·9, P = 0·045) (Figs 3 and 4). The SVR patients, however, had an enhanced response to IFN such that both activated total (CD3−CD56+CD69+) and activated dim (CD3−CD56+dimCD69+) NK cells were equivalent in both patient groups with IFN stimulation.
Fig. 3.
Activated total (CD3−CD56+CD69+), bright (CD3−CD56+brightCD69+), and dim (CD3−CD56+dimCD69+) NK cells in NR versus SVR patients. NR patients (black vertical lines) had more activated (CD3−CD56+CD69+) NK cells as a percentage of total CD3−CD56+ cells and activated dim (CD3−CD56+dimCD69+) cells as a percentage of total dim (CD3−CD56+dim) NK cells in the absence of IFN-α stimulation than SVR patients (black horizontal lines) (P = 0·04 and P = 0·05, respectively). IFN-induced activation of CD3−CD56+ NK cells and dim (CD3−CD56+dim) NK cells tended to be greater in SVR patients (grey horizontal lines) compared to NR patients (grey vertical lines) (P = 0·10 and P = 0·10, respectively). Error bars represent standard error.
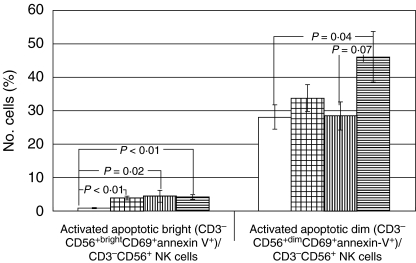
Fig. 4.
Activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK and dim (CD3−CD56+dimCD69+annexin-V+) NK cells. Activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) cells as a percentage of total CD3−CD56+ cells are significantly increased in HCV-infected patients (hatched), NR patients (vertical lines), and SVR patients (horizontal lines) compared to healthy controls (white bar) in the absence of IFN-α stimulation (P < 0·01, p = 0·02, and P < 0·01, respectively). Activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) cells are increased in SVR patients (horizontal lines) compared to healthy controls (white bar) and NR patients (vertical lines) in the absence of IFN stimulation (P = 0·04 and P = 0·07, respectively). Error bars represent standard error.
Up-regulation of annexin-V on activated CD3−CD56+bright/dim cells.
We evaluated expression of annexin-V as an early marker of programmed cell death [21] on activated (CD3−CD56+CD69+) NK cells as a percentage of all NK cells (both bright and dim). Activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells were significantly increased in HCV-infected patients, NR, and SVR patients compared to healthy controls in the absence of IFN (P = 0·003, p = 0·020, P < 0·001, respectively) (Fig. 4). No significant differences were observed between the NR and SVR subgroups with respect to activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells. However, activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells as a percentage of all NK cells were increased in the SVR patients compared with both NR and healthy controls in the absence of IFN (45·9 ± 7·7 versus 28·4 ± 4·2, P = 0·068 and 45·9 ± 7·7 versus 28·0 ± 3·7, P = 0·040, respectively) (Fig. 4). We also assessed the relationship between activated apoptotic NK cell subsets and hepatic necroinflammatory grade assessed on a five-point scale. The percentage of activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells was correlated with necroinflammatory grade (r = 0·6, P = 0·01). The percentage of activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells increased by 2·5 ± 0·7% per increase in grade (P = 0·002).
DISCUSSION
In this study, we asked whether differences in NK cell subsets would correlate with HCV treatment responses and whether PEG-IFN-α stimulation in vitro would correlate with in vivo responses to treatment with PEG-IFN-α-2b and ribivirin. We found that SVR patients had significantly fewer activated dim (CD3−CD56+dimCD69+) NK cells and tended to have increased activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells compared with NR patients. PEG-IFN-α significantly increased the percentage of activated total (CD3−CD56+CD69+) NK cells and activated bright and dim (CD3−CD56+bright/dimCD69+) NK cell subsets in all groups. We also found a significant association between activated bright (CD3−CD56+brightCD69+) NK cells and activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells and liver inflammation.
