Abstract
Hepatitis C virus (HCV) RNA has been localized in antigen-presenting dendritic cells (DCs) from patients with chronic hepatitis C (CHC). DCs from patients with CHC also exhibit impaired functional capacities. However, HCV RNA in DCs and functional impairment of DCs in CHC might be independent or interrelated events. Moreover, the impact of antiviral therapy on the functions of DCs in CHC is not well documented. In order to address these issues, we took advantage of antiviral therapy in these patients. Ten patients with CHC, expressing HCV RNA in circulating DCs, became negative for HCV RNA in circulating DCs after therapy with interferon-α and ribavirin for 4 weeks. The functions of DCs from HCV RNA+ patients (isolated before antiviral therapy) and HCV RNA– patients (isolated 4 weeks after antiviral therapy) were compared in allogenic mixed leucocyte reactions. In comparison to circulating DCs from normal control subjects, DCs from HCV RNA+ patients had a significantly decreased capacity to stimulate allogenic T lymphocytes (P < 0·01) and produce interleukin-12 (P < 0·05). However, the allostimulatory capacity of circulating DCs from HCV RNA– patients was several-fold higher compared to that of HCV RNA+ DCs from the same patient. DC from HCV RNA– patients also produced significantly higher levels of interleukin-12 compared to HCV RNA+ DCs from the same patient (P < 0·01). Taken together, this study is the first to provide experimental evidence regarding the impact of HCV RNA and antiviral therapy on the function of DCs in patients with CHC.
Keywords: antiviral therapy, dendritic cells, HCV RNA, hepatitis C virus
INTRODUCTION
Infection with hepatitis C virus (HCV) is characterized by a high rate of chronicity (80–85%) that, in 20% of cases, leads eventually to serious complications such as liver cirrhosis and hepatocellular carcinoma. Although abundant amounts of HCV RNA are present in the sera and the liver of patients with chronic hepatitis C (CHC), they do not exhibit effective and sustained immune responses to HCV-encoded antigens [1]. Impaired activations of HCV-specific CD4+ T helper lymphocytes and low frequencies of HCV-specific CD8+ cytotoxic T lymphocytes have been reported in the peripheral blood of these patients [2,3]. These factors indicate that circumvention the immune responses of the hosts might be one of the important factors related to HCV persistency.
Induction and maintenance of antiviral immune responses require active participations of the cells of the innate and the adaptive immune systems, the most potent of which is the antigen-presenting dendritic cells (DCs). DCs produce abundant amounts of type-1 interferons (IFNs) and are professionally competent to recognize, capture, processing and presentation of viruses to induce antiviral immunity [4,5].
To gain insights about HCV/DC interaction, some investigators have enriched DCs by culturing monocytes or monocyte-rich population of human peripheral blood with high concentrations of granulocyte-macrophages colony stimulating factor (GM-CSF) and interleukin (IL)-4 in vitro. Their studies have shown expression of HCV RNA and impaired function of monocyte-derived DCs from patients with CHC [6–8]. However, recent advances of DC research indicate that monocytes are seldom converted to DCs in vivo and high levels of GM-CSF and IL-4, required for conversion of monocytes to DCs in vitro, are not usually available in vivo. In fact, recently circulating DCs have been isolated from human blood. These DCs are completely different from monocyte-derived DCs both phenotypically and functionally. Circulating DCs are originated in the bone marrow, enter the peripheral circulation and migrate to the site of localization of viruses. Circulating DCs are professionally competent to capture, process and present viruses to induce antiviral immune responses in vivo. Recently, we have reported the localization of HCV RNA and functional impairment of circulating DCs from patients with CHC [9]. In addition, circumstantial evidences indicate that circulating DCs might constitute a reservoir in which HCV replication takes place during natural infection [10].
However, some important factors about DC/HCV interactions have not been addressed adequately. Although localization of HCV RNA in circulating DCs and functional impairment of circulating DCs from patients with CHC has been reported, there is no experimental evidence that can substantiate that HCV RNA in circulating DCs is responsible for their functional impairment.
