Abstract
Pauci-immune idiopathic small-vessel vasculitis is strongly associated with the presence of antineutrophil cytoplasm autoantibodies (ANCA). Antibodies to PR3 predominate in patients with Wegener's granulomatosis; antibodies to myeloperoxidase (MPO) are found more frequently in patients with microscopic polyangiitis. There is increasing in vivo and in vitro evidence for a pathogenic role of ANCA in systemic vasculitis based on associations of ANCA with disease activity. If ANCA are pathogenic, why is the course of disease different from one patient to another? Antibodies can recognize different binding sites (epitopes) on their corresponding antigens. Differences in binding specificity may influence the pathogenic potential of the antibodies. Differences between epitope specificity of ANCA between patients or changes in epitope specificity of ANCA in time in an individual patient may, accordingly, result in differences in disease expression. This review will focus on epitope specificity of autoantibodies in systemic autoimmune diseases and especially on the epitope specificity of PR3– and MPO–ANCA. We will discuss whether PR3–ANCA or MPO–ANCA recognize different epitopes on PR3 and MPO, respectively, and whether the epitopes recognized by ANCA change in parallel with the disease activity of ANCA-associated vasculitis. Finally, we will speculate if the direct pathogenic role of ANCA can be ascribed to one relapse- or disease-inducing epitope. Characterization of relapse- or disease-inducing epitopes bound by PR3–ANCA and MPO–ANCA is significant for understanding initiation and reactivation of ANCA-associated vasculitis. Elucidating a disease-inducing epitope bound by ANCA may lead to the development of epitope-specific therapeutic strategies.
Keywords: ANCA, ANCA-associated vasculitis epitope spreading, MPO, PR3
INTRODUCTION
Pauci-immune idiopathic small-vessel vasculitis is strongly associated with the presence of antineutrophil cytoplasm autoantibodies (ANCA). In the context of idiopathic vasculitis, ANCA with a cytoplasmic staining pattern by indirect immunofluorescence (c-ANCA) are generally directed against proteinase 3 (PR3), while a perinuclear staining pattern (p-ANCA) is often produced by antibodies to myeloperoxidase (MPO). These target antigens of ANCA are both located in the azurophilic granules of neutrophils and monocytes [1]. Antibodies to PR3 predominate in patients with Wegener's granulomatosis (WG); antibodies to MPO are found more frequently in patients with microscopic polyangiitis (MPA), although these associations are not absolute [2].
There is increasing in vivo evidence for a pathogenic role of ANCA in systemic vasculitis based on associations of ANCA with disease activity. Patients who are persistently or intermittently ANCA-positive during remission are prone to develop relapses [3–5] and in many cases titres of ANCA rise prior to a relapse of WG or MPA [6–9]. In addition, treatment based on changes in ANCA titres was shown to prevent the development of relapses in patients with ANCA-associated vasculitis [9,10].
Over the last decade numerous in vitro data support a direct pathogenic role of ANCA in systemic vasculitis. Following priming of neutrophils, binding of ANCA to either MPO or PR3 on the neutrophil surface leads to neutrophil activation, manifested as oxygen radical production and degranulation which may induce tissue necrosis [11–13]. Furthermore, in vitro interaction between leucocytes, ANCA and endothelial cells can result in damage to the latter cells by toxic products released from the activated leucocytes [14–16]. Recently, an animal model for MPO–ANCA-associated vasculitis has been described that strongly supports a pathogenic role for MPO–ANCA in glomerulonephritis and vasculitis. Antibodies to murine MPO were generated by immunization of MPO knock-out mice with murine MPO. Purified IgG of these mice was transferred to B and T cell-deficient Rag2 knock-out and wild-type mice, which developed mild necrotizing and crescentic glomerulonephritis after a single injection with anti-MPO antibodies [17].
If ANCA are pathogenic, why is the course of disease different from one patient to another? Antibodies can recognize different binding sites (epitopes) on their corresponding antigens. Differences in binding specificity may influence pathogenic potential of the antibodies. Differences between epitope specificity of ANCA between patients or changes in epitope specificity of ANCA in time in an individual patient may, accordingly, result in differences in disease expression. This review will focus on epitope specificity of autoantibodies in systemic autoimmune diseases, and especially on epitope specificity of PR3– and MPO–ANCA. We will discuss whether PR3–ANCA or MPO–ANCA recognize different epitopes on PR3 and MPO, respectively, and whether the epitopes recognized by ANCA change in parallel with disease activity of ANCA-associated vasculitis. Finally, we will speculate if the direct pathogenic role of ANCA can be ascribed to one relapse- or disease-inducing epitope.
EPITOPE SPREADING IN ANTIBODY-MEDIATED AUTOIMMUNE RESPONSES
Autoimmune diseases are considered to result from breakdown of self-tolerance, manifested by the appearance of autoreactive B and T lymphocytes. Systemic autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and ANCA-associated vasculitis (AAV), result from the emergence of both autoreactive T and B cells. Based on the observation that the autoimmune response is not generalized but is directed specifically to one or a restricted number of autoantigens, it has been proposed that molecular mimicry between these antigens and microbial antigens might be one of the pathogenic mechanisms in the induction of autoimmune disease. Exposure to microbial antigens that display conformational epitopes closely resembling those of the autoantigen may elicit an antibody response that cross-reacts with epitopes on the autoantigen [18]. Whereas molecular mimicry is considered a trigger for autoimmunity, epitope spreading has been described as an important factor explaining the diversification and amplification of autoimmunity in an individual. Epitope spreading involves the acquired recognition of new epitopes within the same molecule (intramolecular epitope spreading) as well as epitopes residing in proteins that are associated in the same macromolecular complex (intermolecular epitope spreading) [19–21].
