Abstract
IPEX syndrome is a genetic autoimmune disease characterized by immune-mediated polyendocrinopathy, enteropathy, and X-linked inheritance. We describe a case of IPEX in which lymphocyte phenotypes were assessed at birth, before initiation of Cyclosporin A therapy, and at frequent intervals to 18 months of age. We performed flow cytometry for lymphocyte subtypes and for activation markers (HLA-DR, CD25, and CD69 or CD71). The ratios of both T to B cells and CD4+ to CD8+ cells were elevated at birth, but CD4+ cells were not activated. HLA-DR+ and CD25+ activated T-cells increased in association with two episodes of clinical deterioration: colitis and the onset of type I diabetes mellitus. These results indicate that measures of activation, particularly HLA-DR+ and CD25+ frequency, correlate well with the development of early active disease and may presage clinical episodes. Continuous maintenance of immunosuppression, once started, appears critical for prevention of permanent tissue damage.
Keywords: x-linked, regulatory T cells, immunodeficiency diseases, apoptosis, flow cytometry
INTRODUCTION
Immune-dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX, MIM 304790) syndrome is a rare human disorder caused by mutations in FOXP3, a recently described gene that encodes a protein called Scurfin [1]. Classic IPEX presents in early infancy with a spectrum of illnesses arising from a dysregulated immune response, most commonly type I diabetes mellitus (T1Dm), eczema, severe enteropathy, hypothyroidism and autoimmune cytopenias. These illnesses are usually fatal in the first year of life, absent aggressive anti-immune therapy with Cyclosporine A (CsA) and/or FK506 (tacrolimus). Some patients appear resistant to such therapy. All FOXP3 mutations identified in classic IPEX families change the Scurfin amino acid sequence, and most cluster in the conserved winged-helix domain at the carboxy-terminus.
In 1982, Powell et al. [2] reported a large, X-linked recessive family in which numerous males had experienced similar illnesses with variable onset and sometimes waxing and waning severity. Genetic mapping in this kindred placed the genetic defect in the same X-chromosome region of as two other IPEX families with more classic histories [3], but FOXP3 coding mutations were not found in members of Powell's kindred [4,5]. Subsequently, Bennett et al.[6] reported a nucleotide substitution in the first canonical polyadenylation signal following the FOXP3 termination codon, a change that predicted a reduction in Scurfin mRNA stability.
We describe a previously unreported, affected member of this family, whose lymphocyte profile was followed prospectively from birth, i.e. before, during, and after the onset of clinical disease and during the initiation and adjustment of CsA therapy.
MATERIALS AND METHODS
Case report
With approval of the human subjects committee, we retrospectively reviewed clinical data previously collected from birth to 18 months of age in an IPEX patient from the kindred reported by Powell [2]. We analysed the lymphocyte phenotype before and after the onset of disease, the effect of CsA administration, and assessed measures of lymphocyte activation.
Prenatal diagnosis was performed at 16 weeks gestation because of the extensive family history of males affected with multiple autoimmune disorders [2]. Comprehensive genetic mapping in this family [3] established the basis for prenatal diagnosis by linkage analysis, which indicated a 99% chance that the mother's first pregnancy, a male, was affected. Linkage analysis was used because the events described here occurred before the identification of the underlying gene defect.
The mother delivered a 3085 g infant at term. No abnormalities were noted at birth. Because of the expectation for early onset immune disease, he was followed closely with clinical examinations, urine glucose screening, stool occult blood screening and regular assessment of blood lymphocyte activation status.
At 5½ weeks of age, he was hospitalized for three days after a two-day history of small quantities of clotted red blood in haem-positive stools. Physical examination was unremarkable. The white blood cell count was 10.7 × 109/l, with 78% lymphocytes, 15% neutrophils, 3% monocytes, 2% eosinophils and 1% basophils. The haematocrit was 25·1% with a normal mean corpuscular volume. The following autoantibodies were negative: anti-red blood cell (direct Coombs’ with multivalent anti-human globulin and anti-C3b), anti-insulin, and anti-islet cell.