In this study, we explored the impact of HCV RNA in circulating DCs on their functions. A group of patients with CHC expressing HCV RNA in DCs were selected for this study. They were treated with antiviral agents and serial observation revealed that circulating DCs of some patients became negative for HCV RNA 4 weeks after therapy commencement. This provided us with an excellent opportunity to compare the function of DCs from HCV RNA+ patients (DCs isolated from CHC patients before antiviral therapy) and DCs from HCV RNA– patients (DCs isolated from CHC patients, 4 weeks after the start of antiviral therapy). This study has also provided insights about the impact of antiviral therapy on the functions of DCs in patients with CHC.
MATERIALS AND METHODS
Clinical characteristics of HCV patients
The purpose of this study was to evaluate the impact of HCV RNA and antiviral therapy on the function of circulating DCs from patients with CHC and it was important to isolate DCs when these patients were HCV RNA+ and after they became HCV RNA–. To achieve this, circulating DCs were isolated from several patients with CHC and the expression of HCV RNA in circulating DCs was checked. These patients were treated with antiviral agents. Ten patients with CHC expressing HCV RNA in circulating DCs before therapy became negative for HCV RNA in circulating DCs 4 weeks after therapy commencement. These 10 patients were enrolled in this study. The diagnosis of chronic hepatitis of these patients was made from clinical symptoms and biochemical parameters of liver function tests. All patients were positive for anti-HCV antibody and HCV RNA in the sera. They were negative for markers of hepatitis B virus. They were infected with various HCV serotypes (enzyme immunoassay, Special Reference Laboratory, Osaka, Japan). Viral loads ranged from 190 KIU/ml to >850 KIU/ml at the time of sample collection (amplicor method, Special Reference Laboratory). At the time of blood sample collection, alanine aminotransferase levels ranged from normal levels (<48 IU/l) to high levels (187 IU/l). None of them had received any antiviral therapy during the last 3 years. After written consent has been obtained, liver biopsies were conducted from all patients and the activity of hepatitis was graded according to histological classification, as described by Desmet et al. [11]. Additional information of these patients is shown in Table 1.
Table 1.
Clinical characteristics of patients with chronic hepatitis C
| Sl no. | Age (years)/ sex | ALT* 6–48 IU/l | AST 7–45 IU/l | HCV RNA (KIU/ml) | Levels of hepatitis | Levels of fibrosis |
|---|---|---|---|---|---|---|
| 1 | 43/Male | 30 | 27 | >850 | Moderate | Mild |
| 2 | 48/Male | 73 | 58 | >850 | Moderate | Moderate |
| 3 | 49/Female | 62 | 44 | 190 | Moderate | Moderate |
| 4 | 57/Male | 71 | 68 | >850 | Mild | Moderate |
| 5 | 48/Male | 62 | 30 | 600 | Moderate | Severe |
| 6 | 44/Male | 187 | 101 | 410 | Moderate | Severe |
| 7 | 69/Female | 115 | 113 | 810 | Moderate | Severe |
| 8 | 74/Male | 117 | 115 | 550 | Moderate | Moderate |
| 9 | 45/Male | 72 | 55 | >850 | Moderate | Moderate |
| 10 | 61/Male | 50 | 45 | >850 | Severe | Severe |
ALT, alanine aminotransferase, AST, aspirate aminotransferase. The levels of hepatitis and fibrosis in the live biopsy specimens of each patient were graded according to the International Criteria, described by Desmet et al. (11).
Fourteen HCV-seronegative normal volunteers were also included in this study. The functions of DCs of these normal subjects were compared alongside DC of patients with CHC. An appropriately convened institutional review board approved the protocol.