Evidence that autoimmune responses in systemic autoimmune diseases are dynamic with evolving specificities has been demonstrated mainly for SLE. Here, B cell epitope spreading from a disease-inducing epitope to other areas of the autoantigen (intramolecular epitope spreading) and other autoantigens (intermolecular epitope spreading) have been described [22–25]. With regard to the molecular mimicry model proposed, it is interesting to note that antibodies elicited by the Epstain–Barr (EBV) viral antigen EBNA-2 may cross-react with the D1 peptide of Sm autoantigen, thus suggesting a role for EBV-specific immune responses in the development of SmD1 autoantibodies in SLE patients [26,27]. Therefore, it seems that in SLE autoreactive B cell specificities may originate from one epitope, cross-reactive with an epitope present on microbial antigens, followed by epitope spreading to other areas of the same or different autoantigens. Is the first epitope recognized by antibodies in patients with SLE a disease-inducing epitope? Immunization of experimental animals with a peptide that comprises one of the earliest B cell epitopes in the anti-Sm response found in humans with SLE leads to autoantibody production to other autoantigens described in SLE, among which are antibodies to native DNA. The production of these autoantibodies was associated with the development of symptoms of SLE [23,28].
If, in SLE, mechanisms as molecular mimicry and epitope spreading are important pathogenic mechanisms, could this also be the case in other systemic autoimmune diseases, such as ANCA-associated vasculitis? For AAV a new intriguing pathogenic mechanism was proposed for initiation and progression of AAV. Autoimmunity to PR3 could be initiated through an immune response against the antisense complementary peptide (amino acids 87–172, Fig. 1) of PR3. Antibodies to this complementary PR3 could, through idiotype–anti-idiotype response, induce antibodies to the idiotype of anti-complementary PR3 antibodies. These anti-idiotypic antibodies were shown to cross-react with PR3 and could thus be considered autoantibodies [29]. Parts of the complementary PR3 sequence were also present in microbial antigens, suggesting that molecular mimicry is also an important pathogenic mechanism in AAV. Therefore, in AAV epitope spreading and molecular mimicry also seem important pathogenic mechanisms.
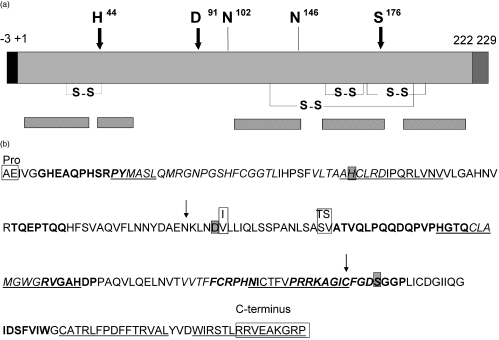
Fig. 1.
The protein structure of PR3. (a) The amino acids, H44, D91 and S176 forming the catalytic triad are indicated with arrows and the two glycosylation sites on N102 and N146 are indicated with lines [74]. SS indicates disulphide bridges. Bars below the protein indicate the location of identified epitopes recognized by PR3–ANCA. (b) Amino acid sequence of PR3 with areas that are surface exposed and have been identified as epitopes recognized by PR3–ANCA from patients with WG. Shown in bold, epitopes identified by Williams et al. [45]; italic epitopes were identified by van der Geld et al. [47]; underlined epitopes were identified by Griffith et al. [46]. In the grey boxes are the amino acids forming the catalytic triad of PR3. In white boxes are the pro- en C-terminal sequence. These sequences are removed upon processing of PR3 to a mature proteolytically active protein. Also in boxes are the two polymorphisms described in the PR3 sequence [74–76]. Arrows indicate the start and end of the sequence corresponding to the complementary peptide of PR3 [29].
CLUES FOR DIFFERENCES IN EPITOPES RECOGNIZED BY ANCA
Several functional characteristics of PR3– as well as MPO–ANCA have been described. Changes in functional characteristics of ANCA occur upon disease progression and may indicate that different epitopes are recognized by ANCA. PR3–ANCA can interfere with the proteolytic activity of PR3 [30–32] and with the binding of PR3 to its physiological inhibitor α1-antitrypsin (α1-AT) [30–33]. Interestingly, disease activity in WG appeared to be related closely to these interfering capacities of PR3–ANCA. Disease activity in WG was related even more closely to these capacities of PR3–ANCA than the titre of these antibodies [31–33]. This, indeed, may indicate that changes in functionality of ANCA during the course of the disease are the result from changes in epitope specificity. PR3–ANCA of most patients during an active phase of WG interfere with the enzymatic activity of PR3 and with PR3/α1-AT complexation, suggesting that PR3–ANCA bind common epitopes which are, at least initially, linked to the active site of PR3.
In contrast to PR3–ANCA, MPO–ANCA do not interfere with the enzymatic activity of MPO [34–37]. Nevertheless, a similar interference to that seen for PR3–ANCA and α1-AT has been reported for MPO–ANCA and its complexation with ceruloplasmin, the physiological inhibitor of MPO [36,38]. MPO–ANCA of patients with MPA interfered with the inhibition of MPO by ceruloplasmin, while MPO–ANCA of patients with WG produced a much less marked effect [36]. This may reflect differences in epitope specificity between both clinical conditions associated with the same antibody. The majority of MPO–ANCA of MPA patients did not inhibit the enzymatic activity of MPO, suggesting that a major epitope may be close to but not involving the active site.
