Abstract
Glomerulonephritis may be triggered by antibody deposits that activate macrophages to promote tissue damage. Macrophage-induced apoptosis of human vascular smooth muscle cells and rodent mesangial cells is potentially relevant to glomerulonephritis. Therefore, studies of macrophage-induced apoptosis were extended to antibody-activated macrophages. That is, we studied antibody dependent cellular cytotoxicity (ADCC). To corroborate results, we studied biochemical versus microscopic measurements, soluble or immobilized immunoglobulin and vascular smooth muscle cells (VSMCs) or mesangial cells (MCs). U937 macrophages and human peripheral blood macrophages provoked antibody-dependent killing of MCs and VSMCs. Macrophage-induced death was apoptotic based on electron microscopy, annexin-V, activated caspase-3 and hypodiploid DNA. ADCC was inhibited by antagonistic antibodies to Fas-L and to CD16 (Fc-γ-RIII) but not to CD64 (Fc-γ-RI). In conclusion, antibody-dependent killing of human MCs by human macrophages was via Fas-L and CD16.
Keywords: ADCC, apoptosis, CD16, glomerulonephritis, macrophage
INTRODUCTION
Macrophages are abundant in the lesions of severe glomerulonephritis: human crescentic glomerulonephritis [1] and rodent nephrotoxic nephritis [2]. Macrophages are demonstrably critical to the pathogenesis of rodent nephrotoxic nephritis [2] and thought to mediate human crescentic nephritis [3]. Glomerulonephritis lesions contain activated macrophages and apoptotic cells [4,5].
Glomerulonephritis is thought to be triggered by glomerular immune complex (IC) deposits via either (or both) complement or via cell receptors for antibody Fc regions (Fc receptors, FcRs). Fc-knockout mice are resistant to antibody-mediated glomerulonephritis, both in the spontaneous autoimmune model NZB/WF1 [6] and after experimental immunization (nephrotoxic nephritis) [7,8].
Moreover, macrophage effector functions are activated by FcRs [9]. Glomerulonephritis is triggered most usually by IgG-containing ICs [3]. Inflammatory cells express stimulatory IgG Fc receptors Fc-γ-RI (CD64) and Fc-γ-RIII (CD16) [9]. Although FcRs CD16 and CD64 may mediate mouse antibody-induced glomerulonephritis, their role in human glomerulonephritis is less clear. CD64 is the high-affinity stimulatory IgG receptor and CD16 is a low-affinity stimulatory IgG receptor [9]. Although CD16 mediates human polymorph activation by soluble immune complexes [10], the relative contribution of CD16 and CD64 to macrophage activation by particulate IgG, such as IgG-coated target cells, is uncertain. Antibody-dependent cellular cytotoxicity (ADCC) occurs when leucocytes are activated by antibody recognition to kill target cells. Although macrophages may mediate ADCC, the mechanism is unclear.
Macrophage-induced apoptosis is emerging as a macrophage effector function. For example, cytokine-activated rodent macrophages induce apoptosis in normal rodent glomerular mesangial cells (MCs) by Fas-ligand and nitric oxide [11]. We have shown that human macrophages, when activated by oxidized lipoproteins [12], kill human vascular smooth muscle cells (VSMC) in direct co-culture via Fas-ligand [13] and nitric oxide [14]. This also showed that HCMED-1E6 VSMCs and primary human VSMCs exhibit similar macrophage-induced apoptosis [12–14]. Similarly, others have shown that cytokine-activated human macrophages induce apoptosis in human vascular smooth muscle cells [15,16]. Taken together with the evidence of macrophage activation by FcRs, and of the roles of macrophage in antibody-triggered glomerulonephritis, we asked whether antibody would provoke macrophage-induced apoptosis.
A major challenge in detecting cell-mediated cytotoxicity is distinguishing in which cell type cell death has occurred. Most biochemical cell death assays do not discriminate effector from target cell death. Therefore, we and others used selective fluorescent labelling of macrophages and fluorescence microscopy to assess killing of VSMCs and MCs by macrophages in direct co-culture [11–16]. However, several papers have now validated biochemical methods for measurement of cell death based on either release [17,18] or retention [19–23] of the polar fluorescent dye calcein loaded into target cells.
In this study, we examined killing by antibody-activated macrophages and show that human macrophages kill human mesangial and vascular smooth muscle cells when stimulated by antibodies via CD16.
METHODS
Cell culture
U937 human monocytes were obtained from European Cell Culture Collection (Porton Down, UK) and cultured in Dulbecco's modified Eagle's medium (DMDM) supplemented with 10% fetal calf serum (FCS), 2 mm L-glutamine, amphotericin, 50 U/ml penicillin, 50 µg/ml streptomycin [10% FCS DMEM + antibiotics, antifungal, glutamine (AAG)] (all from Sigma, Gillingham, UK). U937 cells were differentiated to macrophages by 1·6 nm phorbol myristate acetate [phorbol ester PMA (Sigma, Gillingham, UK)] for 48 h in 16-well glass chamber slides (Nunc, Brentwood, UK). Human peripheral blood monocytes were obtained from the National Blood Service as described [12–14] and differentiated by culture for 24 h in 10% FCS DMEM + AAG in glass chamber slides (Nunc). The human mesangial cell line SV40HMC was the gift of Dr J. Domin (Imperial College), cultured in 10% FCS DMEM + AAG. HCMED1-E6. VSMCs (gift of Prof. M. Bennett).