We cultured NK cells in vitro to evaluate the effect of PEG-IFN-α-2b on activated and apoptotic bright (CD3−CD56+bright) and dim (CD3−CD56+dim) NK cells obtained from chronic HCV-infected patients prior to initiating therapy. To identify NK cells, we used CD56 surface expression, a marker associated with IFN-γ secretion [22,23]. To identify activated cells, we used CD69 surface expression, a marker of NK cell cytotoxicity [24,25]. We used annexin-V, which binds to phosphatidylserine, to identify early apoptotic cells [21]. Exposure of the membrane phospholipid, phosphatidylserine, to the external cellular environment, where it can bind to annexin-V, is an initial step in the apoptotic pathway [26].
In these studies, we found that examination of NK cell subsets (bright versus dim) revealed differences not seen when we evaluated NK cells as a whole. When CD56+ bright and dim subsets were examined, the HCV-infected group had significantly more bright cells compared to healthy controls. Activated CD56+dim cells were significantly reduced in the SVR compared to the NR group in the absence of IFN-α. PEG-IFN-α markedly increased the percentage of activated (CD3−CD56+CD69+) NK cells in all groups leading to a 2-fold increase in the SVR group. Interestingly, activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells were significantly increased in all three HCV-infected groups compared with controls. Activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells as a percentage of all NK cells were similar between NR and control groups in the absence of IFN stimulation, but higher in SVR compared to controls and NR patients.
In agreement with others, we found that CD3−CD56+ NK cells are decreased in HCV-infected patients [6,27]. Furthermore, we found that without PEG-IFN-α-2b stimulation, HCV-infected patients had fewer activated NK cells, activated bright (CD3−CD56+brightCD69+) and activated dim (CD3−CD56+dimCD69+) NK cells compared to controls, suggesting that chronic HCV infection may alter both the immunoregulatory and the cytotoxic function of NK cells. Decreased activated dim (CD3−CD56+dimCD69+) NK cells and increased activated apoptotic dim (CD3−CD56+ dimCD69+annexin-V+) NK cells in SVR patients may indicate that dim (CD3−CD56+dim) NK cell turnover is enhanced in these patients. IFN-α increases CD3−CD56+ cytotoxicity [11] and IFN-γ secretion [13]. By increasing dim NK cell activation and augmenting NK cell cytotoxicity by increasing perforin/granzyme release [11], PEG-IFN-α may augment the process leading to a decrease in HCV RNA. Freshly isolated hepatic NK cells have been shown to be cytotoxic against an NK cell line [28]. In mouse cytomegalovirus infection, NK cell activation is required for virus control, and the virus has evolved mechanisms by which to inhibit NK cell activation [29–31]. In vitro, HCV core protein can up-regulate major histocompatibility complex (MHC) class I expression resulting in significantly down-regulated NK cytotoxic activity [32]. We also evaluated CD3−CD161+ cells and found that CD161 expression did not differ between any of our patient groups (data not shown), corroborating results from others [27], suggesting that changes in CD161 expression are not associated with HCV clearance.
Nonspecific hepatic inflammation is associated with hepatic fibrosis [17]. We found that bright (CD3−CD56+bright) NK cells were increased 2-fold in HCV-infected patients compared with controls. Furthermore, increasing activated bright (CD3−CD56+brightCD69+) NK cells and increasing activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells were both significantly associated with increasing hepatic necroinflammatory grade. To our knowledge, this is the first report associating NK cell subsets with hepatic inflammation. These findings suggest that NK cells may be important regulators of hepatic processes. Decreased numbers of hepatic CD56+ NK cells observed in late-stage HCV disease may be a risk factor for the development of hepatocellular carcinoma [33]. In addition, increased peripheral blood and intrahepatic CD56+ T cells correlate with hepatic inflammation in chronic HCV infection [34,35]. Bright (CD3−CD56+brightCD16−) NK cells, which express the MHC common allelic determinants C94/NKG2A and lack natural killer inhibitory receptors (KIRs), are immunoregulatory and are potent producers of proinflammatory cytokines (interferon-γ and tumour necrosis factor-α) [36]. In contrast, dim (CD3−CD56+dim CD16+) NK cells, a subset of which expresses allele-specific MHC class I KIRs, are principally cytotoxic [36].
In summary in this preliminary study, we found that patients who achieved a successful response to treatment for HCV had fewer activated dim (CD3−CD56+dimCD69+) NK cells and increased numbers of activated apoptotic dim (CD3−CD56+ dimCD69+annexin-V+) NK cells compared to NR patients and healthy controls. In addition, compared with NR patients, SVR patients may have enhanced ability to respond to PEG-IFN. Further studies should evaluate activated dim (CD3−CD56+dimCD69+) NK cell turnover and assess activated dim (CD3−CD56+dimCD69+) NK cell functionality in relationship to a response to HCV treatment.