The aforementioned findings suggest that differences in pathogenic potential of ANCA and differences in disease expression are related to differences in epitope specificity of the antibodies.
EPITOPES RECOGNIZED BY PR3–ANCA
PR3 is a serine proteinase of 29–32 kDa [39,40]. It is a highly folded protein with four disulphide bridges keeping its 3D structure intact [41]. PR3 is processed into a mature form consisting of 222 amino acids [42,43]. First, the N-terminal propeptide of two amino acids of PR3 is removed. After removal of the propeptide the substrate-binding pocket becomes accessible and PR3 becomes potentially enzymatically active. A seven amino acids C-terminal extension is removed during processing of PR3 (Fig. 1a).
Over the last 10 years several attempts have been made to characterize the interactions between ANCA and PR3. Elucidation of the epitopes on PR3 recognized by PR3–ANCA has been hampered by the fact that the majority of PR3–ANCA recognize conformational epitopes [44]. Antibody binding is abrogated by exposure of PR3 to low pH or by reducing its disulphide bonds, and was either lost or diminished considerably after boiling of PR3 in SDS [35,44]. Conformational requirements of PR3 in order to be recognized by PR3–ANCA are supported further by the observation that PR3–ANCA showed no binding to in vitro translated PR3 [44].
Despite the apparent requirement for an intact tertiary structure, there have been four reports of PR3–ANCA binding to linear peptides [35,45–47]. Sera from patients with WG were shown to react with some of these peptides in enzyme-linked immunosorbent assay (ELISA) systems [45–47], but in three of the studies the same peptides were also recognized by control sera, although to a lesser extent [35,45,47].
In 1994 Williams et al. [45] identified several antigenic sites on PR3 which seemed to be exposed at the outside of the molecule. Some of these regions are close to the active site of PR3; one epitope even included part of the catalytic triad of PR3 (Fig. 1b). Using the same test system, Chang et al. could not reproduce their results because of a high level of non-specific binding [35]. In another study using linear peptides covering the entire sequence of PR3, including the signal- and propeptide, four epitope areas were identified that were recognized preferentially by WG sera drawn at initial presentation of disease compared to control sera [47] (Fig. 1b). Two of these epitope areas were located near the active centre of PR3. No epitopes were detected at the C-terminus of PR3 nor in the signal- or propeptide. Finally, Griffith et al. [46] identified five regions on PR3 that were bound by PR3–ANCA (Fig. 1b), four of which were intimately linked with the catalytic site.
The results of the studies discussed above seem inconclusive, as different regions have been identified as epitopes recognized by PR3–ANCA, but some overlap is seen clearly (summarized in Fig. 1b). None the less, the determined regions are still large and one cannot pinpoint amino acid stretches most important for the recognition of PR3 by PR3–ANCA. As mentioned previously, autoimmunity to PR3 could be initiated through an immune response against the antisense complementary peptide (amino acids 87–172, Fig. 1) of PR3 [29]. Only two areas on PR3 identified as epitopes bound by PR3–ANCA from patients at their initial presentation are located in the sequence of PR3 corresponding to the complementary peptide of PR3, implying that the antibody response to complementary PR3 cannot account for the induction of all antibodies to PR3.
Recently, recombinant chimeric molecules of human leucocyte elastase and human PR3 and of human PR3 and murine PR3 were generated. Sera from patients with WG with renal involvement drawn at the time of diagnosis bound to different constructs [48]. Also in this study, patients with WG at the time of diagnosis varied in their repertoire of epitopes bound by PR3–ANCA.
Competition studies of PR3–ANCA with monoclonal antibodies to PR3 indicate further that PR3–ANCA from patients with WG are directed against a restricted number of different epitopes on PR3 [46,49,50]. Also, in cross-inhibition studies of PR3–ANCA from different patients using biosensor technology, PR3–ANCA from vasculitis patients in their first presentation recognized a limited number of overlapping regions on PR3 [46,51]. This area might cover an immunodominant epitope common for PR3–ANCA from all patients at their initial presentation of WG.
All the studies on epitope mapping were performed on native PR3, as up to 40% of PR3–ANCA sera failed to recognize recombinant PR3 [52]. None the less, production of recombinant PR3 in different expression systems and cell lines has also provided more insight into the significance of the various intracellular processing steps of PR3 for recognition by PR3–ANCA [43,52–57]. The N-terminal proform of PR3 is not recognized by all PR3–ANCA [58]. Structural changes resulting from carboxy-terminal processing [59] and glycosylation of PR3 [60] appear less relevant for the recognition of PR3 by PR3–ANCA. Personal observations suggest that PR3–ANCA recognize epitopes on human PR3 which are absent on rat or murine PR3.