Fluorescence microscopy assay for cell death in co-culture
The macrophage : mesangial cell ratio was 10 : 1. Macrophages were differentiated as above in 16-well glass chamber slides (Nunc) and labelled with the fluorescent dye 3′3′-dioctadecyloxacarbocyanine perchlorate (Di[O]) 5 µm in medium for 30 min, followed by three washes in DMEM. Di[O] is a membrane intercalating lipid and gives granular cytoplasmic fluorescence with similar absorption and emission wavelengths to fluorescein. Di[O] is an accepted stable cell label [24] used in studies of cytotoxicity [25] and of vascular cells [26]; 103 mesangial cells were added to 104 activated macrophages and co-cultured for 48 h in 200 µl/well 10% FCS DMEM + AAG. At the end of incubation, cells were stained with propidium iodide (PI) and bisbenzimide (Hoechst 33258, H33258), both 10 µg/ml for 10 min at 37°C. PI is a DNA intercalating agent that stains only if there is loss of membrane integrity and H33342 is a DNA intercalating agent that stains all nuclei. Cells were fixed in 10% formaldehyde at 4°C for 24 h and examined with a fluorescence microscope. Cells were considered macrophages if they fluoresced green with the marker dye Di[O]. In contrast mesangial cells were non-fluorescent or had pale orange cytoplasmic autofluorescence. Mesangial cells were scored as apoptotic as before. Nuclei of live cells were large and pale blue with H33258. Nuclei were considered apoptotic if they were small and bright with H33258, which reflects chromatin condensation. Some of the nuclei of apoptotic cells stained additionally with propidium iodide, indicating increased membrane permeability seen in late apoptosis [27]. Apoptotic scores were counted on the number of condensed nuclei on H33258 staining. This assay is similar to a commercial kit (Vybrant, Molecular Probes Eugene, OR, USA) used to assess macrophage-induced apoptosis.
Biochemical assays for cell death in co-culture
The choice of death assays for use in co-culture is limited by the need to distinguish mesangial (target cell) death from macrophage (effector cell) death. With the microscopy assay, this was carried out by labelling the macrophages green fluorescent. Conversely, the two related biochemical assays were based on green fluorescent loading of viable target cells.
Calcein release assay for cell death in co-culture
The calcein release assay is conceptually similar to the 51Cr release assay. Briefly, mesangial cells are loaded with fluorescent dye calcein by incubating the cells with calcein-acetoxymethylester (calcein-AM). Calcein-AM is membrane permeant and cleaved by intracellular esterases into the non-permeant fluorescent form calcein, which is trapped in the cell. Calcein fluoresces at the same emission and absorption wavelengths as fluorescein. When the cells die, they release calcein. Therefore the amount of cell death is calculated from supernatant fluorescence, using the same equation used for the 51Cr release assay.
HMCs were trypsinized into suspension and washed twice in PBS by centrifugation and resuspension. HMCs were then incubated in 100 µm calcein-acetoxymethylester (Sigma) for 30 min at 37°C 5% CO2. The calcein-loaded cells were then washed twice in phosphate buffered saline (PBS) and added to 96-well plates at 104 cells per well (cpw). U937 cells were washed twice in PBS and added to the wells at 105 cpw. The cells were incubated in 100 µl/well phenol-red free medium for 4 h at 37°C 5% CO2 and then 50 ml medium was aspirated, transferred to a second microtitre plate and fluorescence measured (Tecan Spectrofluor Plus, top mode fluorescence measurement, absorption 480 nm, emission 535 nm, 10 flashes). Maximum (control) release was in the presence of 10 µl 10% Triton X-100 per well, background was with HMCs only.
 |
Calcein retention assay for cell death in co-culture
The calcein retention assay is the converse of the calcein release assay. That is, if cells do not release calcein, they remain bright. This was refined by using the precursor dihydrocalcein-AM, which is converted to calcein in cells but is more stable in storage.
HMCs or VSMCs were trypsinized into suspension and washed twice in PBS by centrifugation and resuspension. HMCs or VSMCs were then incubated in 100 µm dihydro-calcein-acetoxymethylester (Molecular Probes) for 30 min at 37°C 5% CO2. The cells were then washed twice in PBS and added to 96-well plates at 104 cells per well (cpw). U937 cells were washed twice in PBS and added to the wells at 105 cpw. The cells were incubated in 100 µl/well phenol-red free medium for 4 h at 37°C 5% CO2. Then the medium was aspirated and fluorescence measured (Tecan Spectrofluor Plus, bottom mode fluorescence measurement, absorption 480 nm, emission 535 nm, 10 flashes, four reads per well, square format). Background fluorescence was in the presence of 10 µl 10% Triton X-100 per well and maximum (control) was with HMCs or VSMCs only.
Assessment of apoptosis
Electron microscopy, annexin-V flow cytometry and propidium iodide flow cytometry for hypodiploid DNA were carried out as before. For flow cytometry for active caspase 3, cells were fixed in 1% paraformaldehyde 4°C 15 min, permeabilized in 1% Triton X-100 4°C 15 min, washed in Tris-buffered saline (TBS) × 2, stained with 1 : 100 monoclonal rabbit antiactive caspase 3 (Pharmingen, San Diego, CA, USA) for 30 min, stained with 1 : 100 antirabbit-PE for 30 min, washed in TBS × 2 and fixed in 10% formaldehyde fpr 30 min. Cells were analysed with a FACScan flow cytometer, cellquest for acquisition and WinMDI software for analysis.
