Abstract
Progressive immunodeficiency in HIV infection is paralleled by a decrease in IL-12 production, a cytokine crucial for cellular immune function. Here we examine the molecular mechanisms by which HIV infection suppresses IL-12 p40 expression. HIV infection of THP-1 myeloid cells resulted in decreased LPS-induced nuclear factor binding to the NF-κB, AP-1, and Sp1 sites of the IL-12 p40 promoter. By site-directed mutagenesis we determined that each of these sites was necessary for transcriptional activation of the IL-12 p40 promoter. Binding of NF-κB p50, c-Rel, p65, Sp1, Sp3, c-Fos, and c-Jun proteins to their cognate nuclear factor binding sites was somewhat impaired by HV infection, although a role for other as yet unidentified factors cannot be dismissed. The cellular levels of these transcription factors were unaffected by HIV infection, with the exception of a decrease in expression of NF-κB p65, consistent with the observed decrease in its binding to the IL-12 p40 promoter following HIV infection. Analysis of regulation of upstream LPS-induced MAP kinases demonstrated impaired phosphorylation of JNK and p38 MAPK, and suppressed phosphorylation and degradation of IκBα following HIV infection. These results suggest that alterations in nuclear factor binding to numerous sites in the IL-12 p40 promoter, together may contribute to the suppression in IL-12 p40 transcription previously reported. These effects on nuclear factor binding may be a direct effect of HIV infection on the IL-12 p40 promoter, or may occur indirectly as a consequence of altered MAP kinase activation.
Keywords: monocytes/macrophages, cytokines, transcription factors, lipopolysaccharide, gene regulation
INTRODUCTION
Interleukin (IL)-12 is a 70 kD heterodimeric cytokine, consisting of p40 and p35 monomeric subunits covalently linked by a disulphide bond [1,2]. IL-12 p70 is produced mainly by myeloid cells, including monocytes/macrophages, and dendritic cells, in response to CD40 ligation during antigen presentation, or following recognition of bacterial components by CD14 and toll-like receptor (TLR) family members [3–8]. Early production of IL-12 from responding cells following exposure to bacteria or bacterial products is vital for promoting cell-mediated immune responses via evoking IFN-γ production from natural killer cells and T lymphocytes, and via skewing Th cell development towards the Th1 phenotype [9–12]. In this manner, IL-12 is critical for elimination of intracellular bacterial and parasitic infections by organisms including Leishmania major, Toxoplasma gondii, and Mycobacterium tuberculosis, and may be key in elimination of some viral infections [13–18].
Coordinated expression of both IL-12 p35 and p40 subunits is required for synthesis and release of the bioactive p70 molecule, yet the two monomers are differentially regulated. While expression of the p35 subunit is considered to be constitutive and occurs in many cell types, expression of the inducible p40 subunit is restricted to cell types capable of producing bioactive IL-12 p70 [19–21]. In response to numerous stimulatory and inhibitory factors, expression of IL-12 p40 mRNA correlates closely with induction of p70 production, while p35 mRNA levels remain unchanged [22–26]. As IL-12 p40 protein and mRNA closely parallel production of the p70 molecule, understanding regulation of the IL-12 p40 gene is of paramount importance in understanding production of bioactive IL-12 p70.
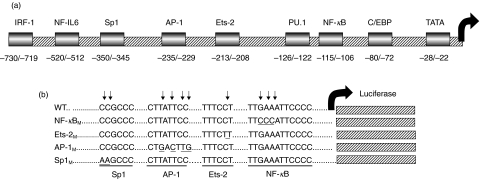
The human IL-12 p40 promoter contains at least 8 putative transcription factor binding sites including C/EBP, NF-κB, PU.1, Ets-2, AP-1, Sp1, NF-IL-6, and IRF-1 (Fig. 1a) [27]; however, the relative contribution of each of these sites in promoter activation remains poorly understood. In the murine RAW 264·7 macrophage cell line, the C/EBP and NF-κB elements exhibit a functional synergy in promoter activation [28]. The presence of the NF-κB element in murine J774 cells, which is known to bind NF-κB p50/p65 and p50/c-Rel heterodimers [29], is essential for pathogen-induced promoter activation [30]. A large multiprotein complex comprised of p50, c-Rel, ICSBP, IRF-1, and Ets-2 proteins forms at the Ets-2 site in stimulated murine RAW 264·7 macrophages [31,32], the inhibition of which contributes to Fc receptor γ-mediated suppression of IL-12 p40 expression [33]. While a requirement for ICSBP and IRF-1 proteins in IL-12 production has been demonstrated in knockout mice [34,35], and IRF-1 and ICSBP synergize to activate the IL-12 p40 promoter in the absence of intact NF-κB or Ets-2 sites [36], the role of the IRF-1 DNA binding element in promoter activation specifically has not been determined. In addition, the contributions of the NF-IL-6, Sp1, and AP-1 sites in p40 transcription have not been evaluated.
Fig. 1.
Proximal human IL-12 p40 promoter constructs. (a) Schematic representation of putative transcription factor binding sites and their location within the proximal human IL-12 p40 promoter. Adapted from X. Ma et al. (1996) Temp. Journal of Experimental Medicine 183: 147–157. (b) Schematic representation of NF-κB, AP-1, and Sp1 IL-12 p40 promoter mutants in the context of a luciferase reporter construct, relative to the wild-type promoter.
The immunodeficiency that occurs during HIV infection is associated with a dramatic decline in cell-mediated immunity, in parallel with impaired secretion of IL-12 [22,37]. This defect in IL-12 production does not reflect a global deficiency in inflammatory cytokine secretion by myeloid cells, as expression of TNF-α and IL-1β is not reduced [37]. In vitro, addition of IL-12 to PBMC from HIV positive individuals partially restores proliferation and IL-2 secretion in response to HIV envelope peptides, or to p24 antigen, and enhances NK cytotoxicity and HIV-specific CTL lytic function [38–40]. Taken together these observations suggest that the defect in cell-mediated immune responses following HIV infection may be somewhat reversible, and that IL-12 may play a significant role in HIV-specific adaptive immune responses. Examination of the mechanism(s) of HIV-mediated suppression of IL-12 synthesis is therefore crucial for furthering our understanding of HIV immunopathogenesis.