Antiviral therapy of patients with CHC
Patients were treated with both ribavirin (600–800 mg daily, orally) and IFN-α (6–10 million unit per day, intramuscularly, once daily for 2 weeks and then thrice weekly). Sera were collected from all patients before and at different points after the start of therapy. Circulating DCs were isolated from these patients at different times after the commencement of antiviral therapy. The antiviral therapy will be continued in all patients for 6 months after finishing this study.
Isolation of circulating DCs from peripheral blood
Peripheral blood mononuclear cells from patients with CHC and healthy volunteers were isolated by density gradient separation using Ficoll-Conray (Pharmacia, San Jose, CA, USA) and were resuspended in RPMI-1640 (Iwaki, Chiba, Japan) plus 10% heat inactivated fetal calf serum (Filtron Pty Ltd, Brooklyn, Australia) containing l-glutamine and gentamycin. Cell viability was checked by trypan blue exclusion (0·1% trypan blue) test.
Circulating DCs were isolated from peripheral blood mononuclear cells by two-step immunomagnetic cells sorting using a commercial kit (DC Isolation Kit, Miltenyi Biotech GmbH, Bergisch Gladbach, Germany), exactly according to the instructions of the manufacturer, and has been described in detail by us [9,12]. Briefly, T cells, monocytes and natural killer cells were depleted from peripheral blood mononuclear cells using magnetic beads coated with monoclonal antibodies against CD3 (clone BW264/56), CD11b (clone M1/70·15·11·5) and CD16 (clone VEP-13) by Auto MACS (Miltenyi Biotech Gmbh). Purified population of circulating DC was isolated from the depleted cell fractions by a positive selection step using a monoclonal antibody against CD4 (clone M-T321, Miltenyi Biotech Gmbh). Flow cytometric analysis revealed that the contaminating T lymphocytes, B lymphocytes and natural killer cells were <1·0% in isolated circulating DC.
T lymphocytes were isolated from peripheral blood mononuclear cells using an affinity column (CollectTM, Biotex Laboratories Inc., Edmonton, Canada) in which B cells were depleted from peripheral blood mononuclear cells during their passage through the affinity column containing polyclonal goat antihuman IgG (H + l). Flow cytometric analysis revealed that the purity of T cell populations was >95%.
Expression of surface antigen, allostimulatory capacity and IL-12 production of circulating DCs
The expression of MHC class II (HLA DR), MHC class I (HLA A, B, C), CD86 and CD40 on circulating DCs was assessed by direct flow cytometry using fluorescein isothiocyanate-conjugated monoclonal antibody to human HLA-DR (MHC class II, clone L243), HLA A, B, C (MHC class I), and phycoerythrin-conjugated monoclonal antibody to human CD86 [clone 2331 (FUN-1)] and CD40 (all from BD Pharmingen, San Jose, CA, USA). Data acquisition and analysis were performed on fluorescein-activated cell sorter (Becton Dickinson Biosciences, San Jose, CA, USA).
We have optimized the culture condition of allogenic mixed leucocyte reaction (MLR) by conducting a series of preliminary experiments as described in previous studies from our laboratory [9,12]. T cells (2 × 105) from allogenic normal control were cultured with γ-irradiated (40 Gy, Hiltex CO, LTD, HW-150, Osaka, Japan) circulating DCs (1 × 104) from patients and normal volunteers for 5 days at 37°C in a humidified incubator containing 5% CO2 in air. The levels of incorporation of [3H]-thymidine during the last 12 h of the 5-day culture were determined in a liquid scintillation counter (Beckman LS 6500, Beckman Instruments, Inc., Harbour Boulevard, Fullerton, CA, USA) as counts per minute (cpm). All assays were performed in 96-well culture plates and the mean cpm of at least 12 wells were calculated.
To assay the production of IL-12 in DC/T lymphocyte cultures, allogenic MLR were performed for 5 days without adding [3H]-thymidine to the cultures. The supernatants were collected and centrifuged. The levels of IL-12 in the supernatants were measured by an enzyme-linked immunosorbent assay using a commercial kit.