Table 4.
Association between activated apoptotic NK cell subsets and treatment response characteristics*
| HCV RNA† | HCV genotype | Virological response‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 × 106 | ≥1 × 106 | P-value | Genotype 1 | Genotype non1 | P-value | NR | SVR | P-value | |
| Activated (CD3−CD56+CD69+) NK cells‡ | |||||||||
| Without PEG-IFN | 38·7 ± 5·1 | 49·2 ± 4·0 | 0·08 | 42·9 ± 4·0 | 48·2 ± 5·1 | 0·30 | 47·5 ± 3·0 | 35·4 ± 4·1 | 0·04 |
| With PEG-IFN | 82·3 ± 3·5 | 82·7 ± 3·3 | 0·73 | 77·2 ± 3·6 | 86·8 ± 2·7 | 0·57 | 78·3 ± 3·7 | 78·7 ± 4·2 | 1·00 |
| Activated bright (CD3−CD56+brightCD69+) NK cells‡ | |||||||||
| Without PEG-IFN | 28·8 ± 4·7 | 30·1 ± 4·2 | 0·78 | 30·4 ± 5·4 | 29·1 ± 4·0 | 0·85 | 33·4 ± 7·1 | 27·3 ± 2·3 | 0·47 |
| With PEG-IFN | 74·4 ± 9·4 | 79·1 ± 3·4 | 0·69 | 76·7 ± 4·4 | 78·4 ± 5·6 | 0·94 | 78·8 ± 5·1 | 68·1 ± 6·3 | 0·20 |
| Activated dim (CD3−CD56+dimCD69+) NK cells‡ | |||||||||
| Without PEG-IFN | 41·7 ± 6·2 | 53·2 ± 4·3 | 0·13 | 46·3 ± 4·3 | 52·1 ± 5·7 | 0·34 | 51·8 ± 2·5 | 37·8 ± 4·9 | 0·04 |
| With PEG-IFN | 83·4 ± 2·5 | 83·4 ± 3·6 | 0·73 | 77·3 ± 4·0 | 88·2 ± 2·4 | 0·29 | 78·1 ± 4·8 | 80·7 ± 4·0 | 0·52 |
| Activated apoptotic bright (CD3−CD56+brightCD69+annexin-V+) NK cells¶ | 4·1 ± 1·3 | 3·6 ± 0·9 | 0·78 | 4·1 ± 1·2 | 3·6 ± 1·0 | 0·75 | 4·5 ± 1·7 | 4·2 ± 0·8 | 0·65 |
| Activated apoptotic dim (CD3−CD56+dimCD69+annexin-V+) NK cells¶ | 32·6 ± 9·1 | 34·2 ± 4·4 | 0·53 | 35·3 ± 6·5 | 32·4 ± 5·2 | 0·63 | 28·4 ± 4·2 | 45·9 ± 7·7 | 0·07 |
All values represent mean+-standard error and were compared using Wilcoxon rank sum analysis.
HCV RNA categorized by greater or less than 1 million copies/ml (upper limit of assay detection).
Only includes patients with known response to therapy (n = 11) ‡Given as percentage annexin-V+ cells out of total of respective subset cell population
Given as percentage annexin-V+ cells of total CD3−CD56+ NK cell population NK, natural killer; HCV, hepatitis C virus; NR, nonresponder; SVR, sustained virological responder; PEG-IFN, pegylated-interferon
Acknowledgments
We thank Drs Mark Laughlin and Michael Grace as well as Mark Delorenzo for supplying PEG-IFN-α-2b. We also thank Dr Marija Zeremski for critical review of the manuscript. This research was supported, in part, by a General Clinical Research Center Grant (M01-RR00047 and NIH-NCCR M01-RR6020) from the National Center for Research Resources, National Institutes of Health grants DK02573 (AHT), and the Greenberg Foundation for Medical Research.