Inhibitors and stimulants
Anti-Thy1·1 was clone ER4, mouse IgG2A (kind gift of Professor T. Cook) and was added to macrophage/mesangial co-cultures at the same time as the mesangial cells. Anti-CD16 (clone LNK-16, mouse IgG1, Abcam, Cambridge, UK) and anti-CD64 (clone 10·1, IDS Ltd, Newcastle, UK) were 1 mg/ml stock in azide-free PBS. Anti-Fas-L (clone NOK-1 mouse IgG1, Pharmingen) was 1 mg/ml stock in azide-free PBS. Control antibody was MOPC31C murine non-binding IgG1 (Sigma) in azide-free PBS. ICs were formed by mixing equal volumes of 1 mg/ml mouse IgG (Sigma) and 200 µg/ml rabbit antimouse (Sigma) at 4°C for 18 h, and added to macrophages at 1 : 200. Ionomycin (Sigma) was stock 1 mg/ml in PBS. Immobilized IgG was prepared by adding the indicated amount of IgG [human polyclonal IgG, Sandoglobulin (Sandoz, Basel, Switzerland) or murine IgG1 (clone), murine IgG2A (clone), murine IgG3 (clone) BLA36, AA1, Ks20–8, BNH-9 Dako, Glostrup, Denmark] in PBS to wells and incubating at 37°C for 48 h prior to the death assay.
Marker staining by flow cytometry
Binding of antibody to the surface of mesangial cells was assessed with flow cytometry. SV40HMCs were trypsinized to suspension, incubated with 10 µg/ml anti-Thy1·1 at 4°C for 30 min, washed and then incubated with 1 : 200 sheep-antimouse fluorescein. Thy1·1 is expressed by human and rat mesangial cells [28]. To detect α-smooth-muscle-actin, SV40-HMCs were permeabilized by fixing in 1% paraformaldehyde for 10 min then incubated in 0·1% Triton X-100 for 15 min, washed and incubated in 10 µg/ml anti-actin (clone α−1 − A4) washed and then incubated in sheep-antimouse fluorescein. Cells were then post-fixed in 4% formaldehyde at 4°C for 30 min and analysed by flow cytometry was as before.
Statistics
Statistical calculations were carried out on a PC with sigmastat (SPSS Software, Chicago, IL, USA). Data are graphed as mean ± standard error. Normally distributed data were significance tested by anova with adjustment for multiple comparisons with Bonferroni's inequality. Data that were not normally distributed were significance tested with Dunn's test (non-parametric anova, a modification of Wilcoxon that adjusts for multiple simultaneous comparisons). For dose–response analysis, the significance of observed trend was tested for with the χ2 test for trend.
RESULTS
Anti-Thy1·1 antibodies label human mesangial cells
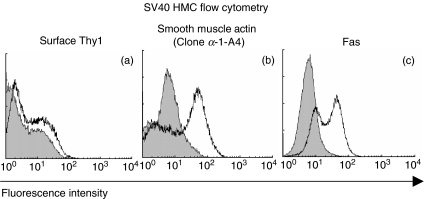
For direct ADCC, antibody must recognize the mesangial cell surface. Binding of antibody to the surface of mesangial cells was assessed with flow cytometry (Fig. 1). Anti-Thy1·1 (clone ER4) showed small but distinct amounts of binding to the surface of SV40HMCs (Fig. 1a) (Thy1·1 is expressed by rat mesangial cells and at low levels by human mesangial cells [28]). Permeabilized SV40HMCs showed specific labelling with antismooth-muscle-actin antibodies confirming a mesangial phenotype (Fig. 1b) [28]. The death receptor Fas is a candidate mediator of macrophage-induced mesangial cell apoptosis and was assessed. There was surface expression of Fas on SV40HMCs (Fig. 1c).
Fig. 1.
(a–d) Flow cytometry of SV40-HMCs. (a–c) For each histogram, x-axis, fluorescence intensity (FL1) on a 4-quadrant log scale, y-axis, cell number, leftward shaded histogram control, rightward open histogram specific staining. (a) Surface staining for Thy1 (clone ER4, gift of Professor T. Cook); (b) staining of permeabilized SV40-HMC for smooth muscle actin (clone α−1 − A4, Sigma); (c) surface staining of SV40-HMC for Fas (clone ♯F22120, Transduction Laboratories).
Fluorescence microscopy assay for cell death
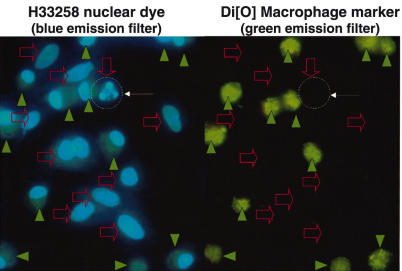
In macrophage/mesangial cell co-cultures, H33258 nuclear fluorochrome dye showed nuclear condensation, consistent with apoptosis (Fig. 2). Macrophages and mesangial cells could be distinguished by the green Di[O] fluorescence (Fig. 2). Nuclei of dead cells showed condensed and fragmented chromatin without staining for propidium iodide (Fig. 2), indicating apoptosis rather than primary necrosis, tested further (below).
Fig. 2.
Fluorescence microscopy of ADCC co-cultures. The two images are the same field photographed with different fluorescence filters. Co-cultures were carried out as detailed in Methods. Briefly, macrophages were selectively and stably labelled by incubation in Di[O], which is a membrane intercalating agent giving green granular cytoplasmic fluorescence. The macrophages were then co-cultured with the mesangial cells in the presence of antimesangial cell antibody Thy1·1 and nuclei of all cells were labelled with H33258, a blue florescent nuclear dye. With H33258 live nuclei are large and pale blue but apoptotic nuclei are small, bright and show nuclear fragmentation. As this fragmentation is blue and nuclear, it is not confused with the green cytoplasmic granularity of Di[O] staining. Filled green arrowheads: macrophages which are identified by the Di[O] green fluorescent granular cytoplasmic label that they were selectively incubated with. Open red arrows: nuclei of mesangial cells, which do not have Di[O] labelling so are not green fluorescent. These do not show on the green fluorescent channel. Fine white arrows and dashed circle: an apoptotic mesangial cell is identified by the lack of Di[O] green fluorescent labelling and by the characteristic condensed fragmented nuclear morphology. For the colour version of this figure, please see http://www.blackwell-synergy.com.