Detection of HCV RNA and polymerase chain reaction (PCR) amplification
The methodology of detection of HCV RNA in circulating DCs has been described in details in a previous communication from our laboratory [9]. In short, intracellular total RNA and viral RNA were extracted from 100 µl of sera or cell pellets (10 000–50 000 DCs/pellet) using a two-step extraction with phenol/guanidium thiocyanate and chloroform, followed by a precipitation with 99·5% ethanol. RNA pellets were resuspended in 10 µl of distilled water. For the detection of HCV positive-strand RNA, complementary DNA was synthesized from 1·0 µl of RNA using random primer and Moloney murine leukaemia virus reverse transcriptase. The PCR amplification of HCV RNA was performed according to the method of Okamato et al. [13]. Oligonucleotide primers were designed to amplify the 5′ non-coding region of HCV. The sequences of the sense and antisense primers for the first-round PCR were 5′-CTGTGAGGAACTACT GTCTT-3′ and 5′-AACACTACTC GGCTAGCAGT-3′, respectively, and those for the second-round PCR were 5′-TTCACGCA GAAAGCGTCTAG-3′ and 5′-GTTGATCCAAGAAAGGA CCC-3′, respectively. Half of the obtained cDNA was amplified using specific primers. Conditions for the nested PCR amplification were as follows; initial cycle at 95°C for 9 min, 30 cycles at 95°C for 1 min, 45°C for 90 s and 72°C for 2 min; and final extension at 72°C for 7 min. Typically, PCR product was visualized on an agarose gel (2%). The expected size of the amplified fragment was 145 base pairs.
Statistical analysis
The levels of cpm and IL-12 were shown as mean ± standard deviation. Means were compared with unpaired t-test. In case of differences (as assessed by an F-test), t-tests were adjusted for unequal variances (Mann–Whitney's U-test). P < 0·05 was considered to be statistically significant. Statistical calculations were performed using the StatView (version 5·0) statistical program in an Intel Computer (Pentium 4).
RESULTS
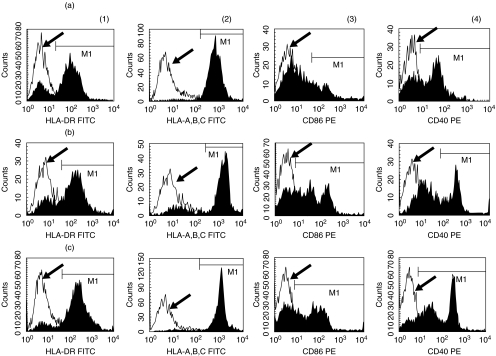
Circulating DCs used in the present study were isolated from 80 to 100 ml of peripheral blood by magnetic cell sorting without use of any cytokines. It took only 4 h to obtain circulating DCs after collecting peripheral blood. Circulating DCs were negative for the markers of T lymphocytes, B lymphocytes, monocytes and natural killer cells. Circulating DCs expressed HLA DR, HLA-A, B, C, CD40 and CD86 (Fig. 1). However, these DCs expressed almost no CD83 (data not shown). Flow cytometric analysis revealed that circulating DCs expressed either CD11c or CD123. These antigens are expressed mainly on DCs. The function of circulating DCs was evaluated in allogenic MLR.
Fig. 1.
The expression of MHC class II (HLA DR) (a), MHC class I (HLA A, B, C) (b), CD86 (c) and CD40 (d) on circulating dendritic cells. The flow cytometric profiles of a representative patient with CHC before therapy (a) and 4 weeks after therapy (C) are shown. The profile in (C) shows the flow cytometric data of a representative normal control. The open histogram indicates the flow cytometric profile of isotype-matched control (arrow). The closed histogram shows the staining with respective antibodies.
