Abstract
One of the most intriguing aspects of tuberculosis is that the outcome of an infection with M. tuberculosis (TB) is highly variable between individuals. The possibility of differences in virulence between M. tuberculosis strains or genotypes has only recently been studied. There is evidence of multifactorial genetic predisposition in humans that influences the susceptibility to tuberculosis. A better understanding of differences in virulence between M. tuberculosis genotypes could be important with regard to the efforts at TB control and the development of improved antituberculosis vaccines. Survival, lung pathology, bacterial load and delayed type hypersensitivity (DTH) responses of BALB/c mice after intratracheal infection with any of 19 different M. tuberculosis complex strains of 11 major genotype families were studied. The results indicate that among genetically different M. tuberculosis strains a very broad response was present with respect to virulence, pathology, bacterial load and DTH. ‘Low’-responders were the H37Rv, Canetti, Beijing-1 strains, while Beijing-2,3, Africa-2 and Somalia-2 strains were ‘high’-responders. A severe pathological response correlates with a high mortality and a high CFU counts in lungs, but poorly with the degree of the DTH response.
Keywords: M. tuberculosis, pathology, genotypes, IS6110 RFLP typing, immunology, CFU
INTRODUCTION
Worldwide, there are 8–10 million new cases of tuberculosis and an estimated two million deaths due to this disease each year [1]. The magnitude of this problem is increasing with the rising incidence of human immunodeficiency virus (HIV) infection and the emergence of multidrug resistant strains of Mycobacterium tuberculosis. It is estimated that one third of the world's population is latently infected with M. tuberculosis [2], constituting the reservoir from which future cases of TB will arise.
One of the most intriguing aspects of this disease is that the outcome of an infection with M. tuberculosis is highly variable between individuals. The majority of infected persons (about 90%) will never develop disease [3], the immune system apparently being capable of permanent containment of the infection. In the 10% of individuals who do develop active TB disease, this is usually after a latent interval that can vary from weeks to many decades, and there is a whole spectrum of clinical presentations, ranging from subclinical to rapidly fatal and almost any organ can be involved. While the precise pathogenesis of TB and the factors determining the highly variable course of infection remain only partly understood, it is generally thought that this depends on a complex interplay between environmental and host characteristics. Environmental factors that affect the susceptibility to tuberculosis include poverty, malnutrition, stress, overcrowding and exposure to environmental mycobacteria [4–8].
Regarding the host, there is evidence of multifactorial genetic predisposition in humans that influences the susceptibility to tuberculosis [9,10]. While an association between the presence of polymorphisms in single genes, e.g. the human NRAMP gene, and tuberculosis has been made [11], this was not found in another geographical region [12]. Until recently, the possibility of differences in virulence between M. tuberculosis strains or genotypes had not been studied.
In fact, until DNA fingerprinting was introduced in the early 1990s, very limited possibilities were available to distinguish between different strains of the genetically highly conserved M. tuberculosis complex. Therefore, most of the immunological research has been based on the use of a limited number of laboratory strains, such as H37Rv and Erdman, with H37Ra as an avirulent laboratory variant. However, 10 thousands of different M. tuberculosis complex strains have been distinguished by DNA fingerprinting techniques over the last decade [13–16]. Based on fingerprinting results, it was found that the population structure of M. tuberculosis in high-prevalence areas is much more conserved than in areas with a low incidence of tuberculosis [17]. This suggests that selective advantages of the predominant M. tuberculosis genotypes may play a role in the spread of tuberculosis in high-incidence settings.
In a previous study that aimed to assess differences in virulence between M. tuberculosis genotypes, a marked difference was observed in survival, lung bacterial load, lung histopathology, and cellular immune responses after infection of mice with different isolates from a spectrum of M. tuberculosis complex genotypes [13]. It was found that M. tuberculosis of the Beijing genotype, which is highly prevalent in Asia and the former USSR republics, was most virulent and elicited a nonprotective immune response. In contrast, M. canetti was associated with a more favourable course while other genotypes caused intermediate clinical and pathological effects. Importantly, vaccination with BCG afforded significantly less protection against infection with M. tuberculosis of the Beijing genotype compared with the other genotype strains. A better understanding of differences in virulence between M. tuberculosis genotypes could be important with regard to the efforts at TB control and the development of improved antituberculosis vaccines.
In the present study, we investigated survival, lung pathology, bacterial load and delayed type hypersensitivity responses of BALB/c mice after intratracheal infection with any of 19 different M. tuberculosis complex strains of 11 major genotype families. The results indicate that among genetically different M. tuberculosis strains a very broad response was present with respect to pathology, virulence, bacterial load and delayed type hypersensitivity. A severe pathological response correlates with a high mortality, a high CFU counts in lungs and a high DTH reaction.