Numerous agents inhibit IL-12 production by alteration of binding of nuclear factors to the IL-12 p40 promoter [41–44], and we have previously demonstrated that HIV infection directly impairs the rate of transcription of the IL-12 p40 gene [45]. The purpose of the current investigation is therefore to thoroughly examine the effect of HIV on LPS-inducible DNA binding activity of nuclear factors to each of the putative transcription factor binding sites in the IL-12 p40 promoter in HIV-infected THP-1 myeloid cells. As bacterial stimulation of IL-12 is defective in HIV-infected myeloid cells independent of IFN-γ priming [22], this report addresses the molecular mechanism underlying this defect in LPS-induced IL-12 production in HIV-infected myeloid cells without additional IFN-γ stimulation. The effect of HIV infection on upstream LPS-induced MAPK/SAPK activation was also determined to provide additional information regarding the molecular events following HIV infection that may contribute to decreased expression of IL-12 p40. Subsequent promoter mutagenesis analysis was performed to investigate the functional significance of the affected binding sites in p40 promoter activation. Taken together, the results of this study present a comprehensive evaluation of key nuclear factor binding sites involved in induction of the IL-12 p40 gene in response to LPS stimulation, and the impact of HIV infection on these elements and on upstream LPS-induced signalling mediators, and thus contributes to our understanding of the molecular events contributing to HIV pathogenesis.
METHODS
Virus propagation
The dual-tropic HIV-1 clinical isolate, cs204, was a gift from Dr F. Diaz-Mitoma (Children's Hospital of Eastern Ontario, Ottawa, Canada) and is noncytopathic in THP-1 cells, with little to no alteration in the growth rate or viability of infected THP-1 cell cultures (unpublished observations). This virus isolate was propagated in the THP-1 cell line (American Type Culture Collection; Manassas, VA, USA) and TCID50 determinations were made for the virus stock against THP-1 cells.
In vitro HIV infection
THP-1 cells were incubated with cell-free HIV-1 at a multiplicity of infection of 0·002 for 6 h at 37°C. Cells were washed with phosphate-buffered saline (PBS) and resuspended in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco/Invitrogen; Burlington, Ontario, Canada). Cultures were maintained for 7 days with replenishment of medium every 3–4 days. This infection protocol results in >90% suppression of LPS-induced IL-12 p40 expression in THP-1 cells, with a reproducible rate of infection of 80–90% as determined by flow cytometry and immunofluorescence microscopy, as previously reported [4].
Nuclear extract preparation
Mock- and HIV-infected THP-1 cells were cultured with medium or 1 µg/ml LPS (Salmonella enteriditidis; Sigma; Oakville, ON, USA) for 2 h prior to extraction of nuclear DNA binding proteins. THP-1 cells were harvested and washed with ice-cold PBS. Cell pellets were resuspended in hypotonic lysis buffer (10 mm HEPES, 1·5 mm MgCl2, 10 mm KCl, pH 7·9) and cytoplasmic membranes were disrupted by mechanical lysis. Nuclear DNA-binding protein was stripped from pelleted nuclei by incubation in high-salt buffer (20 mm HEPES, 25% glycerol, 420 mm KCl, 1·5 mm MgCl2, 2 mm EDTA, pH 7·9), followed by addition of a neutralization buffer (20 mm HEPES, 25% glycerol, 0·2 mm EDTA, pH 7·9). Each buffer was supplemented with 2 µg/ml pepstatin A, 10 µg/ml aprotonin, 2 µg/ml leupeptin, 0·5 mm DTT, and 0·575 mm PMSF (Sigma) prior to use. Supernatants containing nuclear DNA binding proteins were aliquoted and stored at −80°C until use in DNA binding assays.
Electrophoretic mobility shift assays
To assess DNA binding activity in nuclear extracts against putative transcription factor binding sites in the human IL-12 p40 promoter, 5 µg of nuclear extract from each treatment was incubated in a reaction mixture (1 mm Tris-HCl pH 7·9, 0·1 mm EDTA, 4 mm NaCl, 0·1 mmβ-mercaptoethanol, 0·4% glycerol, 1 mm DTT, 50 µg/ml poly dI·dC, 50 µg/ml BSA) containing a 32P-labelled double-stranded oligonucleotide probe, representing one of the individual nuclear factor binding sites. Oligonucleotide probes used in electrophoretic mobility shift assays are listed in (Table 1). Independent EMSA analysis of the PU.1 site was not performed as the NF-κB oligonucleotide overlaps this element. End-labelling of oligonucleotides with 32P-ATP was performed with T4 polynucleotide kinase (Amersham) for 1 h at 37°C in a reaction mixture containing 50 mm Tris-HCl pH 7·5, 10 mm MgCl2, 5 mm DTT, and 50 µg/ml BSA.
Table 1.
Oligonucleotide probe sequences for EMSA analysis of nclear factor binding to the human IL-12 p40 promoter
| Binding site | Location in IL-12 p40 promoter | Oligonucleotide sequence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C/EBP | −72/−80 | 5′-TTG | TTT | TCA | ATG | TTG | CAA | CAA | GT-3′ |
| NF-κB | 106/−115 | 5′-AGG | AAC | TTC | TTG | AAA | TTC | CCC | CAGAAGGTTTT-3′ |
| Ets-2 | −208/−213 | 5′-AAA | GTC | ATT | TCC | TCT | TAG | TTC | ATTA-3′ |
| AP-1 | −229/−235 | 5′-TCC | TTC | CTT | ATT | CCC | CAC | CCA-3′ | |
| Sp1 | −345/−350 | 5′-GTC | TGA | CCG | CCC | CTT | GGC-3′ | ||
| NF-IL-6 | −512/−520 | 5′-GTA | TGT | TTC | TGA | AAT | TAA | TGT | AGG-3′ |
| IRF-1 | −719/−730 | 5′-TGA | GGG | TAT | TTC | ACT | TTC | TGC | TCC-3′ |
Subsequent supershift analyses were conducted to identify specific proteins whose binding capability was altered by acute HIV infection of myeloid cells. EMSA reactions were set up as described above, but included specific antibodies directed against relevant nuclear factors for the sites affected by HIV infection. Specifically, nuclear extracts were combined with the NF-κB oligo in the presence of NF-κB p50, p65, or c-Rel antibodies; with the AP-1 oligo in the presence of c-Fos or c-Jun antibodies; or with the Sp1 oligo and Sp1 or Sp3 antibodies. All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and were included in the EMSA/Supershift reaction mixtures at 20 µg/ml.