Clearance of HCV RNA from circulating DCs due to antiviral therapy with IFN-α and ribavirin
The patients expressing HCV RNA in the sera and circulating DCs were treated with IFN-α and ribavirin. The expression of HCV RNA in the sera and circulating DCs were checked in these patients at different times after therapy commencement. Ten patients with CHC became negative for HCV RNA in circulating DCs, 4 weeks after therapy commencement. Interestingly, HCV RNA was detected in the sera in five of these 10 patients at this time.
This provided us with an opportunity to compare the functions of DCs from HCV RNA+ patients (DCs isolated before the start of antiviral therapy) and DCs from HCV RNA– state of the same patient (DCs isolated 4 weeks after the start of antiviral therapy) with CHC. It also allowed comparison of the role of antiviral therapy on the functions of DCs.
Loss of HCV RNA in circulating DCs resulted in increased expression of surface antigens and up-regulation of allostimulatory capacity of circulating DCs
The expressions of HLA DR, HLA-A, B, C, CD86 and CD40 on circulating DCs from representative subjects are shown in Fig. 1. The expression pattern of HLA DR, MHC class I, CD86 and CD40 on circulating DCs showed a shift to the right in patients with CHC during antiviral therapy (Fig. 1b) compared to before the start of antiviral therapy (Fig. 1a). A flow cytometric profile of these antigens on circulating DCs of a representative normal control subject is shown in Fig. 1c.
Increased expressions of surface antigens on circulating DCs were reflected in increased functional capacities of circulating DCs during antiviral therapy. The function of circulating DCs was evaluated by their capacity to induce proliferation of allogenic T cells in allogenic MLR and production of IL-12 in cultures. As T cells were used from a single normal volunteer, the levels of blastogenesis in allogenic MLR reflected the stimulatory capacity of circulating DCs. The mean level of blastogenesis in cultures containing circulating DCs from patients with CHC before commencement of antiviral therapy was 3063 ± 3324 cpm (mean ± standard deviation, n = 10) (Table 2). Four weeks after the start of antiviral therapy, HCV RNA was no more detectable in circulating DCs of these patients. The levels of blastogenesis of cultures containing DCs, 4 weeks after commencement of antiviral therapy were significantly increased (35605 ± 20930 cpm, n = 10, P < 0·01) (Table 2). This was almost comparable to the levels of blastogenesis of cultures containing circulating DCs from normal control subjects (30345 ± 7654 cpm, n = 14) (Table 2).
Table 2.
Up-regulation of allostimulatory capacity of dendritic cells from patients with chronic hepatitis C due to antiviral therapy
| Sources of dendritic cells | No. of subjects | Levels of blastogenesis (cpm) |
|---|---|---|
| (a) Patients with CHC before therapy | 10 | 3 063 ± 3324 |
| (b) Patients with CHC, 4 weeks after antiviral therapy | 10 | 35 605 ± 20 930* |
| (c) Normal controls | 14 | 30 345 ± 7654 |
Circulating dendritic cells (DC) were isolated from 10 patients with chronic hepatitis C (CHC) before the start of antiviral therapy (a) and 4 weeks after commencement of antiviral therapy (b). Circulating DCs were also isolated from 14 normal volunteers (C). The levels of blastogenesis [expressed as counts per minute (cpm)] of allogenic mixed leucocyte reaction reflected the T cell stimulatory capacity of circulating DCs. Data are shown as mean and standard deviation of cpm.
P < 0·01 compared to allostimulatory capacity of DC prior to antiviral therapy.
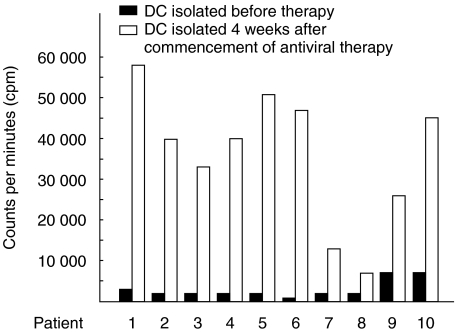
The levels of blastogenesis in allogenic MLR containing of circulating DCs before the commencement of antiviral therapy and 4 weeks after antiviral therapy from the same patient with CHC are shown in Fig. 2. The allostimulatory capacity of circulating DCs increased in all patients with CHC after antiviral therapy compared to before antiviral therapy.