MATERIALS AND METHODS
M. tuberculosis strains
Nineteen different strains representing the major M. tuberculosis genotype families were selected from the global database at the National Institute of Public Health and the Environment (RIVM), that contains 8000 different IS6110 restriction fragment length polymorphism (RFLP) patterns from a wide range of geographical origins [14,15,17]. The selected strains are listed in Table 1. Of seven genotypic groups, at least two genetically similar strains were included to allow that comparison of the effects of evolutionary related strains. The Africa, Beijing, and Somalia strains represent prevalent M. tuberculosis genotype families [18] in Central Africa [19], Asia [20] and Somalia, respectively, while the Haarlem strains constitute one of the most widespread genotype families found worldwide [18]. The Amsterdam strain is involved in a relatively large chain of transmission of tuberculosis in risk groups in Amsterdam, capital of the Netherlands. The Canetti strain is a representative of the recently described subspecies M. canettii [21]. The ‘IS-in-Ori’ is the designation of two strains that have an IS6110 element inserted in the genomic dnaA-dnaN region, a characteristic shared with Beijing genotype strains. The ‘less-transmissible’ strains are M. tuberculosis isolates from patients with pulmonary TB who had been smear-positive for a relatively extended period before the diagnosis was made, but unexpectedly had not transmitted the infection to their close contacts. The ‘zero-copy’ strains are genuine M. tuberculosis strains lacking IS6110 DNA. Furthermore, the H37Rv and Erdman strains that were used in essentially all previous studies of TB in animal models were used as controls [3,22–24].
Table 1.
List of various M. tuberculosis strains used in this study and their pathology: mean score of histological parameters and the summation of these scores in the lungs of mice 28 days after infection
| Genotype | Strain/isolate | Peribronchiolitis | Perivasculitis | Alveolitis | Granuloma | Sum |
|---|---|---|---|---|---|---|
| Africa 1 | 3529 | 2* | 2 | 2·3 | 3·7 | 10·0 |
| Africa 2 | 3008 | 2 | 2·7 | 4 | 3 | 11·7 |
| Amsterdam 1 | 2000241 | 2·3 | 2·3 | 3 | 2·3 | 9·9 |
| Beijing 1 | 9402008 | 2 | 2·7 | 2 | 2·7 | 9·4 |
| Beijing 2 | 17919 | 1·7 | 2·7 | 3·7 | 4 | 12·1 |
| Beijing 3 | 9401707 | 2·3 | 2·3 | 4 | 2·7 | 11·3 |
| Canetti | 9600046 | 2 | 3 | 2·3 | 0 | 7·3 |
| Erdman | 9900342 | 2 | 2·3 | 2·7 | 2 | 9·0 |
| H37Rv | 1·3 | 2 | 1·7 | 0 | 5·0 | |
| Haarlem 1 | 9401431 | 2 | 3 | 3 | 4 | 12·0 |
| Haarlem 2 | 9400104 | 1·7 | 2·7 | 4 | 3 | 11·4 |
| IS-in-Ori 1 | 9601922 | 0·7 | 1·7 | 1·3 | 0·7 | 4·4 |
| IS-in-Ori 2 | 9601933 | 2 | 2·7 | 3 | 2·3 | 10·0 |
| Less-trans 1 | 9700464 | 2 | 2·3 | 2 | 3 | 9·3 |
| Less-trans 2 | 9900172 | 1·3 | 2·7 | 2·3 | 4 | 10·3 |
| Somalia 1 | 2000216 | 2 | 2·7 | 2·7 | 2·3 | 9·7 |
| Somalia 2 | 2000367 | 2·3 | 3 | 4 | 3·7 | 13·0 |
| Zerocopy 1 | 9401016 | 2 | 2 | 1·3 | 2 | 7·3 |
| Zerocopy 2 | IN4 | 1·3 | 1·7 | 1·7 | 1·3 | 6·0 |
The mean of three individual scores is shown for each parameter. The histopathological parameters were semiquantitatively and blindly evaluated and scored as: absent (0), minimal (1), slight (2), moderate (3), marked (4) and strong (5).
The bacteria were grown in agitation at 37°C in Middlebrook 7H9 broth (Difco, Detroit, MI, USA) enriched with glycerol and albumin, catalase and dextrose (Becton Dickinson, Cockysville, MD, USA). Growth was monitored by densitometry. Cell suspensions were aliquoted and frozen at −70°C as soon as they reached the stationary phase. Re-culture procedures were kept to a minimum to avoid any loss of virulence. The bacterial suspensions were counted by using fluorescent-microscopy and Newbauer counting chambers and adjusted to 2·5 × 105 viable cells per 100 µl PBS.