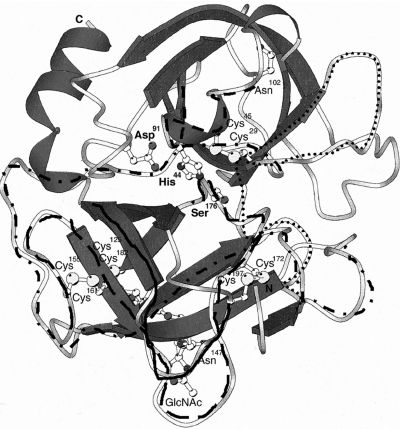
From the above-mentioned studies on epitope mapping of PR3–ANCA, it can be concluded that PR3–ANCA from patients with WG, especially in their first presentation, recognize a restricted number of immunodominant epitopes. None of the identified epitopes are located in the signal peptide nor in the C-terminal peptide of PR3. Two of the epitopes are located near the active site residues histidine and serine of PR3 and binding of these epitopes by PR3–ANCA will have functional consequences for the molecule. PR3–ANCA mainly recognize conformation-dependent epitopes on PR3, but some linear epitopes are bound as well. In Fig. 2 the position of the identified epitopes on PR3 are shown in the 3D structure. The different epitope areas identified on PR3 probably form one or two discontinuous epitopes, as some of these areas are located close to each other in the 3D structure of PR3. These epitopes might be the initial epitopes recognized by PR3–ANCA and are, possibly, cross-reactive with an epitope present on microbial antigens or with another source of antigen such as complementary protein fragments. However, whether these epitopes are disease-inducing has to be investigated further.
Fig. 2.
Epitopes bound by PR3–ANCA in a three-dimensional model of PR3. Three-dimensional model of PR3 according to the coordinates provided by Fujinaga et al. [41]. Amino acids are numbered in agreement with the PR3 sequence published by Campanelli et al. [76]. α-Helixes are shown in coiles and the Β sheets are shown as arrows. The amino acids His44, Asp91 and Ser176 form the catalytic triad of PR3. Epitope regions of Fig. 1b are given; first epitopic region (dots), second region (stripes), third region (stripe-dot strip), fourth region (long lines) and fifth region (heavy line).
EPITOPES RECOGNIZED BY MPO–ANCA
MPO is a 140–150 kDa dimer composed of subunits consisting of one heavy chain (59 kDa) and one light chain (13·5 kDa) and carries two identical prosthetic haem groups. The two heavy chains are joined by a disulphide link. MPO is synthesized as a 89 kDa precursor which undergoes subsequent processing via intermediate forms to yield mature subunits [61] (Fig. 3a).
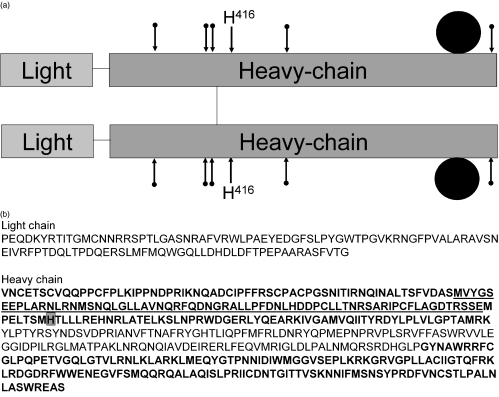
Fig. 3.
MPO protein structure. (a) The peptide subunits of MPO. MPO is a covalently linked dimer, composed of two subunits each with one heavy and one light chain. Each half contains a haem group. The active site, histidine 416, is indicated. The five potential glycosylation sites are indicated with an arrow. (b) Amino acid sequence of MPO [77] with three areas (bold) that have been identified by Fuji et al. [63] as epitopes recognized by MPO–ANCA from patients with MPA. The overlap between two identified regions is underlined. Grey box: the histidine important for the enzymatic activity of MPO.
Also for MPO, although to a lesser extent than for PR3, attempts have been made to clarify the interactions between ANCA and the molecule. Twelve years ago it was suggested that MPO–ANCA react mainly with conformational epitopes, as the recognition of MPO by MPO–ANCA seemed resistant to mild denaturation via SDS or reduction, but was destroyed by thermal denaturation [34,35]. In addition, MPO–ANCA did not bind to overlapping linear peptides covering the sequence of MPO [35].
Despite the suggested requirement for an intact tertiary structure, there are reports on epitope mapping of MPO–ANCA using recombinant deletion mutants of MPO [62,63]. Most MPO–ANCA reacted with up to three epitope regions on the heavy chain part of MPO while none of the MPO–ANCA reacted with the light chain (Fig. 3b). After grouping MPO–ANCA from patients in two groups, one group that recognized one or two epitope regions and another group that recognized all three regions, the relapse rate of patients with MPO–ANCA from the first group was significantly higher than that of patients with MPO–ANCA from the latter group [63]. Therefore, MPO–ANCA restricted to one or two major epitope regions may be associated with a worse prognosis than those recognizing three major epitope regions.
Competition studies of MPO–ANCA with monoclonal antibodies to MPO indicate further that MPO–ANCA are directed against a restricted number of epitopes on MPO [64]. In contrast, cross-inhibition studies using MPO–ANCA from different patients using biosensor technology showed that MPO–ANCA recognize an immunodominant epitope on the surface of MPO [65]. However, this study was performed using recombinant MPO, while 30% of MPO–ANCA sera failed to recognize recombinant MPO [64]. The importance of antigen source has been highlighted recently, as it was suggested that MPO–ANCA recognize epitopes on human MPO which are absent on rat MPO [66].
From the above-mentioned studies on epitope mapping of MPO–ANCA it can be concluded that MPO–ANCA recognize a restricted number of epitopes on MPO that are located on the heavy chain of MPO. Whether these epitopes are recognized only in the initial stage of the disease was not investigated.