Fig. 2.
Increased allostimulatory capacity of circulating dendritic cells (DCs) from patients with chronic hepatitis C (CHC) due to antiviral therapy. Circulating DCs were isolated from patients with CHC before the start of therapy and four weeks after the start of antiviral therapy. Levels of incorporation of [3H]-thymidine (in cpm) reflected the levels of blastogenesis in allogenic mixed leucocyte reaction (MLR) containing T cells (2 × 105) from normal volunteer and circulating DCs from patients with CHC (1 × 104). The levels of blastogenesis in cultures containing DCs from patients with HCV before antiviral therapy (DCs from HCV RNA+ patients) are shown as black bars. The levels of blastogenesis in allogenic MLR containing DCs from the same patient, 4 weeks after the start of antiviral therapy (DCs from HCV RNA– patients) are shown by clear bars.
Antiviral therapy resulted in increased production of IL-12 in DC/T cell cultures
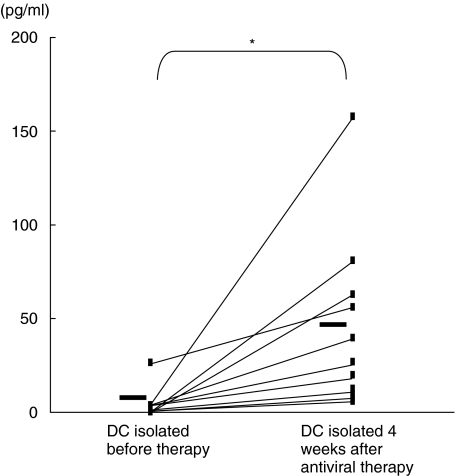
The levels of IL-12 in DC/T cell cultures containing DCs from patients with CHC before antiviral therapy and those containing DCs 4 weeks after commencement of antiviral therapy are shown in Fig. 3. Detectable levels of IL-12 were not seen in most of the cultures containing DCs from HCV RNA+ patients with CHC. However, IL-12 was detected in all cultures containing DCs from HCV RNA– state of the same patients with CHC when circulating DCs were isolated from these patients 4 weeks after commencement of antiviral therapy. The mean level of IL-12 was also significantly higher in cultures containing DCs from HCV RNA– patients compared to the cultures containing DCs from HCV RNA+ patients (Fig. 3).
Fig. 3.
The levels of interleukin-12 in culture supernatants of allogenic mixed leucocyte reaction (MLR) containing dendritic cells (DCs) from patients with chronic hepatitis C (CHC). Circulating DCs were isolated from patients with CHC before (DCs from HCV RNA+ patients) and 4 weeks after the start of antiviral therapy (DCs from HCV RNA– patients). DCs were cultured in allogenic MLR with T lymphocytes from normal volunteers for 5 days. The levels of interelukin-12 in supernatants of allogenic MLR were measured. A single symbol represents individual value. Horizontal bars represent group means *P < 0·05.
DISCUSSION
Although DCs play a cardinal role during the induction of antiviral immune responses, the outcome of DC/virus interactions vary considerably among viruses. The function of DCs is down-regulated in subjects chronically infected with human immune deficiency virus [14] and hepatitis B virus (HBV) [15]. On the other hand, DCs produce abundant amounts of antiviral agents such as type-1 IFN in response to herpes simplex virus [16] and influenza virus [17].