Western blotting of nuclear factors and signalling kinases
To examine the impact of HIV infection on myeloid cell nuclear factor expression, mock- and HIV-infected THP-1 cells (106/ml) were stimulated with medium or LPS (1 µg/ml) for 1 h, washed twice in ice-cold PBS, and lysed in RIPA buffer (50 mm Tris-HCl pH 7·4, 1% NP-40, 0·25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 µg/ml each aprotonin, leupeptin, pepstatin A, 1 mm Na3VO4, 1 mm NaF). Total cell lysates were stored at −80°C until further use. Fifty µg of cell lysate from each treatment were separated by SDS-PAGE, transferred to PVDF membrane (Immobilon-P, Millipore, Bedford, MA, USA), and blotted with primary antibodies against NF-κB p50, p65, c-Rel, Sp3, or c-Jun (Santa Cruz), or against Sp1, or c-Fos (Upstate Biotechnology, Lake Placid, NY, USA) at concentrations ranging from 0·2 to 0·8 µg/ml. Membranes were washed in TBST buffer (20 mm Tris-HCl pH 7·5, 500 mm NaCl, 0·05% Tween-20) followed by incubation with secondary antibodies (α-mouse-HRP or α-rabbit-HRP; Bio-Rad Laboratories, Mississauga, Ontario, Canada) at 0·2 µg/ml for 1 h at room temperature. After washing, bound antibodies were detected by chemiluminescence using Supersignal West Pico Chemiluminescent Substrate (Pierce; Rockford, IL, USA), and visualized by autoradiography. Following exposure, membranes were stripped (TBST + 0·7%β-mercaptoethanol) at 50°C for 20 min, washed, and reprobed with α-PCNA antibody (Santa Cruz) to ensure uniform loading of samples.
Similarly, the expression and phosphorylation status of MAP kinases in mock- or HIV-infected THP-1 cells were monitored by first blotting membranes with phospho-specific antibodies against p38 MAPK (p38α), JNK 1 and 2 MAPK, ERK 1/2 MAPK, or IκBα (Santa Cruz) followed by stripping and reprobing with antibodies against the native, unphosphorylated state of these proteins (Santa Cruz).
Wild-type IL-12 p40 promoter/luciferase recombinant adenovirus construction
As THP-1 cells have proven to be difficult to transfect transiently with larger plasmids, we have constructed recombinant adenoviral vectors containing the wild-type IL-12 p40 promoter, or mutants of this promoter at the NF-κB, AP-1 or Sp1 sites, to facilitate our investigation of the roles of these sites in promoter activation. A 3·3- kb IL-12 p40 promoter-containing luciferase reporter vector (3·3kb-luc) was a kind gift of Dr Giorgio Trinchieri and Dr Xiaojing Ma. A XhoI restriction fragment encompassing the entire IL-12 p40 promoter region was subcloned into the smaller high-copy pGL3 basic luciferase vector (Promega; Maddison, WI, USA). From this pGL3p40 vector, the entire promoter/luciferase cassette was subcloned as a SalI fragment into the E1 region of pRP2159 resulting in formation of pKC2a shuttle vector. Thus, pKC2a contains an Ad5 inverted terminal repeat, packaging signal, IL-12 p40 promoter, luciferase cassette, polyadenylation sequence, and lox site. This shuttle plasmid was cotransfected using Superfect (Qiagen Inc.; Mississauga, ON, USA) along with the pBHGloxΔE1, 3Cre adenoviral backbone plasmid [46] into 293 cells. Following Cre-mediated recombination between loxP sites in the shuttle and adenoviral backbone vector, E1/E3 deleted, replication-defective IL-12 p40 promoter/luciferase-containing recombinant adenovirus was rescued and purified by caesium chloride buoyant density centrifugation.
Promoter mutagenesis and recombinant adenovirus construction
From the pKC2a vector, a 1·4 kb SmaI restriction fragment containing the proximal IL-12 p40 promoter region was subcloned into a pUC19 vector to facilitate mutagenesis. Individual mutants of the IL-12 p40 promoter construct at the NF-κB, AP-1, Ets-2, and Sp1 sites were generated by site-directed mutagenesis using the QuickChange Mutagenesis Kit (Stratagene; La Jolla, CA, USA) according to manufacturers’ instructions. Mutants were confirmed by sequencing and the 1·4 kb mutant IL-12 p40-containing SmaI fragments were substituted back into the parent pKC2a vector. The wild-type and mutant sequences are listed in Fig. 1b. Following ClaI digestion of mutant IL-12 p40/luciferase containing plasmids, fragments containing the entire IL-12 p40 mutant promoter/luciferase constructs were inserted into the pΔE1sp1a shuttle plasmid [47], and cotransformed with pRP2014 adenoviral vector backbone plasmid into the E. coli BJ5183 strain. Following homologous recombination between overlapping portions of the shuttle and backbone vectors, recombinant E1/E3-deleted IL-12 p40 mutant promoter/luciferase-containing adenoviral genome plasmids were purified by alkaline lysis, and transfected into 293 cells for packaging of replication-defective recombinant adenovirus particles, which were purified by caesium chloride buoyant density centrifugation. All virus stock preparations were determined to have incorporated the desired promoter constructs, and the titre of each vector was determined concurrently by plaque assay on 293 cells.
IL-12 p40 luciferase assays
THP-1 cells (2 × 106/ml) were infected with recombinant adenovirus vectors containing the wild-type or mutant IL-12 p40 promoter/luciferase vectors (m.o.i. = 50), for 6 h at 37°C prior to washing with PBS and plating in RPMI + 10% FBS at 2 × 106/ml. After 24 h, THP-1 cells were stimulated with medium or LPS (1 µg/ml) for an additional 48 h, determined in preliminary experiments to be optimal for luciferase detection from the LPS-stimulated wild-type promoter. Cells were lysed and assayed for luciferase activity, using the Enhanced Firefly Luciferase Assay Kit (BD Biosciences/Pharmingen; Mississauga, ON, Canada) according to manufacturers’ instructions. Luciferase activity among the different experimental conditions was normalized according to the total protein content of cellular extracts, and the multiplicity of infection of each recombinant adenovirus remained constant between assays.
RESULTS
Nuclear factor recruitment to the human IL-12 p40 promoter in HIV-infected THP-1 Cells
Several putative transcription factor binding sites have been identified within the human and murine IL-12 p40 promoters, including C/EBP, NF-κB, PU.1, Ets-2, AP-1, Sp1, NF-IL-6, and IRF-1, yet the significance of each site in promoter activation is poorly understood. Since inhibitors of IL-12 synthesis can act via targeted disruption of nuclear factor binding to at least the NF-κB and Ets-2 promoter elements [33,41–41], we have surveyed each of the identified binding sites in the human IL-12 p40 promoter for alterations in nuclear factor recruitment following HIV infection. To examine the effect of LPS on nuclear factor binding to the IL-12 p40 promoter, and the effect of HIV infection on this binding, EMSA analyses were performed on nuclear extracts from mock- and HIV-infected THP-1 cells, using 32P-labelled oligonucleotide probes representing each of the individual putative transcription factor binding sites represented in the human IL-12 p40 promoter.