REFERENCES
- 1.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001;120:250–60. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 2.Biron CA, Dalod M, Salazar-Mather TP. Innate Immunity and Viral Infections. In: Kaufmann SH, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. Washington DC: ASM Press; 2002. pp. 139–60. [Google Scholar]
- 3.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells. a unique innate immunoregulatory role for the CD56 (bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 4.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 6.Deignan T, Curry MP, Doherty DG, et al. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101–8. doi: 10.1016/s0168-8278(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 7.Par G, Rukavina D, Podack ER, et al. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol. 2002;37:514–22. doi: 10.1016/s0168-8278(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 8.Yamagiwa S, Ichida T, Okoshi S, et al. Serial Phenotypic profiling of intrahepatic lymphocytes in patients with chronic hepatitis C before and after combination interferon alfa-2B and ribavirin therapy. Hepatology. 2003;38:460A. [Google Scholar]
- 9.Talal AH, Shata MT, Markatou M, et al. Virus dynamics and immune responses during treatment in patients coinfected with hepatitis C and HIV. J Acquir Immune Defic Syndr. 2004;35:103–13. doi: 10.1097/00126334-200402010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Appasamy R, Bryant J, Hassanein T, Van Thiel DH, Whiteside TL. Effects of therapy with interferon-alpha on peripheral blood lymphocyte subsets and NK activity in patients with chronic hepatitis C. Clin Immunol Immunopathol. 1994;73:350–7. doi: 10.1006/clin.1994.1209. [DOI] [PubMed] [Google Scholar]
- 11.Kaser A, Enrich B, Ludwiczek O, Vogel W, Tilg H. Interferon-alpha (IFN-alpha) enhances cytotoxicity in healthy volunteers and chronic hepatitis C infection mainly by the perforin pathway. Clin Exp Immunol. 1999;118:71–7. doi: 10.1046/j.1365-2249.1999.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malaponte G, Passero E, Leonardi S, et al. Effect of alpha-interferon on natural killer cell activity and lymphocyte subsets in thalassemia patients with chronic hepatitis C. Acta Haematol. 1997;98:83–8. doi: 10.1159/000203603. [DOI] [PubMed] [Google Scholar]
- 13.Jinushi M, Takehara T, Kanto T, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–56. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 14.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C. a randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 15.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 16.Talal AH, Irwin CE, Dieterich DT, Yee H, Zhang L. Effect of HIV-1 infection on lymphocyte proliferation in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr. 2001;26:208–17. doi: 10.1097/00042560-200103010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR and DOSVIRC Groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto H, Okada S, Sugiyama Y, Kurai K, Iizuka H, Machida A, Miyakawa Y, Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J General Virol. 1991;72:2697–704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds P, Alberti A, Alter HJ, et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321–4. [PubMed] [Google Scholar]
- 20.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J General Virol. 1993;74(11):2391–9. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 21.Raynal P, Pollard HB. Annexins. the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 22.Bennett IM, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B. Definition of a natural killer NKR−P1A+/CD56−/CD16− functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med. 1996;184:1845–56. doi: 10.1084/jem.184.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loza MJ, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (interferon gamma) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood. 2002;99:1273–81. doi: 10.1182/blood.v99.4.1273. [DOI] [PubMed] [Google Scholar]
- 24.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207:85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 25.Borrego F, Robertson MJ, Ritz J, Pena J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97:159–65. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golden-Mason L, Asrafel H, Rosen HR, Madrigal-Estebas L, Doherty DG, Hegarty JE, O'Farrelly C. Peripheral Natural Killer (NK) cells are depleted and functionally impaired in chronic HCV infection. Hepatology. 2003;38:458A. [Google Scholar]
- 28.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 29.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–6. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 30.Smith HR, Heusel JW, Mehta IK, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–31. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown MG, Dokun AO, Heusel JW, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–7. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 32.Herzer K, Falk CS, Encke J, Eichhorst ST, Ulsenheimer A, Seliger B, Krammer PH. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56(+) T Cells and Natural Killer Cells in Cirrhotic Livers With Hepatitis C May Be Involved in Their Susceptibility to Hepatocellular Carcinoma. Hepatology. 2000;32:962–9. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yonekura K, Ichida T, Sato K, et al. Liver-infiltrating CD56 positive T lymphocytes in hepatitis C virus infection. Liver. 2000;20:357–65. doi: 10.1034/j.1600-0676.2000.020005357.x. [DOI] [PubMed] [Google Scholar]
- 35.Panasiuk A, Prokopowicz D, Zak J, Wysocka J. Peripheral blood T, B, and NK cells in relation to histological hepatitis activity and fibrosis stage in chronic hepatitis C. Hepatogastroenterology. 2003;50:178–82. [PubMed] [Google Scholar]
- 36.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]