The upper gastrointestinal tract was normal by endoscopy, but flexible sigmoidoscopy revealed one patch of erythematous papules in the proximal sigmoid colon. The small bowel biopsy was histologically normal, but a biopsy of the abnormal sigmoid patch showed high numbers of infiltrating lymphocytes in the lamina propria and intermittent disruption of the normal mucosal architecture, consistent with changes seen in other affected members of his family. At discharge, he was started on oral cyclosporin A (CsA) with a therapeutic blood level target range of 200–300 ng/ml.
Except for a viral respiratory infection at 4½ months of age, he did well until about 7 months of age when CsA levels dropped below 100 ng/ml. Shortly thereafter (73/4 months), he was admitted when he displayed decreased activity, polydypsia, possibly polyuria and new glycosuria. Eczematoid rash and retroauricular lymphadenopathy were the only abnormalities on examination, but blood glucose was 395 mg/dl. The WBC was 17.2 × 109/l with 42% neutrophils, 43% lymphocytes, 11% monocytes, and 5% eosinophils. Hematocrit was 31%. Blood and urine ketones were negative. Anti-islet cell, antithyroid, and antiadrenal antibodies were negative. He was treated with insulin for new type I diabetes mellitus (T1Dm), though blood glucose levels remained erratic. Oral immunosuppression was switched briefly to intravenous (55 mg/day) before substituting Neoral by mouth when a level of 167 ng/ml was obtained. Subsequent blood sugar control remained difficult, but CsA levels stabilized on a 150 mg oral dose.
At one year of age, he was a well-grown, normally developed, muscular boy with moderately well controlled diabetes and dry eczema that worsened when CsA levels dipped. Stool blood screens were negative. By 16 months, his CsA therapeutic level target had been increased to 250 ng/ml to avoid skin eruptions and he had no significant episodes of diarrhoea or stool occult blood. He remained on insulin therapy with moderately good control. The patient was never given childhood immunizations due to the history of catastrophic responses to immunization in some affected relatives. The family moved from the area at 18 months of age.
Immunophenotyping of peripheral blood lymphocytes
Cord or peripheral blood was examined weekly for the first eight weeks, and then monthly for 16 additional months. Intestinal biopsies for routine pathology were obtained at 4 weeks of age during the first episode of bloody diarrhoea. The intestinal biopsies were from the duodenum and rectosigmoid colon. Cell suspensions were made by mincing the biopsies in RPMI tissue culture medium.
Heparinized whole blood and biopsy cell suspensions were immunostained with fluorescent antibodies by standard methods. Antibodies specific for CD3, CD4, CD8, CD25, CD69, CD71, and HLA-DR (Becton-Dickinson, San Jose, CA, USA), CD26, CD29, CD45RA, and CD95 (Fas) (Beckman Coulter, Miami, FL, USA) were used to identify lymphocyte subpopulations and expression of activation markers. The whole blood was lysed (Q-Prep, Beckman Coulter) and specimen analysis was performed on a FACScan flow cytometer (Becton-Dickinson). Immunophenotyping results are reported as either absolute number of cells per microlitre (µl) or as percent of live, lymphocyte gated, cells. Age matched normal controls were also analysed to establish normal ranges.