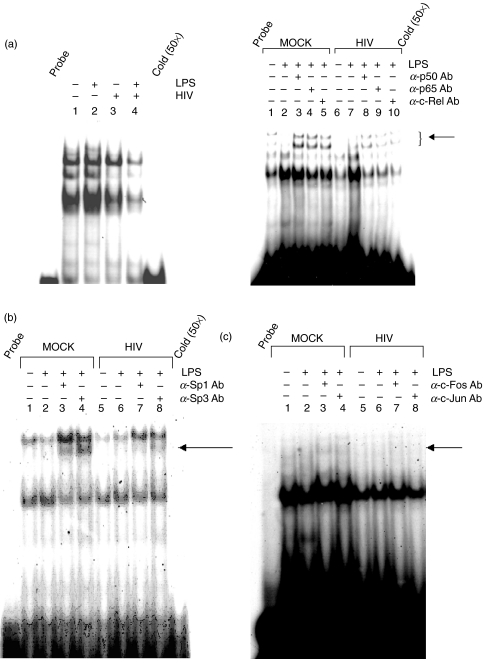
As depicted in Fig. 2, THP-1 cells infected in vitro with HIV-1 exhibited a decrease in the level of LPS-inducible binding of nuclear factors to the NF-κB site within the IL-12 p40 promoter relative to mock-infected LPS-stimulated controls (Fig. 2a left panel, lane 2 versus lane 4). This decrease in nuclear factor binding to the NF-κB site was accompanied by a reduction in binding of p50, p65, and c-Rel proteins (Fig. 2a right panel, lanes 3–5 versus lanes 8–10, respectively). By densitometry this corresponded to a 57·07% decrease in p50, 36·72% decrease in p65, and 86·75% decrease in c-Rel binding to the NF-κB site in LPS-stimulated, HIV-infected THP-1 cells relative to mock-infected, LPS-stimulated controls.
Fig. 2.
Supershift analysis of nuclear factor binding to the human IL-12 p40 promoter in mock- and HIV-infected THP-1 cells. THP-1 cells were mock- or HIV-infected for 7 days prior to stimulation with medium or LPS (1 µg/ml). Nuclear extracts were harvested and incubated with 32P-labelled oligonucleotide probes containing transcription factor consensus sites for (a) NF-κB (b) Sp1 or (c) AP-1, in the presence or absence of nuclear factor-specific antibodies as indicated. Images shown are representative of three independent experiments. Arrows indicate the presence or absence of supershifted complexes.
Similarly, HIV infection produced a minor decrease in LPS-inducible nuclear protein binding to the Sp1 site (Fig. 2b, lane 2 versus 6, a 9·4% reduction by densitometry), with a concomitant decrease in binding of Sp1 and Sp3 to this site. By densitometric analysis, binding of Sp1 and Sp3 was reduced by 43·6% and 61·4%, respectively, in LPS-stimulated HIV-infected THP-1 cells, compared to LPS-stimulated mock-infected controls. The effects of HIV infection on nuclear factor binding at the AP-1 element in THP-1 cells is more striking than the effects at the NF-κB and Sp1 sites. HIV infection reduced the overall level of complex binding at this element (Fig. 2c, lane 2 versus lane 6), and additionally reversed the effects of LPS on nuclear factor binding. Specifically, the LPS-induced decrease in protein binding that occurs at the AP-1 site in mock-infected cells (lane 2 versus lane 1) is not observed in the presence of HIV infection (lane 6 versus lane 5), in addition to the overall reduction in the level of nuclear factor recruitment to the AP-1 site. Supershift analysis revealed reduced LPS-induced binding of c-Fos, and c-Jun proteins following HIV infection (44·1% and 32·4% reduction, lane 3 versus 7 and lane 4 versus 8, respectively) although their participation in binding to the AP-1 site appears minimal. As with the NF-κB and Sp1 sites which did not exhibit complete retardation in nuclear complex mobility by incubation with nuclear factor specific antibodies, it is likely that additional factors interact with AP-1 site in addition to the cognate AP-1 family binding proteins, which may be subject to additional regulation by HIV infection. Binding of nuclear factor-directed antibodies was specific, as supershifted complexes were not observed using a control goat-IgG antibody (data not shown).
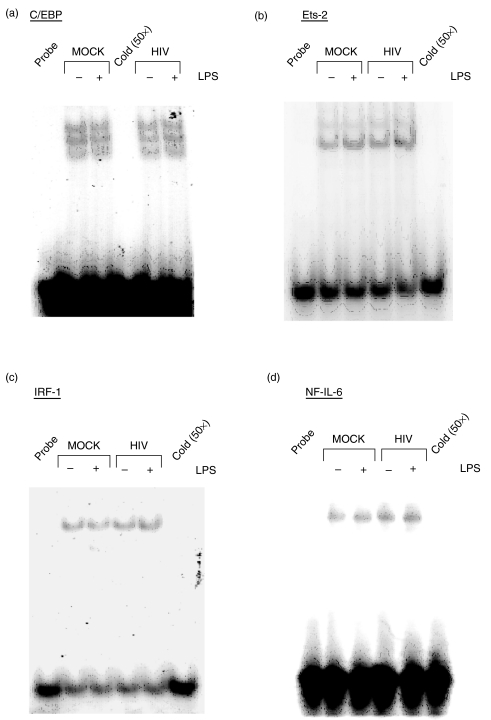
In contrast to the effects of HIV on nuclear factor binding to the NF-κB, AP-1, and Sp1 elements of the IL-12 p40 promoter, acute HIV infection of THP-1 cells did not cause alteration in protein binding to the C/EBP, Ets-2, IRF-1 or NF-IL-6 (Fig. 3a–d, respectively). Together, these observations illustrate that the central NF-κB, AP-1 and Sp1 region (−350/−105 relative to the transcription start site) of the IL-12 p40 promoter is a significant target of transcriptional inhibition of the IL-12 p40 gene following acute HIV infection in the THP-1 myeloid cell line.
Fig. 3.
HIV infection of THP-1 cells does not affect recruitment of nuclear factors to the C/EBP, Ets-2, IRF-1, or NF-IL-6 elements of the IL-12 p40 promoter. THP-1 cells were mock- or HIV-infected, medium- or LPS-stimulated as in Fig. 3. Nuclear extracts were incubated with 32P-labelled probes containing putative (a) C/EBP (b) Ets-2 (c) IRF-1 or (d) NF-IL-6 binding sites represented in the human IL-12 p40 promoter. Gels shown are representative of three independent trials.