Understanding about DC/virus interaction provides insights about the mechanism of pathogenesis of chronic viral infection and might help to develop interventional strategy. The understanding of DC/HBV interactions in murine HBV carrier and patients with chronic hepatitis B has exposed a new field of immune therapy for these subjects [18–20]. Up-regulation of the functions of DCs by vaccine containing hepatitis B surface antigen (HBsAg) (vaccine therapy) or administration of HBsAg-pulsed DCs resulted in clearance of HBsAg, development of antibody to HBsAg and reduced replication of HBV in murine HBV carriers [18,19]. Vaccine therapy has also resulted in reduced HBV replication and production of antibody to hepatitis Be antigen in patients with chronic hepatitis B [20].
HCV is notorious for causing chronic infection. More than half of patients with CHC develop progressive liver diseases such as liver cirrhosis and hepatocellular carcinoma. Antiviral agents are used to treat patients with CHC; however, very few patients benefit from these costly therapies. The development of a better therapeutic regimen for CHC is dependent on proper understanding of the pathogenesis of CHC. Accordingly, investigators have attempted to dissect the causes underlying the impaired immune responses of CHC patients by studying DC/HCV interactions. These studies have revealed that DCs from patients with CHC harboured HCV RNA, had impaired T cell stimulatory capacity and produced reduced levels of IL-12 [6–8]. However, these studies have two major limitations. First, the investigators have used in vitro-cultured, monocyte-derived DCs for analyses, which are not truly representative of functional DCs in vivo. Secondly, these studies have reported HCV RNA in DCs and impaired functions of DCs. However, it is unknown whether localization of HCV RNA in DCs and impaired function of DCs are interrelated or an independent phenomenon. Finally, the role of antiviral therapy on the function of DCs has not been evaluated.
In this study, we have shown that antiviral therapy caused clearance of HCV RNA from circulating DCs in some, but not all, patients with CHC. This allowed us to examine the function of DCs from HCV RNA+ and HCV RNA– patients with CHC. The study has shown that the functions of DCs from HCV RNA+ patients were significantly decreased compared to DCs from HCV RNA– patients. The unique point of this study lies in the fact that we compared the functions of DCs of the HCV RNA+ state and the HCV RNA– state of the same patients with CHC. Moreover, these patients were chronically infected with HCV due to natural infections. This study is fundamentally different from other similar patients in whom in vitro-generated, cytokine-enriched DCs were transfected with vector encoding parts of HCV genes [21].
However, some aspects regarding DC/HCV interaction deserves further investigation. The presence of negative strand HCV RNA in circulating DCs has not been evaluated in this study. It is also important to study the underlying mechanisms of action. Although clearance of HCV RNA from circulating DCs resulted in increased functional capacity of circulating DCs, antiviral agents may also directly up-regulate the function of DCs. However, the role of antiviral agents, especially that of IFN on maturation and activation of DCs, is controversial. Gabriel et al. have reported that IFN-α promotes the rapid differentiation of monocytes into activated DCs from patients with myeloid leukaemia [22]. On the other hand, McRae et al. have shown that IFN-α and -β inhibit the in vitro differentiation of DCs from normal control subjects [23]. Both these investigators used monocyte-derived DCs and the experimental protocol was also different. These studies indicate that there is a need to re-evaluate the roles of IFN on circulating DCs, especially in patients with CHC. IFN-α may play a diverse role in the functions of circulating DCs. It may stimulate directly circulating DCs in patients with CHC and can also induce other proinflammatory cytokines, which might up-regulate the functions of circulating DCs. Therapy with IFN-α may also induce improved DC function by reducing the load of HCV in vivo.
In this study, we examined the functions of DCs before and 4 weeks after therapy commencement. However, these patients will receive antiviral therapy for 6 months and the final evaluation of therapeutic response will be conducted 12 months after the commencement of antiviral therapy. The functions of DCs of these patients will again be evaluated at 6 and 12 months after therapy commencement to evaluate if the improved function of DCs remained as such or altered with time.