RESULTS
Major lymphocyte subpopulations in IPEX
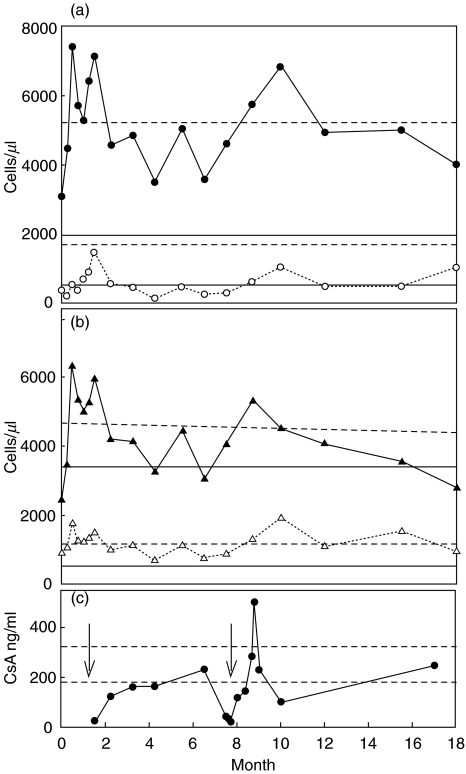
The surface phenotypes of circulating lymphocyte subpopulations were determined by flow cytometry of cord blood at birth, and of peripheral blood at intervals to 18 months of age (Fig. 1). The absolute numbers of CD3+ T cells were generally high normal to elevated. CD19+ B cells remained low in absolute number (Fig. 1a), except for a brief peak following the early colitis episode.
Fig. 1.
Absolute numbers of circulating lymphocytes versus patient age in months. (a) • CD3+ T cells (horizontal dashed lines represent the normal range, 1500–6500 cells/µl); ○ CD19+ B cells (horizontal solid lines represent the normal range); (b) ▴ CD4+ T cells (horizontal dashed lines represent the normal range); Δ CD8+ T cells (horizontal solid lines represent the normal range). (c) • CSA levels (ng/ml), data available up to 17 months of age. The dashed lines indicate the therapeutic range and the arrows denote clinically significant events: bloody diarrhoea at 4 weeks and the development of T1Dm at 7·7 months.
Among CD3+ T cells, the percentage and absolute number of CD8+ cells was relatively stable throughout (Fig. 1b). In contrast, the number of CD4+ cells varied considerably between 2450 and 6300 cells/µl (Fig. 1b), and were sporadically above normal (normal range 1000–4200 cells/µl). The absolute number of CD4+ cells peaked at two weeks of age (6300 cells/µl), about two weeks before the first clinical episode of colitis (first arrow in Fig. 1c). An elevated percentage and absolute number of CD4+ cells persisted through this episode but corrected when therapeutic CsA immuno-suppression was achieved. A normal percentage of CD4+ T cells (33–55%) was observed only when CsA levels were well above 200 ng/ml, i.e. at 6·5 months and 17 months of age (Fig. 1b,c). This was reflected in an improved CD4/CD8 ratio of 2·4 at 17 months compared to 4·1 at one month of age. The majority (75–95%) of CD4+ T cells maintained a naïve phenotype (CD45RA+) throughout the study (data not shown).
Activated and regulatory T lymphocytes
A variety of surface marker combinations were followed to evaluate activated lymphocytes and regulatory T cells in cord and peripheral blood including: CD25+CD4+ (regulatory and activated T cells), CD69+CD4+, HLA-DR+CD4+, CD26+CD3+, CD71+, and CD95+(FAS). In addition, lymphocyte infiltration in intestinal biopsies was evaluated by routine histology and by immunophenotyping.
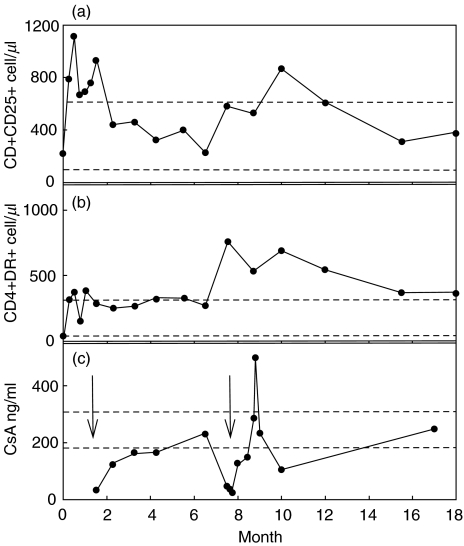
The peak in total CD4+ T cells at two weeks of age (Fig. 1b) was accompanied by a relative increase in the number and frequency of HLA-DR+ and of CD25+ cells, which had been normal at birth and stable at one week of age (Fig. 2a,b; Table 1). This trend continued through the episode, but reversed when therapeutic CsA suppression was achieved (Fig. 2c).