Functional significance of IL-12 p40 transcription factor binding sites in LPS-induced promoter activation
While numerous transcription factor binding sites have been mapped in the human and murine IL-12 p40 promoters by virtue of their consensus sequences, it is unclear what the relative contribution of each individual site is to the activation of the promoter. To determine whether sites exhibiting alterations in nuclear factor binding following HIV infection of myeloid cells are actually functional in activation of the IL-12 p40 promoter by LPS, site-directed mutagenesis of the NF-κB, AP-1, and Sp1 sites was performed.
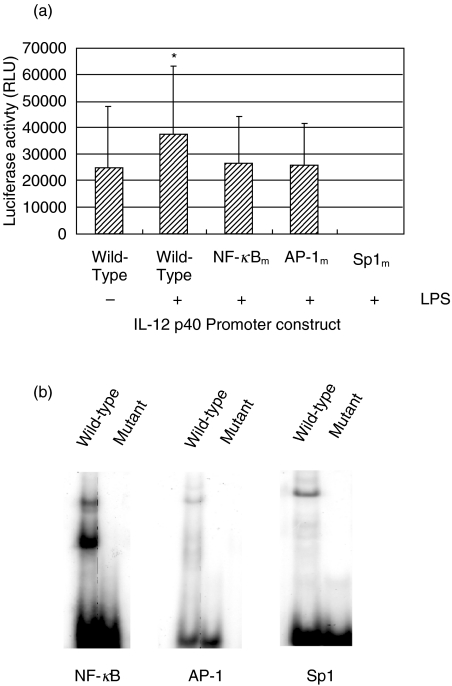
A 3·3 kb IL-12 p40 promoter construct (a kind gift of Dr Giorgio Trinchieri and Dr Xiaojing Ma) was subcloned into a pGL3 basic luciferase vector. Site-directed mutants of the individual NF-κB (NF-κBm), AP-1 (AP-1m), or Sp1 (Sp1m) sites were generated, and are shown relative to the wild-type promoter sequence in Fig. 1b. The wild-type and mutant IL-12 p40 promoter constructs were assembled into recombinant adenoviral vectors for delivery to THP-1 cells. As demonstrated in Fig. 4, mutation of the NF-κB or AP-1 elements reduced LPS-induced transcriptional activation of the human IL-12 p40 promoter in THP-1 cells to the level of the unstimulated wild-type promoter (Fig. 4a). In addition, mutation of the Sp1 site completely inhibited transcriptional activation of the IL-12 p40 promoter. By EMSA analysis comparing nuclear factor binding to the mutant versus wild-type oligonucleotides for each of the NF-κB, AP-1 and Sp1 sites, we verified that each individual binding site mutation significantly inhibited nuclear factor binding to the IL-12 p40 promoter (Fig. 4b). This complete absence of luciferase activity in THP-1 cultures infected with the Sp1 mutant IL-12 p40 promoter recombinant adenovirus is not due to a failure to package recombinant IL-12 p40 promoter/luciferase containing genomes into adenovirus particles (data not shown). These results confirm that each of the sites within the human IL-12 p40 promoter which were affected by acute HIV infection of myeloid cells are vital for LPS-induced activation of the IL-12 p40 promoter, highlighting the significance of these sites as targets of modulation of IL-12 p40 expression by HIV.
Fig. 4.
The NF-κB, AP-1, and Sp1 elements of the human IL-12 p40 promoter are required for LPS-induced promoter activation. Recombinant adenoviral vectors containing a wild-type IL-12 p40 promoter, or a promoter containing mutations of the NF-κB (NF-κBm), AP-1 (AP-1 m), or Sp1 (Sp1m) sites individually, driving expression of a luciferase reporter cassette were used to infect THP-1 cells (m.o.i. = 50). Cells were stimulated with medium or LPS (1 µg/ml) for 48 h prior to lysis and quantification of luciferase activity. (a) This graph compares luciferase activity in THP-1 cells infected with adenovirus carrying the wild-type IL-12 p40 promoter or NF-κBm, AP-1 m, or Sp1m mutant promoters. Bars represent mean±standard deviation; n = 3, *P = 0·033 by Student's T-test comparing medium versus LPS-induced activation of the wild-type IL-12 p40 promoter. Raw luciferase activity is reported as RLU (relative light units) and was normalized between samples according to total protein content of cellular extracts. (b) EMSA analysis of oligonucleotides representing mutated versus wild-type NF-κB, AP-1 and Sp1 binding sites, confirming ablation of LPS-induced nuclear factor binding by site-directed mutagenesis at these sites.
Effect of HIV infection on nuclear factor expression in myeloid cells
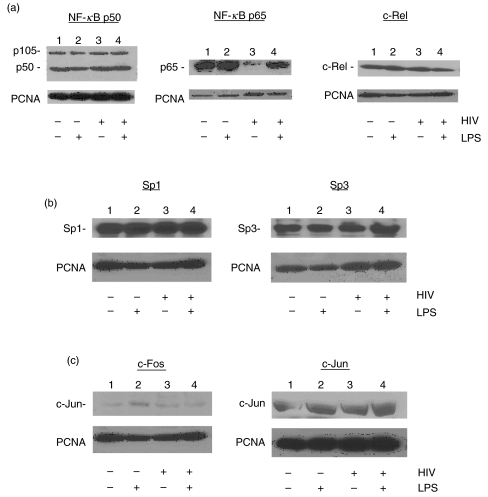
Following our observation that acute HIV infection altered binding of numerous nuclear factors to the NF-κB, AP-1 and Sp1 sites in THP-1 cells, we examined the impact of HIV infection on overall expression of these defined nuclear factors to lend further insight into the mechanism of their altered binding to the IL-12 p40 promoter. Whole-cell lysates were isolated from mock- or HIV-infected THP-1 cells after 7 days in culture, and levels of nuclear factor expression were determined by Western Blotting. HIV infection showed no effect on NF-κB p50 or c-Rel expression, although p65 expression was decreased relative to the mock-infected control (Fig. 5a), potentially contributing to the reduced p65 binding at the NF-κB site observed in Fig. 2. By densitometric analysis of Western Blots, this represented a 75·8% (± 20·4%) decrease in steady-state levels of p65 protein in HIV infected unstimulated cells (lane 3 versus lane 1), and a 76·9% (± 3·8%) decrease in p65 expression in LPS-stimulated, HIV-infected THP-1 cells relative to LPS-stimulated mock-infected THP-1 (lane 4 versus lane 2). The overall cellular expression of the Sp1 family proteins Sp1 and Sp3, as well as the AP-1 proteins c-Fos and c-Jun were also unaffected by HIV infection of THP-1 cells (Figs 5b,c, respectively). These data suggest that with exception reduced p65 expression, modulation of expression of nuclear factors does not appear to explain the altered binding of these factors to the IL-12 p40 promoter observed following acute HIV infection of myeloid cells (Fig. 2).