PMA-differentiated U937 macrophages show ADCC to mesangial cells (fluorescence microscopy assay)
ADCC was assessed first with U937-macrophages (Fig. 3a). For this we used a direct co-culture microscopy method (Methods). Briefly, by an extension of our previous methods [12–14], macrophages were labelled green for identification and nuclei were labelled with H33258. Apoptotic mesangial cells were defined as bright condensed nuclei that were not green.
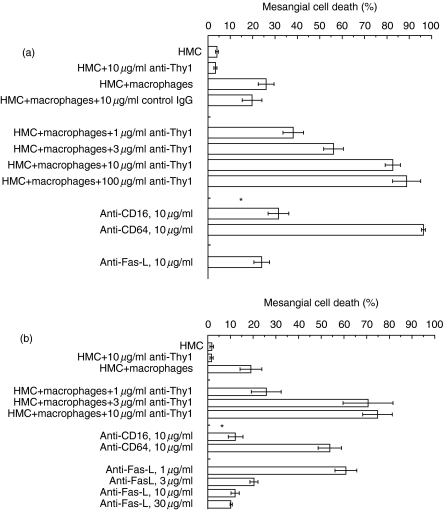
Fig. 3.
Mechanism of antibody-dependent killing of mesangial cells by macrophages. The assay details are in the Methods. Assay images are in Fig. 2. Briefly, macrophages were labelled with the green fluorescent dye Di[O] and excess dye washed off. Macrophages were then cultured with MCs in the presence or absence of antimesangial cell antibody anti-Thy1·1 (clone ER4) and nuclei stained with the blue fluorescent dye H33258. Mesangial cell death was the number of Di[O]-negative cells with apoptotic nuclei that divided by the total number of Di[O]-negative cells. (a) Antibody-dependent killing of mesangial cells by U937 macrophages. x-Axis, mesangial cell death index. Data are mean ± s.e.m.; y-axis, treatment. *Significantly different to macrophages MCs + 10 µg/ml anti-Thy1·1 antibody, P < 0·05, anova. (b) Antibody-dependent killing of mesangial cells by blood-derived macrophages. x-Axis, mesangial cell death index; y-axis, treatment. Data are mean ± S.E.M. * significantly different to macrophages + MCs + 10 µg/ml anti-Thy1·1 antibody, P < 0·05, anova.
U937-macrophages killed SV40HMCs, and this was increased in concentration-related manner by co-incubation with anti-Thy1·1. Killing was inhibited by antagonistic antibodies to Fas-L, antagonistic antibodies to CD16 (P < 0·05, Fig. 3a). Killing was not inhibited by anti-CD64 antibodies (P > 0·05, Fig. 3a).
Primary blood derived human macrophages show ADCC to mesangial cells (fluorescence microscopy assay)
To confirm relevance, studies with U937 macrophages were repeated with macrophages established by 24-h culture from human blood monocytes (Fig. 3b). This was performed as for the U937 macrophages. Briefly, macrophages were labelled green for identification, nuclei were labelled with H33258 and apoptotic mesangial cells were defined as bright condensed nuclei that were not green. Blood-derived macrophages killed SV40HMC mesangial cells, death increasing in a concentration-related manner with anti-Thy1·1. As with the U937 macrophages, killing was inhibited by antagonistic antibodies to Fas-L, antagonistic antibodies to CD16. Killing was not inhibited by anti-CD64 antibodies (P > 0·05, Fig. 3b).
Macrophage–mesangial cell ADCC is by apoptosis
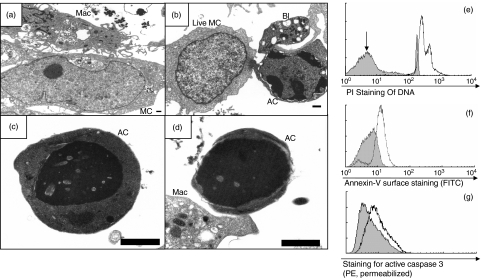
Whether ADCC involved apoptosis or necrosis was determined. Electron microscopy of co-cultures showed the classical features of apoptosis (Fig. 4a). There was early cytoplasmic and membrane blebbing with peripheral electron-dense chromatin condensation (Fig. 4b). In the later stages, there was uniform electron-dense nuclear condensation and cytoplasmic condensation (Fig. 4c), with loss of cytoplasm to form a corpse comprising mainly condensed nucleus (Fig. 4d). There was no evidence of primary necrosis (Fig. 4a).
Fig. 4.
Evidence that ADCC involves apoptosis. (a–d) Electron microscopy. In each micrograph, a 1 µm scalebar is shown (the images are a different magnifications to show different features most clearly). (a) A macrophage (Mac) with numerous processes contacts a mesangial cell (MC). (b) A live mesangial cell (live MC) contrasts with a cell in early apoptosis (AC) showing peripheral chromatin condensation and marked blebbing (Bl). Blebbing is characteristic of apoptosis. (c) An apoptotic cell showing electron-dense chromatin condensation and cytoplasmic collapse (contrasting with necrosis in which there is cytoplasmic swelling). (d) A late apoptotic cell (AC) which shows electron-dense chromatin condensation and cytoplasmic condensation but has lost most of the cytoplasm. (e, f, g) Flow cytometry. Data are representative of n = 3 experiments each. (e) PI quantification of cell DNA. Cells were fixed and permeabilized in ethanol prior to PI staining. x-Axis, fluorescence intensity with PI (FL2-H, 4 quadrant log scale), y-axis, cell number. Shaded histogram, control mesangial cells. Open histogram, ADCC co-culture. A hypodiploid peak is indicated in the co-culture (arrow). The fraction of hypodiploid cells in the control SV40-HMC co-cultures is approximately 1% and in co-cultures is approximately 90%. (f) Annexin-V-FITC staining, shaded histogram, control staining; open histogram, annexin-V staining. (g) Active caspase-3 staining, shaded histogram, control staining, open histogram, anti-caspase-3 staining.