Fig. 2.
Absolute cell numbers of CD4+ T cell subsets associated with activation versus patient age in months. (a) CD4+CD25+ cells/µl. (b) CD4+HLA-DR+ cells/µl. (c) CSA levels (ng/ml), data available up to 17 months of age. Horizontal dashed lines indicate the normal ranges (a and b) and the therapeutic range of CSA (c). The arrows indicate clinically significant events as in Fig. 1.
Table 1.
Percentage of CD25+ cells and of HLA-DR+ cells among circulating CD4+ lymphocytes
| Age | CD25+ (%)* | HLA-DR+ (%)† | Clinical event |
|---|---|---|---|
| 0 (cord blood) | 8·3 | 1·7 | Birth |
| 2 weeks | 17·6 | 5·8 | None |
| 5½ weeks | 13·8 | 7·7 | Colitis |
| 6 weeks | 15·6 | 4·7 | CSA initiated |
| 41/4 months | 10·0 | 10·0 | Fever |
| 73/4 months | 14·3 | 18·6 | Onset of T1Dm |
| 83/4 months | 10·0 | 10·0 | High CSA level |
Normal newborn range is <10%
Normal newborn range is <5%.
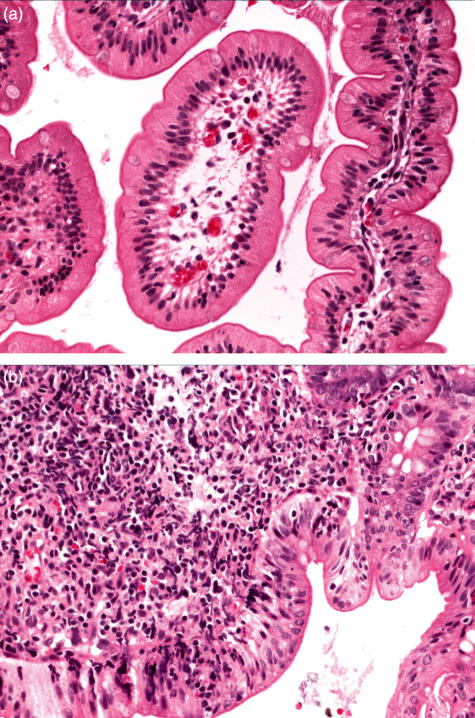
Two biopsies of intestinal epithelium (duodenum and rectosigmoid colon) were obtained during an episode of bloody diarrhoea at 4 weeks of age. Both the sigmoidoscopy and routine pathology evaluations showed that the duodenal epithelium was normal, while the colon epithelium showed evidence of colitis (Fig. 3). Histological analysis of both biopsies exhibited reactive changes with foci of eosinophil and lymphocyte infiltration in the colonic mucosa only (Fig. 3a,b).
Fig. 3.
Hematoxylin and eosin stain of sectioned endoscopic biopsies (400×). Micrograph of a section of (a) duodenum showing normal histology and (b) colon showing extensive eosinophil and lymphocyte infiltrate.
T lymphocytes extracted from the biopsies of duodenum and colon, during the colitis episode were also examined by flow cytometry. A much larger number of lymphocytes were obtained from the colon biopsy, as expected from the histological evaluation. However, HLA-DR+ cells represented a large fraction of the CD4+ T cells recovered from both the duodenum and colon (65–70%), consistent with an activated, inflammatory state at both sites. In addition, the expression of CD25 on CD4+ T cells was also examined as an indicator of both activated and regulatory T cells. The fraction of CD4+ cells that were CD25+ was normal in the duodenum (30%; normal range for tissue 10–30%[7]), but it was unexpectedly reduced to 10% in the colon. In addition, these cells were only dimly positive (Table 2), which indicates fewer CD25 molecules on the surface of CD4+ T cells in the colon.