Murine model of progressive pulmonary tuberculosis
The experimental model of pulmonary tuberculosis has been described in detail previously [3]. Pathogen free male BALB/c mice were used at 6–8 weeks of age. Animals were anaesthetized intraperitoneally with pentobarbital. The trachea was exposed via a small midline incision followed by injection of 2·5 × 105 viable cells in 100 µl PBS. After suturing the incision, infected mice were kept in a vertical position until the effect of anaesthesia had passed. In total, 19 groups of 41 mice each were infected with different M. tuberculosis complex strains and 30 mice of each group were left undisturbed to record survival from day 8 up to day 112 after infection. All procedures were performed in a laminar flow cabinet in a bio-safety level III facility.
Histopathology
At day 1, 3, 16, 23, 28 and 56 after infection, three mice (or all survivors if less than three) per group infected with a certain M. tuberculosis strain were killed by exsanguination. The right lung lobe was fixed with ethanol and embedded in paraffin. The left lung lobe, the spleen and serum were rapidly frozen in liquid nitrogen and kept at −70°C for microbiological studies. Three µm haematoxilin-eosin (HE)-stained paraffin sections were examined by light microscopy. Sections were viewed and scored without knowledge of the M. tuberculosis strain. The histopathological parameters peribronchiolitis, perivasculitis, alveolitis and granuloma formation were each semiquantitatively scored as absent, minimal, slight, moderate, marked or strong, noted as 0, 1, 2, 3, 4, and 5, respectively. In this score the frequency as well as the severity of the lesions were incorporated. Granuloma formation was scored by estimating the occupied area of the lung section. For each time point, the lungs of three animals were examined and the mean score of each of the four histological parameters was calculated. To evaluate the strength of the total pathological response the mean scores of the mentioned four parameters were added, thus the maximal sum was twenty.
Colony forming unit (CFU) counts
One lung lobe of three mice per sacrifice time point (day 1, 3, 16, 23, 28 and 56) was used for CFU-counting. Lungs were homogenized with a Polytron homogenizer (Kinematica, Luzern, Switzerland) in sterile tubes containing 1 ml of PBS with 0·05% Tween 80. Ten µl of the original concentration and five dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H11 agar (Difco Laboratories, Detroit, MI, USA) enriched with Bactoglycerol and oleic acid, albumin, catalase and dextrose (OADC-enrichment 100 ml/l). The number of colonies was counted after incubation at 37°C for 15 days.
Delayed type hypersensitivity
Delayed type hypersensitivity responses were measured in three mice per infected group at each time point (day 1, 3, 16, 23, 28, 56 and 112) after infection by injection with H37Rv in the hind foot-pad, one day before exsanguination. Culture filtrate was harvested from M. tuberculosis H37Rv after 4–5 weeks of growing in Proskauer and Beck medium modified by Youmans [25,26]. The culture filtrate antigens were precipitated with 45% (w/v) ammonium sulphate, washed and re-dissolved in PBS. Our culture filtrate used as challenge antigen appeared to be reproducible and gave a stronger DTH response than a commercial PPD preparation. For evaluation of DTH each mouse received an injection of 20 µg of antigen in 40 µl of PBS into the hind foot-pad. In order to achieve very low nonspecific background swelling, the needle perforated the skin near the ‘heel’. Then the needle travelled under the skin so that antigen was injected over the ‘palm’, where the readings of swelling were also made. We have found that ensuring the absence of a skin puncture wound at the site where the DTH is read reduces background and variability. The swelling at the ‘palm’ was measured with an engineer's micrometer before and 24 h after the injection [8]. At 24 h after challenge the response was stronger than after 48 h. An ‘early’ DTH response at 4 h after challenge disappeared within the next four hours. The measurements per strain per day were pooled.
RESULTS
Histopathology
Only a minimal mononuclear inflammatory infiltrate was noted in a few animals at autopsy performed at days 1 and 3 after infection. At these two early time points, histopathological parameters such as peribronchiolitis, perivasculitis, and alveolitis were absent in most animals, and minimal in some animals, while granuloma formation was always absent. Thus, the histopathological parameters scored at day 1 and 3 were not discriminatory and were omitted from the total evaluation.