Fig. 5.
Effect of acute HIV infection on nuclear factor expression in THP-1. THP-1 cells were mock- or HIV-infected and after 7 days, stimulated with medium or LPS (1 µg/ml) as in Fig. 3. Fifty µg of whole-cell lysate were electrophoretically separated by SDS-PAGE, transferred to PVDF membrane and blotted with antibodies against (a) NF-κB p50, p65, and c -Rel proteins; (b) Sp1 and Sp3; (c) c-Fos and c-Jun proteins; or against PCNA as a loading control. HRP-conjugated secondary antibodies were detected by chemiluminescence and subsequent autoradiography. Gels shown are representative of three independent experiments.
Inhibition of activation of NF-κB and JNK and p38 MAP kinases by HIV infection
LPS-stimulation of myeloid cells via toll-like receptor 4 (TLR4) initiates a broad array of intracellular signalling events, culminating in activation of NF-κB via IκBα phosphorylation and degradation, and activation of p38, JNK and ERK MAP kinases and their downstream transcription factor substrates [48]. Since alterations in the level of expression of nuclear factors by HIV does not appear to be the principal mechanism of disruption of nuclear factor binding to the promoter, we next addressed the impact of HIV infection on LPS-induced MAP kinase and NF-κB activation by Western Blotting with antibodies against MAP kinases and IκBα in their native and phosphorylated states.
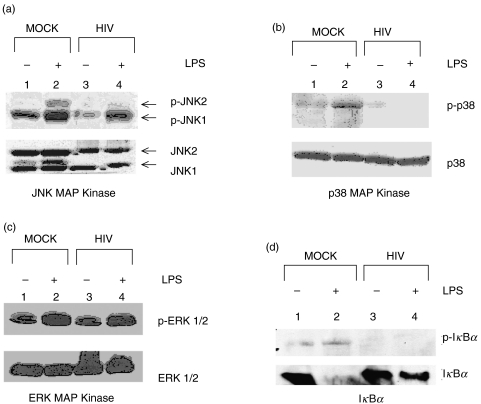
As shown in Fig. 6a, acute HIV infection of THP-1 cells resulted in diminished levels of JNK MAP kinase in HIV-infected versus mock-infected THP-1 cells (unstimulated cells 58·6% ± 21·98%, lane 3 versus lane 1; LPS-stimulated 51·5% ± 4·7%, lane 4 versus lane 2), and a similar reduction in phosphorylated JNK in LPS-stimulated HIV infected THP-1 compared to LPS-stimulated mock-infected THP-1 (48·9% ± 21·3%, lane 4 versus lane 2). In addition, LPS-induced phosphorylation of p38 MAPK was also suppressed by 73·5% (± 15·8%) in HIV-infected THP-1 cells, without an alteration in p38 MAPK expression (Fig. 6b, lane 4 versus lane 2). While phosphorylation and expression of ERK 1/2 MAPK was unaffected by HIV infection, phosphorylation and degradation of IκBα and therefore, NF-κB activation, was impaired in LPS-stimulated HIV-infected THP-1 cells (Figs 6c,d, respectively). Specifically, LPS-induced phosphorylation of IκBα was reduced by 59·1% (± 15·7%, lane 4 versus lane 2) following HIV infection. While steady-state levels of IκBα protein was unaffected by HIV infection, we observed a significant increase in cellular IκBα in LPS-stimulated HIV-infected THP-1 cells compared to mock-infected controls (lane 4 versus lane 2), indicative of impaired phosphorylation-induced degradation of IκBα in HIV-infected THP-1 cells. Taken together, these observations suggest that HIV infection of myeloid cells leads to selective impairment of LPS-induced JNK and p38 MAPK activation, and additionally suppresses NF-κB activation via inhibition of IκBα degradation. Overall, these LPS signalling defects likely contribute to the reported observations of altered nuclear factor binding following acute HIV infection.
Fig. 6.
Effect of acute HIV infection on MAP kinase and IκBα expression and activation in THP-1 Cells. THP-1 cells were mock- or HIV-infected and after 7 days, stimulated with medium or LPS (1 µg/ml) for 1 h prior to whole-cell lysis. Fifty µg of whole-cell lysate were electrophoretically separated, transferred to PVDF membrane and blotted with antibodies against (a) JNK MAP kinase (b) p38 MAP kinase (c) ERK 1/2 MAP kinase or (d) IκBα proteins. After chemiluminescent detection and autoradiography, membranes were stripped and reprobed with phospho-specific antibodies as indicated. Images shown are representative of 3 independent experiments.
DISCUSSION
Given its role in cellular immunity, understanding molecular aspects of IL-12 regulation is of paramount importance in understanding HIV immunopathogenesis. Previous investigations have demonstrated that suppression of IL-12 p40 by HIV infection of monocytic cells requires active cellular infection and occurs at least in part at the level of transcription of the IL-12 p40 gene [45]. In this report we show that acute HIV infection of myeloid cells impairs nuclear factor binding to the central NF-κB/AP-1/Sp1 region of the human IL-12 p40 promoter, and reversed the effects of activation by LPS on nuclear factor binding to the AP-1 site. The observed changes in nuclear factor binding may be a consequence of impaired LPS-induced activation of p38 and JNK MAP kinases, or interference with NF-κB activation.
EMSA analysis of THP-1 cells revealed decreases in nuclear factor binding to the NF-κB, Sp1, and AP-1 elements of the IL-12 p40 promoter following LPS stimulation of HIV infected cells. In addition to reducing the overall level of protein binding to the AP-1 site, HIV infection reversed the effects of LPS stimulation on nuclear factor binding to this element. LPS-inducible nuclear factor binding to the C/EBP, Ets-2, NF-IL-6 and IRF-1 sites of the IL-12 p40 promoter was unaffected by HIV infection. Recruitment of nuclear proteins to their cognate NF-κB, Sp1 and AP-1 binding sites was marginally impaired by HIV infection, indicating that other as yet undefined nuclear proteins likely bind to these elements and may participate in HIV-mediated suppression of LPS-induced IL-12 p40 promoter activation at these sites. Altered binding of these specific factors could not be explained by altered expression, with the exception of a decrease in intracellular p65 protein levels in HIV-infected THP-1 cells. Each of the NF-κB, AP-1, and Sp1 elements seem to be indispensable for promoter activation as illustrated by the mutagenesis experiments.