Table 2.
CD25 expression on T cells from intestinal biopsies
| Tissue source | Duodenum | Sigmoid-colon |
|---|---|---|
| Total CD25 positive | 32·9 | 10·5 |
| % CD25 dim | 17·0 | 9·1 |
| % CD25 bright | 15·9 | 1·4 |
Following stabilization on CsA therapy, blood lymphocyte proportions stabilized. However, the subsequent onset of T1Dm (second arrow Fig. 1) was presaged by a marked increase in the absolute numbers of HLA-DR+ and CD25+CD4 cells in peripheral blood, although the absolute numbers of T cells and CD3+ T cells increased only slightly (Figs 1,2). This was followed by a dramatic drop in CsA levels, which was attributed to decreased drug absorption from the gut. As before, restoration of CsA levels above 200 ng/ml correlated with a reversal of the lymphocyte activation state and restored general health, although diabetes persisted.
Expression of activation markers CD26, CD69, CD71 and CD95 were also followed. CD26+ cells (as a proportion of CD3+ cells) and CD95+ cells (as a proportion of total lymphocytes) increased at 8 months of age after the onset of T1Dm but did not change thereafter. The percentage of CD69+ cells (as a proportion of CD4+ cells) and of CD71+ cells (as a proportion of total lymphocytes) was variable and did not correlate well with the clinical course in this patient (data not shown).
DISCUSSION
While it is difficult to generalize from the experience of a single IPEX patient, our observations are in line with the current model of autoimmune pathogenesis in FOXP3 deficiency, based largely on studies in the genetically equivalent scurfy mouse model. Scurfy mice present a similar spectrum of immune-mediated tissue inflammation and destruction, and die at a very early age, much like classic IPEX [8,9]. The immunological profile is characterized by lymphoproliferation; substantial increases in the proportion of T cells expressing activation markers, including CD25 and CD69, and high levels of numerous cytokines. Seminal studies showed that the disease is mediated by CD4+ T cells that are hyper-responsive to T cell receptor stimulation and resistant to down-regulation by CsA [10,11]. Some evidence suggests that antigen stimulation via the T cell receptor is required to initiate the pathogenic changes [12,13].
The findings in our IPEX patient are parallel and consistent. We observed that the T to B cell ratio and the CD4/CD8 ratio were already increased at birth, prior to exposure to exogenous antigens, yet the frequency of T cells with activated phenotypes (CD25+, HLA-DR+) was not initially remarkable. The number and frequency of CD25+ and of HLA-DR+CD4+ T cells did increase at two weeks of age, followed by clinical presentation with colitis. A similar increase was later observed prior to presumed insulitis leading to T1Dm. Since development of murine CD4+CD25+ T regulatory cells does not occur in the absence of normal FOXP3[14–16], the observed increases in CD25+ cells in our FOXP3 deficient, IPEX patient probably reflect increases in activated T cells rather than an expansion of regulatory T cells.
The first increase in activation markers preceding colitis occurred in the absence of immunosuppressive medication; the second preceded diminished CsA levels, which we believe fell because of unrecognized malabsorptive inflammatory enteropathy. Acute onset of diabetes quickly followed. In both clinical events, CsA blood levels of at least 200 ng/ml seemed necessary to return homeostasis and relative health, suggesting that the disease-causing immune cells were somewhat resistant to levels considered therapeutic for some conditions.
The experience of this patient suggests that significant changes in activation markers may be useful in predicting the onset of autoimmune illness in IPEX, even in the presence of immunosuppressive therapy. At a minimum, they appear more sensitive than total and CD4+ T cell numbers. In addition, it appears that continual maintenance of therapeutic CsA (or other immunosuppressive drug) levels is critical to prevent the development of additional autoimmune IPEX manifestations.