EPITOPE CHANGES DURING THE COURSE OF DISEASE
The functional characteristics of PR3–ANCA differ between quiescent and active disease [31–33], suggesting that epitopes recognized by these autoantibodies change over time. Several studies, especially on PR3–ANCA, demonstrate that epitopes recognized by ANCA change during the course of the disease.
Within individual patients, cross-inhibition studies of PR3–ANCA at the moment of diagnosis and during the most recent relapse were performed using biosensor technology. These studies showed changes in epitopes recognized by PR3–ANCA between the moment of disease presentation and the moment of the most recent relapse. Interestingly, in two patients an epitope spreading was observed during the course of the disease, whereas in the other patients a narrowing of epitopes was seen in time [51]. In another study, in one patient an epitope spreading was also seen upon remission [46]. Furthermore, as mentioned previously, the N-terminal proform of PR3 is not recognized by all PR3–ANCA [58]. Nevertheless, PR3–ANCA that do recognize the proform and the mature form of PR3 (85% of all PR3–ANCA) correlate better with disease activity of WG than PR3–ANCA that bind mature PR3 only [67]. This also indicates that there are changes in epitopes recognized by PR3–ANCA during the course of the disease.
Only one report studied changes in epitope specificity of MPO–ANCA during the course of MPA. In one patient with MPO–ANCA-associated vasculitis an epitope shift was seen between sequential relapses [68].
Changes in epitope specificity of PR3–ANCA and/or MPO–ANCA appear to occur during the course of the disease. As ANCA are supposed to be pathogenic in vivo, epitope spreading in patients with AAV following remission may have resulted in a change in their pathogenicity. In contrast to SLE, where B cell specificity originates from one epitope and spreads to other areas on the same and even to different autoantigens [22–25], epitope spreading for PR3–ANCA and/or MPO–ANCA in AAV was observed only within PR3 or MPO, respectively. Epitope spreading in one autoantigen as seen for AAV during the course of the disease seems to be a common phenomenon in autoimmune disease. This leaves open the question of whether one particular epitope of PR3 or MPO induces an immune response that induces the disease.
IS THERE A DISEASE-INDUCING EPITOPE?
PR3–ANCA of most WG patients during the active phase of the disease interfere with the enzymatic activity of PR3 and with PR3/α1-AT complexation. Could this enzyme-inhibiting epitope recognized by PR3–ANCA constitute a disease-inducing epitope or a relapse-inducing epitope?
Interestingly, PR3–ANCA present in remission still inhibit the proteolytic activity of PR3 and are, in fact, more effective in doing so than ANCA obtained from patients with active disease, as the relative capacity of PR3–ANCA to interfere with the proteolytic activity of PR3 was higher for patients during remission [32]. Upon remission, those patients that remain ANCA-positive still have enzyme-inhibiting PR3–ANCA in their serum. This suggests that these enzyme-inhibiting antibodies do not add substantially to the pathogenicity of ANCA in AAV. Indeed, antibodies that inhibit the enzymatic activity of PR3 may act as alternative inhibitors, preventing PR3-induced degradation of extracellular matrix proteins [69]. In vitro PR3 can cleave ANCA [70], disagreeing with the action of ANCA as alternative inhibitors of PR3, but if this digestion of ANCA by PR3 also happens in vivo at the site of inflammation has not been determined. Taken together, the particular epitope specificity of PR3–ANCA that induces the disease has not yet been elucidated.
For MPO–ANCA-associated vasculitis it was shown that MPO–ANCA against one or two major epitopes are associated with more relapses than those recognizing three major epitopes [63]. This suggests that a more restricted pattern of recognition is more relapse-inducing. Until now, the interfering activity of on the inhibition of MPO by ceruloplasmin has not been correlated with disease activity of MPO–ANCA-associated vasculitis [36]. The clinical significance of this effect is not clear. As MPO–ANCA do not interfere with the enzymatic activity of MPO they might sustain the enzymatic activity of MPO at the site of inflammation by interfering with the inhibition of MPO, leading to increased MPO-induced endothelial and tissue damage. Further insight into the role of epitope specificity of MPO–ANCA may be gained from the recently developed murine model for MPO–ANCA-associated vasculitis [17].
CONCLUSION
PR3–ANCA as well as MPO–ANCA recognize a restricted set of epitopes on PR3 and MPO, respectively. In AAV restricted epitope spreading was observed for PR3– and MPO–ANCA within their corresponding autoantigens and no epitope spreading, in contrast to SLE, was observed towards other autoantigens. Several studies, especially on PR3–ANCA, demonstrate that epitopes recognized by ANCA change during the course of the disease, although these changes are not consistent. Whether the pathogenic role of ANCA may be ascribed to one, or some, relapse- or disease-inducing epitopes bound by PR3–ANCA and MPO–ANCA has, as yet, not been elucidated.
Characterization of relapse- or disease-inducing epitopes bound by PR3–ANCA and MPO–ANCA, although complicated, is significant for understanding the initiation and reactivation of AAV. Elucidating a disease-inducing epitope bound by ANCA may lead to the development of epitope-specific therapeutic strategies, of which successful examples have been described in experimental models for SLE [71–73]. In addition, it may answer the question of which epitope, possibly mimicking a foreign epitope, is responsible for triggering the disease in susceptible people.