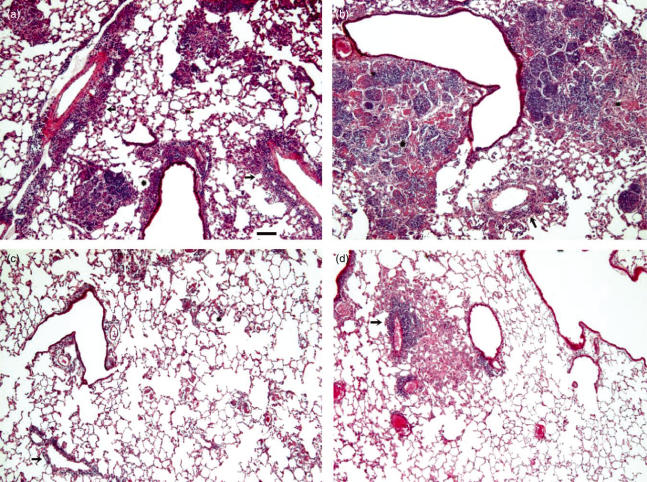
All histopathological parameters increased in severity in the course of infection. Large differences in the time-dependent induction of lung pathology were observed between the different strains. Figure 1 illustrates some of the differences in the histopathological parameters in the course of infection with different genotypes. E.g., at day 23 after infection, the Beijing-2 strain caused a moderate peribronchiolitis, perivasculitis and alveolitis and a strong lepromatous-like granuloma formation (Fig. 1a,b), whereas at day 56 post infection the Canetti strain and the IS-in-Ori-1 strain caused, in the absence of granuloma formation, only a mild peribronchiolitis, perivasculitis and alveolitis (Fig. 1c,d). The perivascular and peribronchiolar infiltrates of the Canetti infected animals consisted mainly of histiocytes and some lymphocytes. In the regions with alveolitis, mainly alveolar macrophages and neutrophilic granulocytes were recognized, besides some lymphocytes.
Fig. 1.
Microscopic images of lung of mice 23 days after infection with Beijing-2 (a, b) and of mice 56 days after infection with Canetti (c) and with IS-in-Ori-1 (d). Examples are presented of the different lung pathological parameters and their score. In (a) a moderate peribronchiolar (score 3) (asterix) and perivascular (score 4) (arrow) infiltrate is present with some granuloma of moderate size (score 2). In panel b two marked granuloma (score 4) with areas of necrosis (asterix) surround a bronchiole. Slight perivascular infiltrate is present (arrow). In panel c only slight perivascular infiltrate (score 2) (arrow) and minimal alveolitis (score 1) (asterix) are present in this area, while in (d) a moderate perivascular inflammation (score 2) (arrow) is lying next to a small granuloma (score 2). HE, 60×. Bar = 100 µm.
Strains such as Canetti and H37Rv didn’t induce lung pathology during the first three weeks of infection, whereas the strains Zerocopy 1 and 2 induced minimal pathology during this period. Most of the strains, however, were able to induce a moderate lung damage within 4 weeks after infection. Moreover, differences were observed in the formation of granulomas, sometimes independently from the other parameters. A high score for granulomas paralleled always a moderate to marked pneumonia both at day 28 as at day 56 (Haarlem 1, Somalia 2) whereas no or a few granulomas at day 56 were present in combination with moderate (Canetti, Fig. 1c) or minimal (Is-in-Ori 1, Fig. 1d) pneumonia.
At day 16 a minimal granuloma formation was restricted to the strain Somalia 1, the IS-in-Ori 1 strain, Beijing 2 and Africa 2, and a slight granuloma formation was present in Amsterdam 1-infected mice. At day 23, the Africa 2, IS-in-Ori 2, Somalia 1 and Zerocopy 1 infected animals showed slight granuloma formation, and Beijing 2 and Amsterdam 1 mice a moderate granuloma formation. In all animals the area occupied by granulomas increased in the course of time. Necrotizing granuloma containing noncaseous necrosis, histiocytes, polymorphonuclear granulocytes and cell debris in the abscense of a well-circumscribed edge, giant cells and fibrosis, indicating that these were more lepromatous-like granulomas. At day 23 one out of three mice infected with Africa 2 showed necrotizing granulomas whereas all mice infected with Beijing 2 showed a moderate area of this type of necrosis at that time. One mouse at day 28 and another at day 56 infected with strain Less-transmissible 2, and one mouse infected with Erdman at day 56 showed necrotizing granulomas.
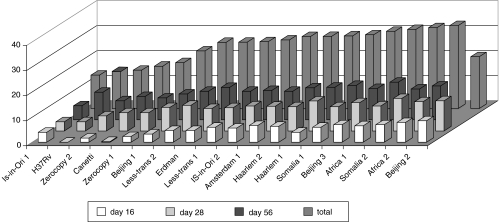
Table 1 shows the mean score of the four histological parameters and their sum per bacterial strain at day 28. As the scores for the different histopathological parameters could be dissociated, e.g. at day 56, the Less-transmissible 2 strain showed a granuloma score of 5 and an alveolitis score of 2·7, while the strain Beijing 3 presented a reversed score, we used the sum of all parameters in the evaluation. The sum of all histological parameters was arbitrarily divided into three classes: divided at day 16 into scores <3, 3–7 and >7 (score range 0·3–8·6), at day 28 by the score <7, 7–10, >10 (score range 3·7–12) and at day 56 by the scores <8, 8–11 and > 11 (score range 5·6–15). Figure 2 illustrates the scores at day 16, 28 and 56 and the summarized one for the 19 different strains (data from day 23 were not evaluated in this summary as only a slight difference was observed compared with those obtained at day 28). Finally, strains were categorized into three groups as based on cumulative scores at 3 different follow-up time points (indicated by cumulative score):
Category 1 (L low-pathogenic): strains inducing minimal to slight pathology (cumulative score <25): IS-in-Ori 1, H37Rv, Zerocopy 2, Canetti, Zerocopy 1 and Beijing 1.