The scurfy phenotype can be rescued by transgenic expression of wild type FOXP3, by fractional bone marrow transplant, or by a single injection of normal T-enriched splenocytes or sorted CD4+CD25+ regulatory T cells [10,14–17]. Furthermore, transgenic over-expression of Scurfin in normal mice decreases T cell responsiveness and number [10,16]. These findings indicate that Scurfin is required, and is perhaps sufficient, for the development of functional regulatory T cells, notably, the CD4+CD25+ regulatory subset. Therefore, the lack of Scurfin due to mutations in FOXP3 may result in autoimmunity due to lack of dominant suppression by such cells. The observation of clinical remission in IPEX patients following bone marrow transplantation (BMT) despite limited donor cell engraftment [1,18], is consistent with this model.
We found it interesting that the lymphocytes in the colitis biopsy had reduced, rather than elevated, frequency and density of CD25, despite HLA-DR+ evidence of activation. The basis for this is unknown, but it contrasts with increased CD25+ expression among circulating cells and the few T cells obtained from the relatively quiescent duodenal biopsy. CD25 is the Interleukin-2 (IL-2) receptor alpha subunit, which is induced early on activated T cells, but is also present on CD4+ regulatory T cells [15]. It is likely that the CD25+ cells in the colon are not regulatory T cells for two reasons. First, the actual level of CD25 expression on the CD4+ T cells is critical in distinguishing regulatory T cells with higher levels of CD25 from nonregulatory T cells [19]. Secondly, this patient lacks FOXP3 mRNA as reported by Bennet et al. [6]. Therefore, since Scurfin (FOXP3 protein) is required for the development of CD4+CD25+ regulatory T cells, but not for CD25+ activated T cells [15], the CD4+CD25+ population in IPEX patients likely represents only activated T cells. Thus, it is curious that CD25 is less frequent among the gut infiltrating lymphocytes at the site of active inflammation and may indicate a valuable clue to the pathogenesis of the disease. Genetic deficiency of CD25 in both mice and humans leads to a phenotype not unlike Scurfy and IPEX [20–22]. CD25 knockout mice respond to the addition of transplanted CD4+CD25+ regulatory T cells [23], as do scurfy mice [17]. In addition, CD4+CD25+ regulatory T cells are greatly expanded when human peripheral blood mononuclear cells are stimulated first with transforming growth factor-beta followed by IL-2 [24]. Thus, it is likely, that CD25 is also necessary for the development or function of regulatory T cells. The observed reduction in CD25+ T cells at the site of ongoing inflammation in the colon suggests several possible explanations for the pathogenesis of the disease. There may be fewer activated CD4+ T cells present at such a site due to the timing of the response. Either the cells in the colon are past the initial stimulation phase (and therefore lack significant CD25), or, perhaps, this phase lasts a shorter time in FOXP3 deficiency. Another potential explanation is that cells that would become regulatory T cells, if they had FOXP3, are summoned in great numbers to the site of the inflammation. However, because they lack high levels of CD25, they cannot function as T regs. Understanding this pathogenesis is the subject of future studies.
It is important to note that this patient may not provide an accurate representation of IPEX patients in general. Powell et al. [2] reported that disease severity in affected members of this family was quite variable, compatible in some cases with survival to adulthood without immunosuppressive drugs. While our patient became ill early, he responded to CsA therapy. This also contrasts with classic IPEX patients who generally succumb between a few weeks and several months of life and may not respond well to CsA [1]. Of those studied, classic IPEX patients all have mutations in the coding region of FOXP3, whereas the mutation reported in our patient's family is noncoding and is believed to decrease the stability of an mRNA that encodes a normal scurfin (FOXP3) protein [6]. Thus, a small amount of normal scurfin protein may be present and serve, at least in some family members, to attenuate the disease, as suggested by Gambineri et al. [25]. However, additional studies will be needed to confirm the presence of small amounts of functional scurfin protein in this patient.