Numerous reports have demonstrated that pharmacological inhibitors of IL-12 synthesis including ASA, 1,25-dihydroxyvitamin D3, VIP and PACAP act via targeted disruption of nuclear complex formation at the NF-κB site [41,43,44]. For the first time our group has demonstrated the involvement of the NF-κB, AP-1, or Sp1 elements of the IL-12 p40 promoter in HIV-mediated suppression of IL-12 expression. Of additional interest is the observation that in mock-infected THP-1 cells, LPS reduces nuclear factor binding to the AP-1 site, indicating that c-Fos, c-Jun, or other AP-1 binding proteins may function in an inhibitory manner directly or indirectly via recruitment of inhibitory factors in unstimulated cells, maintaining an inactive promoter. The nature of such factors, or whether this represents cooperation between these elements and the recently identified GA-12 repressor element at position −155 in the IL-12 p40 promoter remains to be determined [49]. While there little current literature evaluating the role of the AP-1 binding site in human IL-12 p40 expression or describing specific proteins which may bind this element, c-fos–/– mice have an enhanced capacity for IL-12 expression [50], indicating that the AP-1 site or AP-1 family members may negatively regulate IL-12 p40 transcription. The presence of the AP-1 element may be required for promoter activation via interaction with factors binding to adjacent sites, but may also confer a regulatory mechanism for IL-12 p40 transcription by recruitment of negative regulatory factors, which are released following LPS stimulation.
Despite the decrease in nuclear factor binding to the NF-κB and Sp1 elements in HV-infected THP-1 cells, residual binding of nuclear complexes to these promoter elements may reflect recruitment of inhibitory factors to the endogenous promoter, or may reflect promoter occupancy by transcriptionally inactive or improperly modified NF-κB and Sp1 proteins. These observations may be analogous to glucocorticoid receptor-mediated inhibition of NF-κB-mediated activation of proinflammatory genes where disruption of NF-κB DNA binding does not occur, but may instead be due to tethering inhibitory factors to the promoter [51]. Several studies have illustrated that the transcriptional transactivation capacity of NF-κB proteins can be impaired without effects on nuclear translocation or DNA binding [52,53]. In addition, the Sp1 family member Sp3 has been shown to repress promoter activation by Sp1 and numerous other transcriptional activators [54]. In HIV-infected THP-1 cells, it remains to be determined whether the remaining Sp3 protein associating with the promoter represses transcriptional activation mediated by Sp1 or other adjacent nuclear factors.
HIV infection of THP-1 cells suppressed LPS-induced phosphorylation of JNK and p38 MAPK. In addition, phosphorylation and degradation of IκBα in response to LPS stimulation was impaired in HIV-infected THP-1, consistent with the observed decrease in nuclear factor binding to the NF-κB site. As these kinases or signalling mediators contribute to regulation of activation and function of NF-κB, AP-1, and Sp1 family members [55,56], and NF-κB and p38 MAP kinase have been strongly linked to IL-12 induction [48,57], it is likely that transcriptional inhibition of IL-12 p40 by HIV infection occurs, at least in part, via repression of activation of these LPS-induced signalling mediators. The exact molecular targets of HIV in the LPS-mediated NF-κB and MAPK activation cascade remain to be identified. As HIV Tat and Nef proteins reportedly alter MAPK activation in several nonmyeloid systems [58–60], modulation of host-cell transcriptional machinery via aberrant MAPK activation is a highly plausible mode of suppression of IL-12 p40 expression in HIV-infected myeloid cells.
Recent reports have demonstrated that a nucleosome, positioned over the NF-κB element in the murine IL-12 p40 promoter, spanning the Sp1, AP-1 and Ets-2 sites in the human promoter, is selectively remodeled following LPS/IFN-γ stimulation, allowing access of nuclear factors to chromatin and subsequent promoter activation [61]. Regulation of chromatin structure in the central portion of the IL-12 p40 promoter may contribute to the transcriptional deregulation of this gene, and may add another level of regulation of host cell genes in general by HIV. Although the binding alterations at each of the NF-κB, AP-1 and Sp1 sites reported here may not be dramatic individually, the sum total of these changes in the context of the endogenous promoter with the potential additional effects on cooperation between neighbouring sites and recruitment of transcription cofactors, may have a significant impact on transcriptional activation of the IL-12 p40 promoter.
For the first time, our group has demonstrated the effects of acute HIV infection on molecular regulation of the human IL-12 p40 promoter, which may occur via inhibition of IκBα degradation and p38 and JNK MAP kinase activation in myeloid cells. This is also the first demonstration that in addition to the NF-κB IL-12 p40 promoter element, the AP-1 and Sp1 sites are integral for LPS-induced transcription and are a targets of regulation of IL-12 p40 expression. These findings make a significant contribution to our understanding of the impact of the HIV virus on molecular control of a key immunoregulatory cytokine. Elucidation of interactions of HIV proteins with host cell signalling machinery, and a more in-depth analysis of nuclear factor binding to and cooperation among these sites, will help pinpoint the intracellular molecular mechanism of suppression of IL-12 by HIV. This, in turn will further our understanding of HIV pathogenesis, and open the door to possible new therapeutic approaches for managing the impact of HIV infection on the host immune response.
Acknowledgments
The authors gratefully acknowledge Dr Karen Copeland for helpful discussion, and Dr Michael Montpetit, Robert Meulenbroek, and Kathy Sargent for technical assistance, as well as Dr Giorgio Trinchieri and Dr Xiaojing Ma for providing our laboratory with the IL-12 p40 promoter-containing vector 3·3kb-luc. Jonathan B. Angel is supported by a Scientist Salary Awarded by the AIDS Program Committee (Positive Action Fund), Ontario Ministry of Health. Kelley A. Chambers is a recipient of an OHTN studentship award. This work was supported by operating grants from the Ontario HIV Treatment Network (OHTN) and the Canadian Institutes of Health Research (CIHR HOP-63009).