DNA flow cytometry showed a hypodiploid peak confirming DNA cleavage, consistent with apoptosis (Fig. 4e). Annexin-V and the active site of caspase 3 are both exposed in apoptosis. Surface annexin-V binding was positive in ADCC co-cultures, indicating apoptosis (Fig. 4f). ADCC co-cultures showed specific labelling for antibodies to active caspase 3, also indicating apoptosis (Fig. 4g).
On fluorescence microscopy, dead cells showed chromatin condensation and fragmentation with H33258 in the absence of PI-staining, indicating death with intact cell membranes, indicating apoptosis (Fig. 2). However, late in the apoptotic process, cells become permeable and therefore PI-positive, a well-recognized pattern. Thus, PI stains late apoptotic nuclei in addition to H33258, allowing discrimination of early apoptotic cells and late apoptotic cells. Therefore the proportion of early apoptotic (condensation on H33258, PI-negative) and late apoptotic (condensation on H33258, PI-positive) was evaluated over a time-course. Results are expressed as time; % total mesangial cells ± s.e.m. (n = 8 experiments); 1 h: 82 ± 5% live; 18 ± 5% early apoptotic; 0 ± 0% late apoptotic; 14 h: 54 ± 4·8% live; 46 ± 4·9% early apoptotic; 0·2 ± 0·2% late apoptotic; 48 h: 28 ± 3·9% live; 9·4 ± 3·7% early apoptosis; 61 ± 7% late apoptosis. That is, the expected pattern is obtained with early apoptosis seen early and late apoptosis seen later.
Thus apoptosis is seen in macrophage/mesangial cell co-cultures.
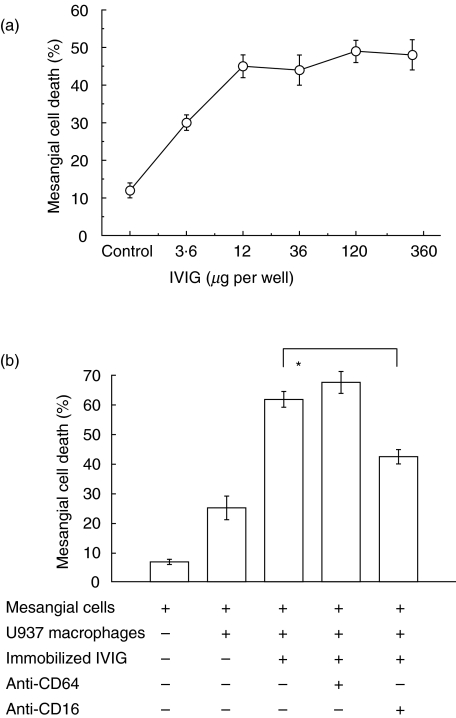
Immobilized polyclonal human IgG enhances macrophage-induced mesangial cell apoptosis via CD16 (fluorescence microscopy assay)
This evidence suggested that macrophages kill mesangial cells when stimulated by antibodies to target cells. However, this limited the antibodies studied to those that react with the target cells, restricting the species and isotype, which could have influenced the requirement for CD16. To overcome this, we studied the effect of precoating wells with immobilized IgG on macrophage-induced apoptosis. We started with polyclonal human IgG (a more natural ligand for human CD16) (Fig. 5). The assay was otherwise performed as for Fig. 3. Briefly, macrophages were labelled green fluorescent with Di[O] then co-cultured with mesangial cells, nuclei stained with H33258 and read microscopically.
Fig. 5.
Immobilized human polyclonal IgG stimulates macrophages to kill human mesangial cells dependent on CD16. (a) y-Axis, % mesangial cell death measured as for Fig. 3; x-axis, amount of human polyclonal IgG adhered to glass microwell. (b) y-Axis, % mesangial cell death measured as for Fig. 3; x-axis, treatments were added as indicated by (+). *P < 0·05 antagonistic anti-CD16 antibodies reduced mesangial cell death compared to control (Dunn's test).
This showed a dose-dependent increase in macrophage-induced SV40HMC apoptosis with the amount of immobilized human polyclonal IgG, with a maximum effect of approximately 50% death (Fig. 5a). Whether this effect was also mediated via CD16 was studied using the same inhibitory antibodies as before (Fig. 5b). Under conditions of culture on immobilized IgG, inhibitory anti-CD16 antibodies reduced SV40HMC apoptosis but inhibitory anti-CD64 antibodies had no significant effect (Fig. 5b). This indicated that our results implicating CD16 but not CD64 in ADCC using a mouse IgG2A antibody were relevant to human IgG.
To examine further the influence of isotype on ADCC, the indirect ADCC model was extended to mouse immunoglobulin. Wells were precoated with 10 ng/well of mouse immunoglobulin of different isotypes, similar to the human immunoglobulin experiment, and mesangial cell death was measured as before. Mesangial cell death was as follows (mean ± s.e.m.): mesangial cells only 6·7 ± 0·9%; macrophages + mesangial cells 25 ± 4%; and macrophages + mesangial cells in the presence of 10 ng/well immobilized immunoglobulins, mouse IgM 49·5 ± 1·3%; mouse IgG1 67·3 ± 4·6%; mouse IgG2 35 ± 2·7%; mouse IgG3 58·3 ± 4·1%. This indicated that mouse IgG2 stimulated human macrophage-induced killing, although less strongly than mouse IgG1 or mouse IgG3 (P < 0·05, Dunn's test).