Category 2 (M moderate pathogenic): strains inducing a moderate pathology (cumulative score ≥25 and <30): Erdman, Less-transmissible 1 and 2, IS-in-Ori 2, Amsterdam 1, Haarlem 1 and 2, and Somalia 1.
Category 3 (H high-pathogenic): strains inducing a severe pathology (cumulative score ≥30): Beijing 2 and 3, Africa 1 and 2, Somalia 2.
Fig. 2.
Score of lung pathology of mice 16, 28 and 56 days (and total score) after infection with 19 different M. tuberculosis strains. Strains are arranged with increasing pathology. From this figure it is clear that after infection pathology increases in time.
Survival
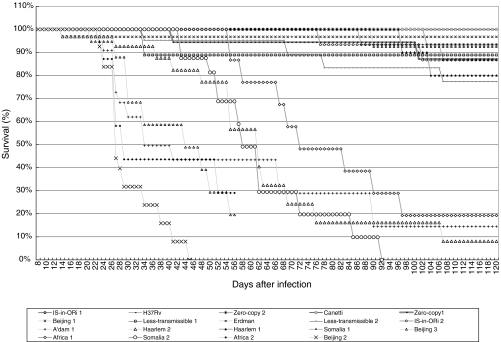
Survival curves of the 19 groups of mice infected with different strains and that were left undisturbed are presented in Fig. 3, demonstrating significant differences in the survival between groups. Based on the survival rate 3 categories could be discerned.
Fig. 3.
Percentage of survival of mice infected with different M. tuberculosis strains. Beijing 3 infected animals did not survive 56 days.
Six strains demonstrated a low (L) virulence (death rate of 0–10%): Zero-Copy 2, Beijing 1, Erdman, Is-in-Ori 1, H37Rv and Canetti, with no deaths occurring during follow-up after infection with the latter three strains (Table 2). Six strains had a moderate (M) virulence (death rate of 10–25%): Zero-Copy 1, Less-transmissible 1 and 2, IS-in-Ori 2, Haarlem 1, and Somalia 1. Seven strains had a high (H) virulence with a death rate of 80–100%. All mice infected with the Beijing 2 and 3 and Africa 2 strains had died within 56 days, and all mice infected with Somalia 2 had died within 92 days. Less than 20% of the animals infected with the strains Amsterdam 1, Haarlem 2 or Africa 1 survived till day 112.
Table 2.
Multiparametric results (histopathology, virulence, CFU and DTH) after infection with 19 different M. tuberculosis strains
| Histopathology | Virulence | CFU | DTH day 16 (X) & DTH day 23 (Y) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| low | mod | high | low | mod | high | low | mod | high | low | mod | high | |
| IS-in-Ori 1 | X | X | X | X | Y | |||||||
| H37Rv | X | X | X | XY | ||||||||
| Zerocopy 2 | X | X | X | X | Y | |||||||
| Canetti | X | X | X | XY | ||||||||
| Zerocopy 1 | X | X | X | X | Y | |||||||
| Beijing 1 | X | X | X | Y | X | |||||||
| Less-trans 2 | X | X | X | X | Y | |||||||
| Erdman | X | X | X | XY | ||||||||
| Less-trans 1 | X | X | X | Y | X | |||||||
| IS-in-Ori 2 | X | X | X | X | Y | |||||||
| Amsterdam 1 | X | X | X | XY | ||||||||
| Haarlem 2 | X | X | X | XY | ||||||||
| Haarlem 1 | X | X | X | XY | ||||||||
| Somalia 1 | X | X | X | Y | X | |||||||
| Beijing 3 | X | X | X | Y | X | |||||||
| Africa 1 | X | X | X | Y | X | |||||||
| Somalia 2 | X | X | X | X | Y | |||||||
| Africa 2 | X | X | X | XY | ||||||||
| Beijing 2 | X | X | X | Y | X | |||||||
The presented survival curves started at day 8 after infection, because deaths occurred during the first 3 days after infection in nearly all groups of mice (except the ones infected by Less-transmissible 2), ranging from 2 to 11 animals per strain. As there was only minimal pulmonary pathology at these early time points, it is unlikely that the infection was the decisive factor for death. It was more probable that trauma due to the experimental procedure per se contributed to death.