In conclusion, this study has shown that circulating DCs from HCV RNA+ CHC patients were almost incapable of stimulating allogenic T lymphocytes and produced very low levels of IL-12. This could account for a typically low anti-HCV immune response in patients with CHC. Antiviral therapy resulted in restoration of function of circulating DCs. The long-term effect of antiviral therapy on the function of DCs and their relation in the context of sustained virological and biological responses of CHC patients are important in assessment of the real importance of DC in CHC.
Acknowledgments
Part of this study was supported by a Grant-in-Aid for Research for New Intractable Liver Diseases by the Ministry of Health, Welfare and Labor, Japan and by a Grant-in-Aid from Ministry of Education, Science and Culture, Japan (C-14570474).
REFERENCES
- 1.Gerlach JT, Diepolder HM, Jung M-C, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 2.Rehermann B, Chang KM, McHutchison JG, Kokka R, Hougton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–40. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, Wong DKH, Dunbur PR, et al. Analysis of successful immune responses in patients infected with hepatitis C virus. J Exp Med. 2002;191:1499–12. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y-J. Dendritic cell subsets and lineage, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 5.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;108:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 6.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 7.Auffermann-Gretzinger S, Keefe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–8. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 8.Bain C, Fatmi A, Zoulim F, Zarski J-P, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 9.Tsubouchi E, Akbar SMF, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004 doi: 10.1007/s00535-003-1385-3. in press. [DOI] [PubMed] [Google Scholar]
- 10.Goutagny N, Fatmi A, De Ledinghen V, et al. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951–8. doi: 10.1086/375350. [DOI] [PubMed] [Google Scholar]
- 11.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 12.Murakami H, Akbar SMF, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504–10. doi: 10.1046/j.1365-2249.2002.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto H, Sugiyama Y, Okada S. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673–9. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 14.Knight SC, Elsley W, Wang H. Mechanism of loss of functional dendritic cells in HIV-1 infection. J Leukoc Biol. 1997;62:78–81. doi: 10.1002/jlb.62.1.78. [DOI] [PubMed] [Google Scholar]
- 15.Arima S, Akbar SMF, Michitaka K, et al. Impaired function of antigen-presenting dendritic cells in patients with chronic hepatitis B. Localization of HBV DNA and HBV RNA in blood DC by in situ hybridization. Int J Mol Med. 2003;11:169–74. [PubMed] [Google Scholar]
- 16.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principle type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 17.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive potent TH1 polarization. Nature Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 18.Akbar SMF, Abe M, Masumoto T, Horiike N, Onji M. Mechanism of action of vaccine therapy in murine hepatitis B virus carriers: vaccine-induced activation of antigen presenting dendritic cells. J Hepatol. 1999;30:755–64. doi: 10.1016/s0168-8278(99)80125-1. [DOI] [PubMed] [Google Scholar]
- 19.Akbar SMF, Horiike N, Onji M. Prognostic importance of antigen presenting dendritic cells during vaccine therapy in murine hepatitis B virus carriers. Immunology. 1999;96:98–108. doi: 10.1046/j.1365-2567.1999.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiike N, Akbar SMF, Ninomiya T, Abe M, Michitaka K, Onji M. Activation and maturation of antigen-presenting dendritic cells during vaccine therapy in patients with chronic hepatitis due to hepatitis B virus. Hepatol Res. 2002;23:38–47. doi: 10.1016/s1386-6346(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 21.Dolganiuc A, Kodys K, Kopasz A, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–24. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 22.Gabriele L, Borghi P, Rozera C, et al. IFN-alpha promotes the rapid differentiation of monocyte from patients with chronic myeloid leukemia into activated dendritic cells tuned to undergo full maturation after LPS treatment. Blood. 2004;103:980–7. doi: 10.1182/blood-2003-03-0981. [DOI] [PubMed] [Google Scholar]
- 23.McRae BL, Nagai T, Semnani RT, van Seventer JM, van Seventer GA. Interferon-alpha and-beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14 (+) precursors. Blood. 2000;96:210–7. [PubMed] [Google Scholar]