REFERENCES
- 1.Borregaard N, Cowland J-B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 2.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41:1521–37. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Gaskin G, Savage COS, Ryan JJ, et al. Anti-neutrophil cytoplasmic antibodies and disease activity during long-term follow-up of 70 patients with systemic vasculitis. Nephrol Dial Transplant. 1991;6:689–94. doi: 10.1093/ndt/6.10.689. [DOI] [PubMed] [Google Scholar]
- 4.Stegeman CA, Cohen Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CGM. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12–7. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Slot MC, Tervaert JW, Boomsma MM, Stegeman CA. Positive classic antineutrophil cytoplasmic antibody (C-ANCA) titer at switch to azathioprine therapy associated with relapse in proteinase 3-related vasculitis. Arthritis Rheum. 2004;51:269–73. doi: 10.1002/art.20234. [DOI] [PubMed] [Google Scholar]
- 6.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 7.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Q J Med. 1995;88:127–33. [PubMed] [Google Scholar]
- 8.Boomsma MM, Stegeman CA, van der Leij MJ, et al. Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43:2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63:1079–85. doi: 10.1046/j.1523-1755.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Tervaert JW, Huitema MG, Hene RJ, et al. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990;336:709–11. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- 11.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991;50:539–46. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 13.Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CGM. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage COS, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992;141:335–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–83. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 16.Radford DJ, Savage CO, Nash GB. Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum. 2000;43:1337–45. doi: 10.1002/1529-0131(200006)43:6<1337::AID-ANR16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang B, Mamula MJ. Molecular mimicry and the role of B lymphocytes in the processing of autoantigens. Cell Mol Life Sci. 2000;57:561–8. doi: 10.1007/PL00000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craft J, Fatenejad S. Self antigens and epitope spreading in systemic autoimmunity. Arthritis Rheum. 1997;40:1374–82. doi: 10.1002/art.1780400803. [DOI] [PubMed] [Google Scholar]
- 20.James JA, Harley JB. B cell epitope spreading in autoimmunity. Immunol Rev. 1998;164:185–200. doi: 10.1111/j.1600-065x.1998.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 21.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 22.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 23.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization. Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A) Proc Natl Acad Sci USA. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbuckle MR, Reichlin M, Harley JB, James JA. Shared early autoantibody recognition events in the development of anti-Sm B/B′ in human lupus. Scand J Immunol. 1999;50:447–55. doi: 10.1046/j.1365-3083.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 26.Incaprera M, Rindi L, Bazzichi A, Garzelli C. Potential role of the Epstein–Barr virus in systemic lupus erythematosus autoimmunity. Clin Exp Rheumatol. 1998;16:289–94. [PubMed] [Google Scholar]
- 27.James JA, Neas BR, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein–Barr virus exposure. Arthritis Rheum. 2001;44:1122–6. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Monneaux F, Muller S. Epitope spreading in systemic lupus erythematosus: identification of triggering peptide sequences. Arthritis Rheum. 2002;46:1430–8. doi: 10.1002/art.10263. [DOI] [PubMed] [Google Scholar]
- 29.Pendergraft WF, III, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3 (105–201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–9. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 30.van de Wiel BA, Dolman KM, van der Meer Gerritsen CH, von Hack CE, dem Borne AEGKr, Goldschmeding R. Interference of Wegener's granulomatosis autoantibodies with neutrophil proteinase 3 activity. Clin Exp Immunol. 1992;90:409–14. doi: 10.1111/j.1365-2249.1992.tb05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daouk GH, Palsson R, Arnaout MA. Inhibition of proteinase 3 by ANCA and its correlation with disease activity in Wegener's granulomatosis. Kidney Int. 1995;47:1528–36. doi: 10.1038/ki.1995.216. [DOI] [PubMed] [Google Scholar]