Lung bacterial counts
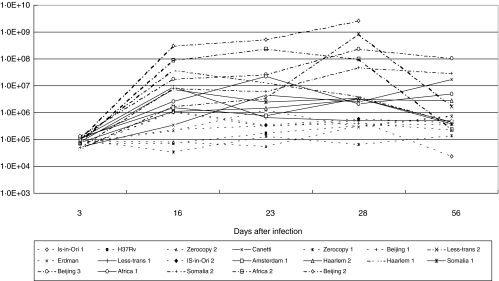
At day 1 and 3 the mean number of CFUs in the lungs of mice infected with different M. tuberculosis strains was about or less than 105. Figure 4 shows the CFU counts at day 3, 16, 23, 28 and 56. In mice infected with Zero-copy 1 and 2, IS-in-Ori 1 and 2, Beijing 1, Erdman, and H37Rv, the CFU-counts remained less than 106 per lung throughout the entire experiment and these strains were categorized as low virulent. In contrast, mice infected with Beijing 2 and 3, Somalia 2, Africa 2, Haarlem 1 and Less-transmissible 2 showed at least a two log higher bacillary load at day 16, 23 or/and 28. These latter strains were considered as high virulent. Such a high bacillary load was also observed at day 56 for mice infected with Beijing 3 and Somalia 2. For all strains, except Canetti and Africa 1, the CFU-counts decreased after day 28.
Fig. 4.
Colony forming unit-counts from lung tissue of mice at 3, 16, 23, 28 and 56 days after infection with different M. tuberculosis strains. Low (-----), moderate (——) and high (–·–·–·–) bacilarry load, can be discerned in time by their nearly horizontal lines from day 16.
Delayed type hypersensitivity
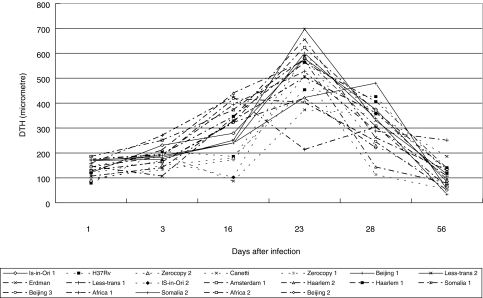
In Fig. 5 the delayed type hypersensitivity (DTH) responses to intracutaneous injection of H37Rv culture filtrate antigens are depicted. All mice showed a very low DTH responses (about 250 µm) at day 1 and 3 after infection. With the exception of Less-transmissible 1, the highest DTH responses were measured 23 days after infection. Irrespective of the infecting strain, none of the mice showed a long lasting DTH responses after infection seen the low levels at days 56 and 108, including the strains that were not associated with death. At day 16 low (<250 µm) and high (>350 µm) responders could be distinghuished with H37Rv, Zero-copy 1 and 2, Canetti, Beijing 1 and IS-in-Ori 2 inducing low responses and Less-transmissible 1, Haarlem 1 and 2, Beijing 3 and Africa 1 and 2 inducing high responses. The distinction of a group of low and high responders at day 16 appeared to be correlated rather well with their histopathology, virulence and CFU: e.g. low DTH responses were measured where absence of pathology and death and a low CFU were found (Table 2). Such a correlation between the height of the DTH response and the severity of other parameters was not likely at day 23 postinfection. At that time point large overlap of DTH responses of mice infected with the different strains was measured. Some genetically related strains as IS-in-Ori 1 and 2, Beijing 1, 2 and 3 and Haarlem 1 and 2 showed an identical DTH response, although the DTH responses of other genetical related strains (Zerocopy 1 and 2, Less-transmissible 1 and 2) gave a very varied reaction.
Fig. 5.
Delayed type hypersensitivity (DTH) responses one day after intracutaneous injection with H37Rv antigen in mice infected with different M. tuberculosis strains at 1, 3, 16, 23, 28 and 56 days after infection. With the exception of Less-transmissible 1 the highest DTH response was noted at day 23. Low (-----), moderate (——) and high (–·–·–·–) responders at day 16 are shown. Low responders remain the lowest during the experimental period, whereas the moderate and high responders show high variation at the other days postinfection.
DISCUSSION
With the aim to study the effect of M. tuberculosis genotype on the course of infection and immunopathology, groups of BALB/c mice were infected intratracheally with one of 19 different M. tuberculosis strains from nine different major genotypes. The results show that these strains varied with regard to virulence, as measured by survival curves, and with regard to histopathology, bacillary burden at different time points after infection and DTH responses. The summary of each of these four outcome parameters for all strains in Table 2 shows that a good correlation exists between the pathology, virulence and bacillary load, all three outcome parameters per strain being in the same or proximate category. All five strains with the highest score for histopathology also induced the highest mortality, with the death of all mice within 92 days after infection, and showed the highest bacillary burden. On the other side of the spectrum all six strains with the lowest score for histopathology caused no or only minimal mortality after 112 days and showed the lowest bacillary burden. DTH responses correlate poorly with these three parameters, except for day 16.