REFERENCES
- 1.Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–68. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–23. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 5.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 7.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowdery JS, Boerth NJ, Norian LA, Myung PS, Koretzky GA. Differential regulation of the IL-12 p40 promoter and of p40 secretion by CpG DNA and lipopolysaccharide. J Immunol. 1999;162:6770–5. [PubMed] [Google Scholar]
- 9.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naïve CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 10.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type I (Th1) -specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chehimi J, Valiante NM, D’Andrea A, et al. Enhancing effect of natural killer cell stimulatory factor (NKSF/interleukin-12) on cell-mediated cytotoxicity against tumor-derived and virus-infected cells. Eur J Immunol. 1993;23:1826–30. doi: 10.1002/eji.1830230814. [DOI] [PubMed] [Google Scholar]
- 13.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–42. [PubMed] [Google Scholar]
- 14.Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, Trinchieri G, Reed SG. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 15.Gazzinelli RT, Hayashi S, Wysocka M, et al. Role of IL-12 in the initiation of cell mediated immunity by Toxoplasma gondii and its regulation by IL-10 and nitric oxide. J Eukaryot Microbiol. 1994;41:9S. [PubMed] [Google Scholar]
- 16.Gazzinelli RT, Wysocka M, Hieny S, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 17.Denis M. Interleukin-12 (IL-12) augments cytolytic activity of natural killer cells toward Mycobacterium tuberculosis-infected human monocytes. Cell Immunol. 1994;156:529–36. doi: 10.1006/cimm.1994.1196. [DOI] [PubMed] [Google Scholar]
- 18.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–24. [PubMed] [Google Scholar]
- 19.D’Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–57. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–11. [PubMed] [Google Scholar]
- 23.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40–CD40 ligand interaction. J Immunol. 1996;156:3932–8. [PubMed] [Google Scholar]
- 24.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–50. [PubMed] [Google Scholar]
- 25.Ma X, Sun J, Papasavvas E, et al. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722–9. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 26.Modlin RL, Barnes PF. IL12 and the human immune response to mycobacteria. Res Immunol. 1995;146:526–31. doi: 10.1016/0923-2494(96)83027-6. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Aste-Amezaga M, Trinchieri G. Regulation of interleukin-12 production. Ann NY Acad Sci. 1996;795:13–25. doi: 10.1111/j.1749-6632.1996.tb52651.x. [DOI] [PubMed] [Google Scholar]
- 28.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–88. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–10. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, Neurath M, Gri G, Trinchieri G. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J Biol Chem. 1997;272:10389–95. doi: 10.1074/jbc.272.16.10389. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Gri G, Trinchieri G. A novel ets-2-related nuclear factor is involved in transcriptional activation of the human interleukin-12 p40 gene promoter in response to interferon-gamma and LPS stimulation of monocytic cells. Ann NY Acad Sci. 1996;795:357–60. doi: 10.1111/j.1749-6632.1996.tb52692.x. [DOI] [PubMed] [Google Scholar]
- 33.Grazia Cappiello M, Sutterwala FS, Trinchieri G, Mosser DM, Ma X. Suppression of Il−12 transcription in macrophages following Fc gamma receptor ligation. J Immunol. 2001;166:4498–506. doi: 10.4049/jimmunol.166.7.4498. [DOI] [PubMed] [Google Scholar]
- 34.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–34. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J Immunol. 1999;163:1529–36. [PubMed] [Google Scholar]
- 36.Masumi A, Tamaoki S, Wang IM, Ozato K, Komuro K. IRF-8/ICSBP and IRF-1 cooperatively stimulate mouse IL-12 promoter activity in macrophages. FEBS Lett. 2002;531:348–53. doi: 10.1016/s0014-5793(02)03556-1. [DOI] [PubMed] [Google Scholar]
- 37.Chehimi J, Starr SE, Frank I, D’Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–4. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 39.Dybul M, Mercier G, Belson M, et al. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J Immunol. 2000;165:1685–91. doi: 10.4049/jimmunol.165.3.1685. [DOI] [PubMed] [Google Scholar]
- 40.Landay AL, Clerici M, Hashemi F, Kessler H, Berzofsky JA, Shearer GM. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996;173:1085–91. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- 41.Leceta J, Gomariz RP, Martinez C, Abad C, Ganea D, Delgado M. Receptors and transcriptional factors involved in the anti-inflammatory activity of VIP and PACAP. Ann NY Acad Sci. 2000;921:92–102. doi: 10.1111/j.1749-6632.2000.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 42.Na SY, Kang BY, Chung SW, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674–80. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 43.Mazzeo D, Panina-Bordignon P, Recalde H, Sinigaglia F, D’Ambrosio D. Decreased IL-12 production and Th1 cell development by acetyl salicylic acid-mediated inhibition of NF-kappaB. Eur J Immunol. 1998;28:3205–13. doi: 10.1002/(SICI)1521-4141(199810)28:10<3205::AID-IMMU3205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 44.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers KA, Parato KG, Angel JB. Active cellular infection of myeloid cells is required for HIV-1-mediated suppression of interleukin-12 p40 expression. Cell Immunol. 2002;215:120. doi: 10.1016/s0008-8749(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 46.Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther. 2000;11:693–9. doi: 10.1089/10430340050015590. [DOI] [PubMed] [Google Scholar]
- 47.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–6. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–12. [PubMed] [Google Scholar]
- 49.Becker C, Wirtz S, Ma X, Blessing M, Galle PR, Neurath MF. Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-kappaB, CCAAT/enhancer-binding protein beta, and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E (2) J Immunol. 2001;167:2608–18. doi: 10.4049/jimmunol.167.5.2608. [DOI] [PubMed] [Google Scholar]
- 50.Roy S, Charboneau R, Cain K, DeTurris S, Melnyk D, Barke RA. Deficiency of the transcription factor c-fos increases lipopolysaccharide-induced macrophage interleukin 12 production. Surgery. 1999;126:239–47. [PubMed] [Google Scholar]
- 51.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–29. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergmann M, Hart L, Lindsay M, Barnes PJ, Newton R. IkappaBalpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription. Requirement for an additional activation pathway. J Biol Chem. 1998;273:6607–10. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- 53.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Luca P, Majello B, Lania L. Sp3 represses transcription when tethered to promoter DNA or targeted to promoter proximal RNA. J Biol Chem. 1996;271:8533–6. doi: 10.1074/jbc.271.15.8533. [DOI] [PubMed] [Google Scholar]
- 55.Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–9. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- 56.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 57.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63:1901–10. doi: 10.1016/s0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 58.Varin A, Manna SK, Quivy V, Decrion AZ, Van Lint C, Herbein G, Aggarwal BB. Exogenous Nef protein activates NF-kappa B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells. Role in AIDS pathogenesis. J Biol Chem. 2003;278:2219–27. doi: 10.1074/jbc.M209622200. [DOI] [PubMed] [Google Scholar]
- 59.Manna SK, Aggarwal BB. Differential requirement for p56lck in HIV-tat versus TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal kinase, and apoptosis. J Immunol. 2000;164:5156–66. doi: 10.4049/jimmunol.164.10.5156. [DOI] [PubMed] [Google Scholar]
- 60.Mischiati C, Pironi F, Milani D, Giacca M, Mirandola P, Capitani S, Zauli G. Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. Aids. 1999;13:1637–45. doi: 10.1097/00002030-199909100-00006. [DOI] [PubMed] [Google Scholar]
- 61.Weinmann AS, Mitchell DM, Sanjabi S, Bradley MN, Hoffmann A, Liou HC, Smale ST. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–7. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]