- 32.Van der Geld YM, Tool AT, Videler J, et al. Interference of PR3–ANCA with the enzymatic activity of PR3: differences in patients during active disease or remission of Wegener's granulomatosis. Clin Exp Immunol. 2002;129:562–70. doi: 10.1046/j.1365-2249.2002.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolman KM, Stegeman CA, van de Wiel BA, et al. Relevance of classic anti-neutrophil cytoplasmic autoantibody (C-ANCA)-mediated inhibition of proteinase 3-alpha 1-antitrypsin complexation to disease activity in Wegener's granulomatosis. Clin Exp Immunol. 1993;93:405–10. doi: 10.1111/j.1365-2249.1993.tb08192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falk RJ, Becker M, Terrell R, Jennette JC. Anti-myeloperoxidase autoantibodies react with native but not denatured myeloperoxidase. Clin Exp Immunol. 1992;89:274–8. doi: 10.1111/j.1365-2249.1992.tb06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang L, Binos S, Savige J. Epitope mapping of anti-proteinase 3 and anti-myeloperoxidase antibodies. Clin Exp Immunol. 1995;102:112–9. doi: 10.1111/j.1365-2249.1995.tb06644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin SV, Chapman PT, Lianos EA, Lockwood CM. The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies. Kidney Int. 1999;55:917–25. doi: 10.1046/j.1523-1755.1999.055003917.x. [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Savige J. Studies to demonstrate inhibition of functional activity of neutrophil lysosomal enzymes with ANCA. Adv Exp Med. 1993;336:97–100. doi: 10.1007/978-1-4757-9182-2_15. [DOI] [PubMed] [Google Scholar]
- 38.Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin Exp Immunol. 1997;108:167–74. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, et al. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao RC, Wehner NG, Skubitz KM, Gray BH, Hoidal JR. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988;82:1963–73. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujinaga M, Chernaia MM, Halenbeck R, Koths K, James MN. The crystal structure of PR3, a neutrophil serine proteinase antigen of Wegener's granulomatosis antibodies. J Mol Biol. 1996;261:267–78. doi: 10.1006/jmbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- 42.Rao NV, Rao GV, Marshall BC, Hoidal JR. Biosynthesis and processing of proteinase 3 in U937 cells. Processing pathways are distinct from those of cathepsin G. J Biol Chem. 1996;271:2972–8. doi: 10.1074/jbc.271.6.2972. [DOI] [PubMed] [Google Scholar]
- 43.Garwicz D, Lindmark A, Hellmark T, Gladh M, Jogi J, Gullberg U. Characterization of the processing and granular targeting of human proteinase 3 after transfection to the rat RBL or the murine 32D leukemic cell lines. J Leukoc Biol. 1997;61:113–23. doi: 10.1002/jlb.61.1.113. [DOI] [PubMed] [Google Scholar]
- 44.Bini P, Gabay JE, Teitel A, Melchior M, Zhou JL, Elkon KB. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 45.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 46.Griffith ME, Coulthart A, Pemberton S, George AJ, Pusey CD. Anti-neutrophil cytoplasmic antibodies (ANCA) from patients with systemic vasculitis recognize restricted epitopes of proteinase 3 involving the catalytic site. Clin Exp Immunol. 2001;123:170–7. doi: 10.1046/j.1365-2249.2001.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Geld YM, Simpelaar A, Van Der Zee R, et al. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 2001;59:147–59. doi: 10.1046/j.1523-1755.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 48.Selga D, Segelmark M, Wieslander J, Gunnarsson L, Hellmark T. Epitope mapping of anti-PR3 antibodies using chimeric human/mouse PR3 recombinant proteins. Clin Exp Immunol. 2004;135:164–72. doi: 10.1111/j.1365-2249.2004.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommarin Y, Rasmussen N, Wieslander J. Characterization of monoclonal antibodies to proteinase-3 and application in the study of epitopes for classical anti-neutrophil cytoplasm antibodies. Exp Nephrol. 1995;3:249–56. [PubMed] [Google Scholar]
- 50.Huang Z, Lockwood CM. Epitope mapping on Wegener's granulomatosis autoantigen proteinase 3. Chin Med J. 2001;114:760–3. [PubMed] [Google Scholar]
- 51.Rarok AA, Van der Geld YM, Stegeman CA, Limburg PC, Kallenberg CG. Diversity of PR3–ANCA epitope specificity in Wegener's granulomatosis. Analysis using the biosensor technology. J Clin Immunol. 2003;23:460–8. doi: 10.1023/b:joci.0000010422.73892.b5. [DOI] [PubMed] [Google Scholar]
- 52.Harmsen MC, Heeringa P, Van der Geld YM, et al. Recombinant proteinase 3 (Wegener's antigen) expressed in Pichia pastoris is functionally active and is recognized by patient sera. Clin Exp Immunol. 1997;110:257–64. doi: 10.1111/j.1365-2249.1997.tb08325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van der Geld YM, Oost Kort WW, Limburg PC, Specks U, Kallenberg CGM. Recombinant proteinase 3 produced in different expression systems: recognition by anti-PR3 antibodies. J Immunol Meth. 2000;244:117–31. doi: 10.1016/s0022-1759(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 54.Szymkowiak CH, Johnston TW, Csernok E, Gross WL. Expression of the human autoantigen of Wegener's granulomatosis (PR3) in a baculovirus expression system. Biochem Biophys Res Commun. 1996;219:283–9. doi: 10.1006/bbrc.1996.0224. [DOI] [PubMed] [Google Scholar]
- 55.Witko-Sarsat V, Halbwachs-Mecarelli L, Almeida RP, et al. Characterization of a recombinant proteinase 3, the autoantigen in Wegener's granulomatosis and its reactivity with anti-neutrophil cytoplasmic autoantibodies. FEBS Lett. 1996;382:130–6. doi: 10.1016/0014-5793(96)00152-4. [DOI] [PubMed] [Google Scholar]
- 56.Specks U, Fass DN, Fautsch MP, Hummel AM, Viss MA. Recombinant human proteinase 3, the Wegener's autoantigen, expressed in HMC-1 cells is enzymatically active and recognized by c-ANCA. FEBS Lett. 1996;390:265–70. doi: 10.1016/0014-5793(96)00669-2. [DOI] [PubMed] [Google Scholar]
- 57.Van der Geld YM, Smook ML, Huitema MG, Harmsen MC, Limburg PC, Kallenberg CG. Expression of recombinant proteinase 3, the autoantigen in Wegener's granulomatosis, in insect cells. J Immunol Meth. 2002;264:195–205. doi: 10.1016/s0022-1759(02)00101-1. [DOI] [PubMed] [Google Scholar]