The granulomas formed after infection with the different strains of M. tuberculosis were classified as type-2 (lepromatous-like), as these lacked a well-circumscribed edge, giant cells and fibrosis, while histiocytes, polymorphonuclear granulocytes and noncaseous necrosis were present [27]. Strains with a high histopathology score, Beijing 2 and 3, Africa 1 and 2, Haarlem 2, were effective inducers of granulomas and were highly virulent as indicated by the short survival time. This was associated with high CFU counts, as is characteristic for the lepromatous type of granuloma [27].
Thus, the histopathological scores were generally positively correlated with CFU counts, the only exception being the Canetti strain that induced a minimal pathology score but relative high CFU-counts at all time points. Clinically manifest tuberculosis due to M. canettii is rare and thus the discrepancy between histopathology and CFU counts illustrates the fact that it is not the bacillary burden per se but the host immune response that determines the clinical effects of infection. In this regard, it is important to realize that the role of the host immune system in the outcome of mycobacterial infection has been studied extensively. It was found that both in mice and in humans the control of mycobacterial infections depends mainly on macrophage activation, through the effect of Th1 type cytokines. Th1 type cytokines provoke inflammation which may lead to the development of tissue pathology by granulomatous inflammation and necrosis. Th1 immunity is therefore not synonymous with protection. Eventually, the containment of the infection depends on intracellular killing of the mycobacteria or at least suppression of growth into a state of latency. The precise mechanisms that are required for containment of M. tuberculosis during in vivo infection are only partly known. In mice this depends on reactive oxygen and nitrogen radicals and NO whereas these do not seem to be the effector mechanism in humans [23,24]. It was found that, in humans, granulysin in combination with perforin was found as an important mycobactericidal mechanism [28]. This protective activity fails if there is a marked release of Th2 type cytokines [29,30], thus the Th1/Th2 balance is thought to determine the outcome of the encounter with the pathogen. This interplay of cytokines is clearly depicted in a BALB/c model of pulmonary tuberculosis following intratracheal inoculation [8,31–33]. In this model, the initial phase is dominated by high production of Th1 cell cytokines, in coexistence with high levels of TNFα and iNOS, which temporarily control the infection. Three weeks after infection a rise in IL-4 production coexists with a drop in cells expressing IL-2, TNFα and iNOS. Pneumonia in coexistence with a high burden of bacteria in the lung, will lead to death.
DTH is a CD4+ T cell mediated response and can be regarded as a reflection of the strength of the Th1 response. In the present study a temporal DTH response was measured indicating that a long lasting protective immunity was not induced in this infection model and in line with this the DTH responses were poorly correlated with CFU-counts or survival. Absence of correlation of DTH responses, tuberculin skin testing, and protective immunity was also described in humans. The development of DTH responses is complex and depends not only on the amount of antigen present but also on the balance of proinflammatory vs. anti-inflammatory cytokines. A well-known phenomenon in severely ill persons with extensive tuberculosis is skin-test anergy while the bacillary burden is very high.
This study included at least two strains of seven different genotypes, allowing the assesment of variation within genotypes. In general, histopathological scores were not much different between genetically related strains (Africa 1 and 2; Haarlem 1 and 2, Zerocopy 1 and 2, Less-transmissible 1 and 2, Is-in-Ori 1 and 2). However, the strain Beijing 1 had a low score, while Beijing 2 and 3 both had high histopathology scores and a large difference was also recorded between the two Somalia strains. Thus, while intragenotypic variation was generally small, this finding indicates even within one genotype mutations in essential genes may change the phenotype of the bacteria affecting the interaction with the host immune defence system and the course of infection.
Together, the results of this study confirm the findings by Lopez et al. [13], who studied the pathological and immunological responses to infection with four M. tuberculosis strains, Beijing, Canettii, Haarlem and H37Rv, in the BALB/c mouse model, and extend those to 16 additional isolates. Manabe et al. also reported differences in responses between two M. tuberculosis strains, reflecting differences in strain genotype [34].
In conclusion, 11 genetically different M. tuberculosis genotypes induced a broad range of clinical and pathological responses in a mouse model of pulmonary tuberculosis. With a few exceptions, the outcome of infection with strains of the same genotype was generally similar and severe histopathology correlated with a high mortality and high CFU counts but poorly with the degree in DTH responses. The observed differences are likely to be secondary to the effects of the particular genotype on the quantitative and qualitative characteristics of the host immune response. Further study into the specific mechanisms or components that underly the differences in outcome between the genotypes are now mandatory as those are highly relevant for the current battle against tuberculosis, both with regard to outbreak control, treatment of individual patients and the development of improved vaccines because highly virulent strains may require different strategies to obtain optimal results.