The reported association of exacerbation in this family with acute immune stimulation [2] is also striking and suggests that potent antigenic stimuli may overcome a hypothetical balance between effector activity and diminished regulatory capacity. The more severe course of classic IPEX, on the other hand, may reflect the inability to maintain regulation even in the face of low-level immune activation. Thus, while study of the immune profile of this patient may not be completely recapitulated in classic IPEX patients, it can provide insights into the critical balance between regulatory and effector T cells that cannot be obtained in the more severe form of the disease.
In summary, we present the first longitudinal study of lymphocyte profiles in an IPEX patient beginning at birth and continuing through the development of enteropathy and T1Dm. Our findings are in line with observations in the scurfy mouse model and are consistent with a lack of regulatory T-cells as the underlying cause for the disease. Correlation with clinical data suggests a possible temporal relationship between increases in T-cell activation markers and subsequent clinical events that could prove clinically useful with further study.
Acknowledgments
The authors wish to acknowledge Neil Buist, MD, and Ron Clarke, MD, for providing clinical specimens and communication with the patient and family. In addition, medical technologists from the OHSU Department of Pathology Special Immunology/Flow Cytometry Laboratory performed the analysis of these specimens.
REFERENCES
- 1.Wildin RS, Smyk-Pearson SK, Filipovich AH. Clinical and molecular features of the immuno-dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–5. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell B, Buist N, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100:731–7. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Yoshioka R, Kiyosawa H, Barker DF, Fain PR, Shigeoka AO, Chance PF. X-linked syndrome of polyendocrinopathy, immune dysfunction, and diarrhea maps to Xp11.23-Xq13.3. Am J Hum Genet. 2000;66:461–8. doi: 10.1086/302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of Foxp3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Brunkow ME, Ramsdell F, et al. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA→AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53:435–9. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 7.Stromberg E, Lundgren A, Edeto A, Lundine S, Svennerholm AM, Landholt C. Increased frequency of activated T-cells in the Helobacter pylori-infected antrum and duodenum. FEMS Immunol Med Microbiol. 2003;36:150–68. doi: 10.1016/S0928-8244(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 8.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. [PubMed] [Google Scholar]
- 9.Godfrey VL, Wilkinson JE, Russell LB X. -linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–87. [PMC free article] [PubMed] [Google Scholar]
- 10.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 11.Blair PJ, Bultman SJ, Haas JC, Rouse BT, Wilkinson JE, Godfrey VL. CD4+CD8- T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J Immunol. 1994;153:3764–74. [PubMed] [Google Scholar]
- 12.Patel DD. Escape from tolerance in the human X-linked autoimmunity–allergic dysregulation syndrome and the Scurfy mouse. J Clin Invest. 2001;107:155–7. doi: 10.1172/JCI11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahorsky-Reeves JL, Wilkinson JE. The murine mutation scurfy (sf) results in an antigen-dependent lymphoproliferative disease with altered T cell sensitivity. Eur J Immunol. 2001;31:196–204. doi: 10.1002/1521-4141(200101)31:1<196::AID-IMMU196>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function on CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, Ziegler SF, Ramsdell F. The amount of scrufin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–20. doi: 10.4049/jimmunol.167.11.6312. [DOI] [PubMed] [Google Scholar]
- 17.Smyk-Pearson SK, Bakke AC, Held PK, Wildin RS. Rescue of the autoimmune scurfy mouse by partial bone marrow transplantation or by injection with T-enriched splenocytes. Clin Exp Immunol. 2003;133:193–9. doi: 10.1046/j.1365-2249.2003.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baud O, Goulet O, Canioni D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X–linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758–62. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 19.Baescher-Allan C, Viglietta V. Hafler DA Human CD4+CD25+ Regualtory T cells. Semin Immunolgy. 2004;16:89–97. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 21.YuA Zhou J, Marten N, Bergmann CC, Mammolenti M, Levy RB, Malek TR. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170:236–42. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]
- 22.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94:3168–71. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells. IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–60. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 24.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–9. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 25.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations in FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]