- 58.Sun J, Fass DN, Viss MA, et al. A proportion of proteinase 3 (PR3)-specific anti-neutrophil cytoplasmic antibodies (ANCA) only react with Pr3 after cleavage of its N-terminal activation dipeptide. Clin Exp Immunol. 1998;114:320–6. doi: 10.1046/j.1365-2249.1998.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capizzi SA, Viss MA, Hummel AM, Fass DN, Specks U. Effects of carboxy-terminal modifications of proteinase 3 (PR3) on the recognition by PR3–ANCA. Kidney Int. 2003;63:756–60. doi: 10.1046/j.1523-1755.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- 60.Specks U. What you should know about PR3–ANCA. Conformational requirements of proteinase 3 (PR3) for enzymatic activity and recognition by PR3–ANCA. Arthritis Res. 2000;2:263–7. doi: 10.1186/ar99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moguilevsky N, Garcia Quintana L, Jacquet A, et al. Structural and biological properties of human recombinant myeloperoxidase produced by Chinese hamster ovary cell lines. Eur J Biochem. 1991;197:605–14. doi: 10.1111/j.1432-1033.1991.tb15950.x. [DOI] [PubMed] [Google Scholar]
- 62.Tomizawa K, Mine E, Fujii A, et al. A panel set for epitope analysis of myeloperoxidase (MPO)-specific antineutrophil cytoplasmic antibody MPO–ANCA using recombinant hexamer histidine-tagged MPO deletion mutants. J Clin Immunol. 1998;18:142–52. doi: 10.1023/a:1023251001261. [DOI] [PubMed] [Google Scholar]
- 63.Fujii A, Tomizawa K, Arimura Y, et al. Epitope analysis of myeloperoxidase (MPO) specific anti-neutrophil cytoplasmic autoantibodies (ANCA) in MPO–ANCA-associated glomerulonephritis. Clin Nephrol. 2000;53:242–52. [PubMed] [Google Scholar]
- 64.Audrain MA, Baranger TA, Moguilevski N, et al. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin Exp Immunol. 1997;107:127–34. doi: 10.1046/j.1365-2249.1997.d01-895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Short AK, Lockwood CM. Studies of epitope restriction on myeloperoxidase (MPO), an important antigen in systemic vasculitis. Clin Exp Immunol. 1997;110:270–6. doi: 10.1111/j.1365-2249.1997.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patry YC, Nachman PH, Audrain MA, Falk RJ, Meflah K, Esnault VL. Difference in antigenic determinant profiles between human and rat myeloperoxidase. Clin Exp Immunol. 2003;132:505–8. doi: 10.1046/j.1365-2249.2003.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell KA, Fass DN, Specks U. Antineutrophil cytoplasmic antibodies reacting with the pro form of proteinase 3 and disease activity in patients with Wegener's granulomatosis and microscopic polyangiitis. Arthritis Rheum. 2001;44:463–8. doi: 10.1002/1529-0131(200102)44:2<463::AID-ANR65>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Chapman PT, Short AK, Lockwood CM. The use of biosensor technology in epitope mapping studies on human myeloperoxidase. Clin Exp Immunol. 1998;112:44. [Google Scholar]
- 69.Van der Geld YM, Limburg PC, Kallenberg CG. Proteinase 3, Wegener's autoantigen: from gene to antigen. J Leukoc Biol. 2001;69:177–90. [PubMed] [Google Scholar]
- 70.Dolman KM, Jager A, von Sonnenberg A, dem Borne AEGKr, Goldschmeding R. Proteolysis of classic anti-neutrophil cytoplasmic autoantibodies (C-ANCA) by neutrophil proteinase 3. Clin Exp Immunol. 1995;101:8–12. doi: 10.1111/j.1365-2249.1995.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaliyaperumal A, Michaels MA, Datta SK. Antigen-specific therapy of murine lupus nephritis using nucleosomal peptides. tolerance spreading impairs pathogenic function of autoimmune T and B cells. J Immunol. 1999;162:5775–83. [PubMed] [Google Scholar]
- 72.Eilat E, Dayan M, Zinger H, Mozes E. The mechanism by which a peptide based on complementarity-determining region-1 of a pathogenic anti-DNA auto-Ab ameliorates experimental systemic lupus erythematosus. Proc Natl Acad Sci USA. 2001;98:1148–53. doi: 10.1073/pnas.98.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hahn BH, Singh RR, Wong WK, Tsao BP, Bulpitt K, Ebling FM. Treatment with a consensus peptide based on amino acid sequences in autoantibodies prevents T cell activation by autoantigens and delays disease onset in murine lupus. Arthritis Rheum. 2001;44:432–41. doi: 10.1002/1529-0131(200102)44:2<432::AID-ANR62>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 74.Sturrock AB, Franklin KF, Rao G, et al. Structure, chromosomal assignment, and expression of the gene for proteinase-3. The Wegener's granulomatosis autoantigen. J Biol Chem. 1992;267:21193–9. [PubMed] [Google Scholar]
- 75.Bories D, Raynal MC, Solomon DH, Darzynkiewicz Z, Cayre YE. Down-regulation of a serine protease, myeloblastin, causes growth arrest and differentiation of promyelocytic leukemia cells. Cell. 1989;59:959–68. doi: 10.1016/0092-8674(89)90752-6. [DOI] [PubMed] [Google Scholar]
- 76.Campanelli D, Melchior M, Fu Y, et al. Cloning of cDNA for proteinase 3: a serine protease, antibiotic, and autoantigen from human neutrophils. J Exp Med. 1990;172:1709–15. doi: 10.1084/jem.172.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashinaka K, Nishio C, Hur SJ, Sakiyama F, Tsunasawa S, Yamada M. Multiple species of myeloperoxidase messenger RNAs produced by alternative splicing and differential polyadenylation. Biochemistry. 1988;27:5906–14. doi: 10.1021/bi00416a013. [DOI] [PubMed] [Google Scholar]