Immobilized polyclonal human IgG enhances macrophage-dependent release of indicator dye from target cells
We next tested whether these data obtained by microscopical examination could be corroborated biochemically using selective dye loading of target cells. Precoating the wells with human polyclonal IgG enhanced macrophage-induced mesangial cell lysis in a dose-dependent manner, when assessed by measuring calcein released into the supernatant [data are percentage specific lysis mean ± s.e.m. (calculated as in methods) U937 cells 37% ± 1·1%; U937 cells plus 1 ng IgG 31% ± 2·7%; U937 cells plus 10 ng IgG 68% ± 2·4%; U937 cells plus 100 ng IgG 74% ± 1·1%; U937 cells plus 1 µg IgG 81% ± 3·6%; representative of n = 3 experiments]. Incubation with anti-CD16 antibodies (clone LNK16, 10 µg/ml) reduced mesangial cell lysis compared with control mouse monoclonal IgG [P < 0·05, Student's t-test, data are percentage-specific lysis mean ± s.e.m. (calculated as in Methods) U937 cells plus 100 ng immobilized human IgG + control soluble mouse IgG1 antibody 16% ± 0·4%; U937 cells plus 100 ng immobilized human IgG + anti-CD16 4% ± 0·1%, representative of n = 3 experiments]. Thus the HMC calcein assay corroborates the HMC microscopy data that antibody stimulates macrophage-induced apoptosis via CD16.
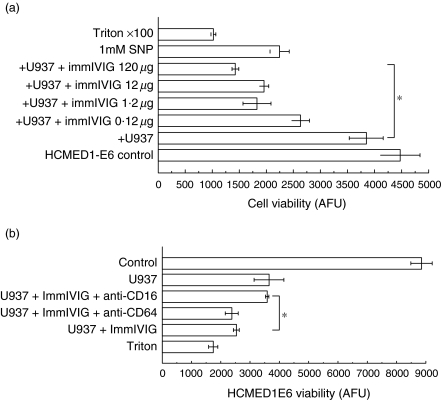
Next, similar experiments were carried out with HCMED1-E6 VSMCs as targets. This was to corroborate both with a biochemical assay and with a cell line that we have characterized previously to show macrophage-induced apoptosis representative of non-immortalized VSMCs. The VSMCs were loaded with dihydrocalcein-AM. Precoating the wells with human polyclonal IgG enhanced macrophage-induced VSMC lysis as assessed by measuring calcein retained by the cells (Fig. 6a). Incubation with anti-CD16 antibodies (clone LNK16, 10 µg/ml) reduced HCMED1E6 VSMC lysis compared with control mouse monoclonal IgG (Fig. 6b), similar to HMCs.
Fig. 6.
Immobilized human polyclonal IgG stimulates macrophages to kill human VSMCs dependent on CD16. VSMCs were loaded with calcein (intracellular fluorescent dye) prior to addition to macrophages and VSMC viability was measured as the retained calcein in arbitrary fluorescence units (AFU) after 4 h co-incubation. (a) x-Axis, HCMED1-E6 viability measured as retained calcein fluorescence (arbitrary fluorescence units, AFU); y-axis, macrophages (U937) and increasing amounts of immobilized human polyclonal IgG were added as indicated. *P < 0·05, χ2 for trend. Triton X-100 (0·1% final) or sodium nitroprusside (1 mm) were added as positive controls. (b) x-Axis, HCMED1-E6 viability measured as retained calcein fluorescence (arbitrary fluorescence units); y-axis, macrophages (U937), immobilized human polyclonal IgG and antagonistic anti-CD16 or anti-CD64 or control soluble mouse IgG1 were added as indicated. Triton X-100 (0·1% final) was a positive control. *P < 0·05 antagonistic anti-CD16 antibodies increased VSMC viability compared to control (Dunn's test).
Human macrophage Fas-L is regulated by immune complexes
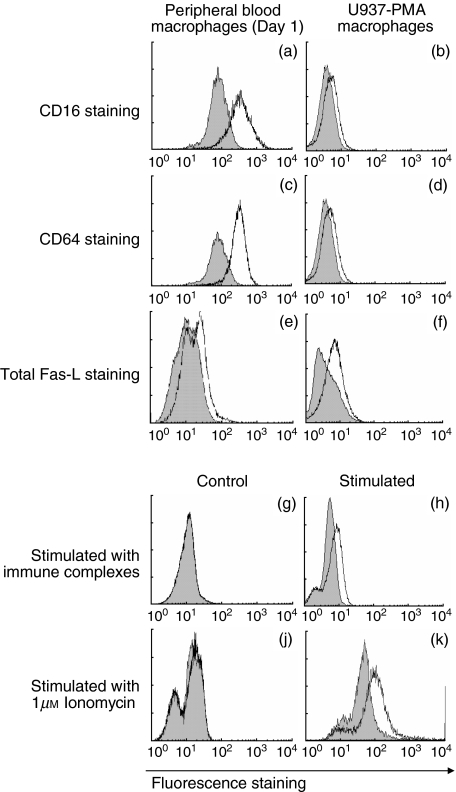
ADCC was inhibited by antagonistic antibodies to Fas-L, CD16 but not by antagonistic antibodies to CD64. Flow cytometry was used to confirm that these were expressed on macrophages (Fig. 7a–d). Both sources of macrophages, peripheral blood macrophages and PMA-differentiated U937 cells, expressed surface CD16 and CD64 at the times added to ADCC cultures (Fig. 7a). Thus, although inhibiting only CD16 reduced ADCC, both CD16 and CD64 were available.
Fig. 7.