REFERENCES
- 1.Delves PJ, Roitt IM. Encyclopaedia of immunology. 2. London: Academic Press; 1998. pp. 1793–7. [Google Scholar]
- 2.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, Bennett's Principles and Practice of Infectious Disease. 4. Vol. 2. New York: Churcill Livingstone; 1995. pp. 2213–9. [Google Scholar]
- 3.Bloom BR, Murray CJ. Tuberculosis commentary on a re-emergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy RC, Ruwende TC, Corrah TK, McAdams KP, Whittle HC, Hill AV. Variation in the NRAMP1 gene and susceptibility to tuberculosis in west-Africans. N Engl J Med. 1998;338:640–4. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 5.Collins FM. Mycobacterial disease, immunosuppression and acquired immuno–deficiency syndrome. Clin Microbiol Rev. 1989;2:360–77. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16:26–35. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 7.Nardell EA. Tuberculosis in homeless, residential case facilities, prisons, nursing homes, and other close communities. Semin Respir Infect. 1989;4:206–15. [PubMed] [Google Scholar]
- 8.Hernández Pando R, Pavón L, Arriaga K, Orozco H, Madrid V, Rook GAW. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–27. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill AV. The immunogenetics of human infectious diseases. Ann Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 10.Stead WW. Genetics and resistance to tuberculosis. Could resistance be enhanced by genetic engineering? Ann Intern Med. 1992;116:937–41. doi: 10.7326/0003-4819-116-11-937. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy R. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1999;1:23–7. doi: 10.1016/s1286-4579(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 12.El Baghdadi J, Remus N, Benslimane A, El-Annaz H, Chentoufi M, Abel L, Schurr E. Variants of the human NRAMP1 gene and susceptibility to tuberculosis in Morocco. Int J Tuberc Lung Dis. 2003;7:599–602. [PubMed] [Google Scholar]
- 13.Lopez B, Aguilar D, Orozco H, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis strains. Clin Exp Immunol. 2003;133:30–7. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale JW, Al-Ghusein H, Al-Hashmi S, et al. Evolutionary relationships among strains of Mycobacterium tuberculosis with few copies of IS6110. J Bacteriol. 2003;185:2555–62. doi: 10.1128/JB.185.8.2555-2562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Z, Morrison N, Watt B, Doig C, Forbes KJ. IS6110 transposition and evolutionary scenario of the direct repeat locus in a group of closely related Mycobacterium tuberculosis strains. J Bacteriol. 1998;180:2102–9. doi: 10.1128/jb.180.8.2102-2109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glynn JR, Whiteley J, Bifani PJ, Kremer K, Van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerging Inf Dis. 2002;8:843–9. doi: 10.3201/eid0808.020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Soolingen D, De Haas PEW, Hermans PW, Groenen PM, Van Embden JD. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:3234–8. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer K, Van Soolingen D, Frothingham R, et al. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–18. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Soolingen D, Hermans PW, De Haas PE, Soll DR, Van Embden JD. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–86. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Soolingen D, Qian L, De Haas PEW, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–8. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Soolingen D, Hoogenboezem T, De Haas PEW, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Sys Bacteriol. 1997;47:1236–45. doi: 10.1099/00207713-47-4-1236. [DOI] [PubMed] [Google Scholar]
- 22.Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Nat Acad Sci. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JYX, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 25.Youmans GP. A method for determination of the culture cycle and the growth rate of virulent human type tubercle bacilli. J Bacteriol. 1946;51:703. doi: 10.1128/jb.51.6.703-710.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence GW. Cultivation of mycobacterium tuberculosis for research purposes. Chapter 6. In: Bloom BR, editor. TuberculosisPathogenesis, Protection and Control. Washington: ASM Press; 1994. pp. 73–83. [Google Scholar]
- 27.Lammas DA, De Heer E, Edgar JD, et al. Heterogenity in the granulomatous resonse to mycobacterial infection in patients with defined genetic mutations in the inteleukin 12-dependent interferon-gamma production pathway. Int J Pathol. 2002;83:1–20. doi: 10.1046/j.1365-2613.2002.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 29.Seah GT, Scott M, Rook GAW. Type 2 cytokine gene activation and its relationship to extend of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 30.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous disease. J Immunol. 2001;166:3432–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 31.Hernández Pando R, Orozco EH, Sampieri A, Pavón L, Velasquillo C, Larriva Sahd J, Madrid MV. Correlation between kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández Pando R, Orozco EH, Arriaga AK, Sampieri A, Larriva Sahd J, Madrid Marina V. Analysis of the local kinetics and localization of interleukin 1α, tumor necrosis factor α and transforming growth factor β during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez Pando R, Schön T, Orozco EH, Serafin M, Estrada GI. Expression of inducible nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp Toxicol Pathol. 2001;53:257–65. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- 34.Manabe YC, Dannenberg AM, Tyagi SK, et al. Different Strains of Mycobacterium tuberculosis Cause Various Spectrums of Disease in the Rabbit Model of Tuberculosis. Infect Immun. 2003;71:6004–11. doi: 10.1128/IAI.71.10.6004-6011.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]