Flow cytometry of macrophages. For each histogram, x-axis, fluorescence intensity (FL1, 4-decade log scale); y-axis, cell number; leftward shaded histograms, control antibody, rightward open histograms, positive staining. Cell culture and staining are in Methods section. (a–d) Surface staining of macrophages for FcγRs CD16 and CD64. (a, c) Macrophage cultured from peripheral blood (culture day 1); (b, d) PMA-differentiated U937 macrophages. (a, b) Staining for CD16, (c, d) Staining for CD64. (e, f) Staining for Fas-L of permeabilized macrophages; (e) cultured from peripheral blood (culture day 1) or (f) PMA-differentiated U937 cells (f). This detects total cellular Fas-L (surface and intracellular). (g–j) Stimulation of macrophage surface Fas-L G-K are all surface staining for Fas-L on (unpermeabilized) macrophages cultured from peripheral blood (culture day 1). (g, j) Unstimulated (control) macrophages. No surface Fas-L staining is seen. (h, k) Macrophages incubated with immune complexes (2 µg/ml) for 24 h (h) or with 1 µm of the calcium channel ionomycin for 10 min (k).
As before [13], there was no surface staining for Fas-L on blood-derived macrophages at the times examined (culture day 1) (not shown). However, permeabilization of the cells revealed an intracellular pool of stainable Fas-L (Figs 7e,f).
We have shown previously that human macrophages acquire an apoptosis-inducing phenotype after 5 days in culture by up-regulating surface Fas-L from an intracellular compartment. We tested whether stimulating the FcRs or their signalling pathways modulated macrophage surface Fas-L (Fig. 7g–k). Surface Fas-L on human blood-derived macrophages was up-regulated by 24 h co-culture with 24 h exposure to 1 µg/ml IgG soluble immune complexes added to crosslink FcγRs (Figs 7g,h). Stimulating macrophages with 1 µm of the fungal calcium channel ionomycin for 10 min also up-regulated surface Fas-L (Figs 7j,k).
DISCUSSION
Immune complex glomerulonephritis is often triggered by IgG immune complexes and involves macrophages, which mediate tissue destruction [3]. Thus we investigated whether human macrophages would kill human cells when stimulated by antibody. Macrophages killed cells via apoptosis increased in a dose-related manner by antibody and inhibited by antagonists of Fas-L, nitric oxide and CD16.
Comparing antagonistic antibodies to CD16 and CD64, we found a strong selective role for CD16, the low-affinity FcγR, in macrophage ADCC, over the high-affinity FcγR, CD64. Although the anti-Thy1·1 antibody used in some of the death assays was mouse isotype IgG2A is not what human macrophages would be expected to see physiologically; we then corroborated the findings more extensively. Thus we found CD16 utilization using (1) U937 macrophages and IgG2a-bound to both human mesangial cells assessed microscopically; (2) peripheral blood macrophages and IgG2a-bound to both human mesangial cells assessed microscopically; (3) immobilized human polyclonal IgG stimulating U937 macrophages killing human mesangial cells assessed microscopically; (4) immobilized human polyclonal IgG stimulating U937 macrophages killing human mesangial cells assessed biochemically; (5) immobilized human polyclonal IgG stimulating U937 macrophages killing human VSMCs assessed biochemically. Thus the role for CD16 in activating cytotoxicity of human macrophages in response to insoluble IgG has been a consistent finding thus far.
The selective involvement of the low-affinity receptor (CD16) over the high-affinity receptor (CD64) is at first surprising, but perhaps understandable. CD16 has very little avidity for monomeric IgG, but binds avidly to IgG immune complexes, because they are multimeric [9]. Thus insoluble IgG may represent a multimeric ligand giving high CD16 binding avidity.
The concentration-dependent inhibition of ADCC by antagonistic anti-Fas-L antibody NOK-1 occurred over the concentration range typical for Fas-L-dependent death in the literature [13]. Cell-surface Fas-L ligates target cell Fas inducing caspase-mediated apoptosis and mediates macrophage-induced apoptosis of human VSMCs [13] and rodent mesangial cells [11]. Thus these data are a logical extension of previous findings to antibody-dependent killing of human glomerular cells in vitro. Consistent with this, U937 macrophages and blood-derived macrophages expressed Fas-L and mesangial cells expressed Fas.
Because macrophage dependent killing was Fas-L-mediated we assessed apoptosis and necrosis in ADCC co-cultures. By electron microscopy, cells had the typical features of apoptosis without evidence of primary necrosis. Electron microscopy morphology is specific for apoptosis: indeed, apoptosis was originally defined by its E.M. features [29]. This was borne out by multiple modern apoptosis tests. Thus DNA flow cytometry, annexin-V binding, caspase-3 active site detection, fluorochrome staining all supported an apoptotic death pathway. In a time-course study short co-cultures had mainly early apoptotic cells (morphologically fragmented, but PI-negative, indicating intact membranes), while prolonged (48 h) co-cultures some late apoptotic cells (PI-positive) could be detected, consistent with a primarily apoptotic process. Thus we concluded that ADCC invoked by macrophages is apoptotic, as with natural killer (NK) cells.
We found Fas-L-mediated antibody-dependent killing with macrophages at culture days 1–2. At this stage, they expressed intracellular Fas-L but did not spontaneously express surface Fas-L as before [13]. However, macrophage Fas-L was up-regulated by IgG immune complexes, which activate macrophages via FcγRs, and by calcium ionophore, which elevates intracellular free calcium, important in FcR post-receptor signalling [30]. Similarly, in NK cells and T lymphocytes, surface Fas-L is elevated by calcium ionophores.
There are always pitfalls in extending in vitro data to the in vivo situation. However, crescentic glomerulonephritis, its most severe form, is characterized by infiltrates of activated macrophages and by mesangial cell apoptosis. We suggest that this model may yield insights into the mechanism of crescentic glomerulonephritis.
In conclusion, this work confirms and extends the findings that macrophages induce Fas-L-mediated rodent mesangial cell apoptosis [11] to antibody stimulation of human macrophages via CD16.
Acknowledgments
I thank the following: Hammersmith Hospital Trust Research Committee for financial support; Dr Jilly Moss for help with E.M. Professor T. Cook discussed the work helpfully and commented on the manuscript. Dr Jan Domin kindly provided SV40HMCs. Dr S. Shaunak and Dr A. Warrens provided spare buffy coat PBMCs. Dr A. Warrens helped with flow cytometry. Prof. M. Bennett provided HCMED1-E6 VSMCs.
References
- 1.Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl. 5):3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- 2.Huang XR, Tipping PG, Apostolopoulos J, et al. Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol. 1997;109:134–42. doi: 10.1046/j.1365-2249.1997.4091307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipping PG, Kitching AR, Cunningham MA, Holdsworth SR. Immunopathogenesis of crescentic glomerulonephritis. Curr Opin Nephrol Hypertens. 1999;8:281–6. doi: 10.1097/00041552-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cook HT, Singh SJ, Wembridge DE, Smith J, Tam FW, Pusey CD. Interleukin-4 ameliorates crescentic glomerulonephritis in Wistar Kyoto rats. Kidney Int. 1999;55:1319–26. doi: 10.1046/j.1523-1755.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- 5.Lai PC, Cook HT, Smith J, Keith JC, Jr, Pusey CD, Tam FW. Interleukin-11 attenuates nephrotoxic nephritis in Wistar Kyoto rats. J Am Soc Nephrol. 2001;12:2310–20. doi: 10.1681/ASN.V12112310. [DOI] [PubMed] [Google Scholar]
- 6.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–4. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 7.Tarzi RM, Davies KA, Claassens JW, Verbeek JS, Walport MJ, Cook HT. Both Fcgamma receptor I and Fcgamma receptor III mediate disease in accelerated nephrotoxic nephritis. Am J Pathol. 2003;162:1677–83. doi: 10.1016/s0002-9440(10)64302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarzi RM, Davies KA, Robson MG, et al. Nephrotoxic nephritis is mediated by Fcgamma receptors on circulating leukocytes and not intrinsic renal cells. Kidney Int. 2002;62:2087–96. doi: 10.1046/j.1523-1755.2002.00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 10.Fossati G, Moots RJ, Bucknall RC, Edwards SW. Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum. 2002;46:1351–61. doi: 10.1002/art.10230. [DOI] [PubMed] [Google Scholar]
- 11.Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 2000;164:2110–9. doi: 10.4049/jimmunol.164.4.2110. [DOI] [PubMed] [Google Scholar]
- 12.Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23:1553–8. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- 13.Boyle JJ, Bowyer DE, Weissberg PL, Bennett MR. Human blood-derived macrophages induce apoptosis in human plaque-derived vascular smooth muscle cells by Fas-ligand/Fas interactions. Arterioscler Thromb Vasc Biol. 2001;21:1402–7. doi: 10.1161/hq0901.094279. [DOI] [PubMed] [Google Scholar]
- 14.Boyle JJ, Weissberg PL, Bennett MR. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler Thromb Vasc Biol. 2002;22:1624–30. doi: 10.1161/01.atv.0000033517.48444.1a. [DOI] [PubMed] [Google Scholar]
- 15.Seshiah PN, Kereiakes DJ, Vasudevan SS, et al. Activated monocytes induce smooth muscle cell death: role of macrophage colony-stimulating factor and cell contact. Circulation. 2002;105:174–80. doi: 10.1161/hc0202.102248. [DOI] [PubMed] [Google Scholar]
- 16.Vasudevan SS, Lopes NH, Seshiah PN, et al. Mac-1 and Fas activities are concurrently required for execution of smooth muscle cell death by M-CSF-stimulated macrophages. Cardiovasc Res. 2003;59:723–33. doi: 10.1016/s0008-6363(03)00514-5. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Meth. 1994;172:227–39. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 18.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–5. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mene P, Pugliese F, Cinotti GA. Adhesion of U-937 monocytes induces cytotoxic damage and subsequent proliferation of cultured human mesangial cells. Kidney Int. 1996;50:417–23. doi: 10.1038/ki.1996.331. [DOI] [PubMed] [Google Scholar]
- 20.Wang XM, Terasaki PI, Rankin GW, Jr, Chia D, Zhong HP, Hardy S. A new microcellular cytotoxicity test based on calcein AM release. Hum Immunol. 1993;37:264–70. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Meth. 1994;177:101–11. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 22.Oral HB, George AJ, Haskard DO. A sensitive fluorometric assay for determining hydrogen peroxide-mediated sublethal and lethal endothelial cell injury. Endothelium. 1998;6:143–51. doi: 10.3109/10623329809072201. [DOI] [PubMed] [Google Scholar]
- 23.Roden MM, Lee KH, Panelli MC, Marincola FM. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. J Immunol Meth. 1999;226:29–41. doi: 10.1016/s0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 24.Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12:333–1. [PubMed] [Google Scholar]
- 25.Toes RE, den Haan JM, Feltkamp MC, et al. In vivo detection of fluorescent tumor-specific cytotoxic T cell clones. J Immunol Meth. 1993;163:23–32. doi: 10.1016/0022-1759(93)90235-y. [DOI] [PubMed] [Google Scholar]
- 26.Ragnarson B, Bengtsson L, Haegerstrand A. Labeling with fluorescent carbocyanine dyes of cultured endothelial and smooth muscle cells by growth in dye-containing medium. Histochemistry. 1992;97:329–33. doi: 10.1007/BF00270034. [DOI] [PubMed] [Google Scholar]
- 27.Zamai L, Canonico B, Luchetti F, et al. Supravital exposure to propidium iodide identifies apoptosis on adherent cells. Cytometry. 2001;44:57–64. [PubMed] [Google Scholar]
- 28.Holthofer H, Sainio K, Miettinen A. The glomerular mesangium: studies of its developmental origin and markers in vivo and in vitro. APMIS. 1995;103:354–66. [PubMed] [Google Scholar]
- 29.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]