Abstract
The molecular basis of common variable immunodeficiency (CVID) is undefined, and diagnosis requires exclusion of other diseases including X-linked lymphoproliferative disease (XLP). This rare disorder of immunedysregulation presents typically after Epstein–Barr virus infection and results from defects in the SAP (SLAM associated protein) gene. SAP mutations have been found in a few patients diagnosed previously as CVID, suggesting that XLP may mimic CVID, but no large-scale analysis of CVID patients has been undertaken. We therefore analysed 60 male CVID and hypogammaglobulinaemic patients for abnormalities in SAP protein expression and for mutations in the SAP gene. In this study only one individual, who was found later to have an X-linked family history, was found to have a genomic mutation leading to abnormal SAP cDNA and protein expression. These results demonstrate that SAP defects are rarely observed in CVID patients. We suggest that routine screening of SAP may only be necessary in patients with other suggestive clinical features.
Keywords: common variable immunodeficiency, SH2D1 A gene, SLAM-associated protein, X-linked lymphoproliferative disease
INTRODUCTION
Common variable immunodeficiency (CVID) is the most frequently diagnosed primary immunodeficiency and is characterized by varying degrees of T and B cell abnormalities [1,2]. Due to the heterogeneity of both immunological and clinical phenotypes within this disorder, the agreed guidelines for diagnosis require (1) a significant decrease in either two of the three major immunoglobulin isotypes, (2) abnormal specific humoral antibody responses to vaccines and (3) the exclusion of molecularly defined causes of hypogammaglobulinaemia [3]. This third criterion may pose practical difficulties as many known causes of primary antibody deficiency now exist, including X-linked and autosomal recessive agammaglobulinaemias [4–8], X-linked and autosomal recessive hyper-IgM syndromes [9–11] and X-linked lymphoproliferative syndrome [12–14]. Thus before a diagnosis of CVID can be confirmed, a significant number of specialized genetic or protein based diagnostic tests need to be performed, many of which are not readily available. It is therefore necessary to ascertain the true prevalence of the well-defined hypogammaglobulinaemia syndromes within the CVID population.
X-linked lymphoproliferatine disease (XLP) is a rare primary immunodeficiency disorder where severe immunodysregulatory phenomena occur typically after exposure to Epstein–Barr virus (EBV) [15]. The gene defective in XLP is SAP (SLAM-associated protein where SLAM is signalling lymphocyte activation molecule) [12–14], a small SH2 domain containing protein involved in signal transduction events downstream of the SLAM family of receptors, which has been shown to regulate T and NK cell function [16,17]. A number of studies have now demonstrated SAP gene mutations and abnormalities of SAP protein expression in a large proportion of patients with XLP. Thus reliable molecular diagnostic tools for XLP exist.
The three major phenotypes seen in XLP are fulminant infectious mononucleosis (FIM), development of B cell lymphoma and dysgammaglobulinaemia [15]. This last presentation has clinical and immunological similarities with CVID, hence the need to exclude XLP as a possible diagnosis in male CVID patients. We and others have previously identified a small number of ‘CVID’ patients with SAP gene mutations [18–20] suggesting that an unknown percentage of CVID males may in fact have XLP. However, to date no formal large-scale analysis has been undertaken to establish the true prevalence of SAP defects in males with CVID.
METHODS
Patient selection
Following ethical approval from LRECs and informed consent from patients, we obtained 5 ml of peripheral blood from 60 males with a clinical and immunological profile of CVID. Patients were selected only on the basis that they met the ESID/PAGID criteria for CVID and no other selection criteria were used. Serum immunoglobulin levels and vaccine responses, where available for each patient, were obtained. Control blood samples, taken contemporaneously in each centre, were used to establish the extent of protein degradation. Patients were then classified as ‘probable’ or ‘possible’ CVID according to the ESID/PAGID CVID diagnostic criteria [3]. Where vaccine responses were unavailable as a result of immunoglobulin replacement, these patients could not be strictly classified as CVID and were therefore termed hypogammaglobulinaemia.
SAP immunoblot analysis
Peripheral blood mononuclear cells (PBMCs) were isolated and lysed in NP40 lysis buffer. Immunoblotting was performed according to previously described protocols [21,22]. Anti-SAP antibody and the secondary HRP conjugated antirabbit antibody (Sigma, UK) were added at final concentrations of 1 : 1000 in milk-phosphate buffered saline (PBS)-T. To determine the amount of protein degradation, each membrane was subsequently incubated with an anti-β-actin antibody (Sigma) and detected using a horseradish peroxidase antimouse antibody (Dako, UK).
Genomic DNA sequencing
Following polymerase chain reaction (PCR) amplification [21], PCR products were purified using either Microspin columns or Exo-SAP-IT (Amersham Biosciences, Bucks, UK) and then sequenced using Big Dye Chemistry and ABI automated sequencers (Applied Biosystems, Brackley, UK). Primers used for forward and reverse sequencing were the same as those used for generating the PCR product. Sequences from patient samples were compared with wild type SAP sequence.
CDNA analysis
PBMCs were lysed in Trizol (Invitrogen, Paisley, UK) and RNA isolated. First strand cDNA was made using oligo-dT (Sigma, UK). This product was then amplified using SAP-specific primers and conditions as described [12]. cDNA was separated on a 1% Nu-Sieve gel (Cambrex, East Rutherford, NJ, USA). Sequencing of SAP cDNA was performed as for genomic DNA sequencing using the primers for generating the cDNA PCR product.
RESULTS AND DISCUSSION
Sixty male patient blood samples were obtained and patients were categorized according to the existing ESID/PAGID CVID criteria. Those patients with two or more reduced immunoglobulin (Ig) isotype serum measurements together with abnormal vaccine responses were classified as probable CVID (n = 35) and those with one reduced Ig measurement were termed possible CVID (n = 3). Three patients underwent immunoglobulin replacement therapy before IgG levels could be ascertained. In 22 patients, immunoglobulin replacement therapy precluded the assessment of vaccine responses. Thus, in order to adhere strictly to the classification criteria, these patients have been labelled as hypogammaglobulinaemia. Patient characteristics, immunoglobulin and vaccine response profiles, significant family history and history of EBV-related disease are shown in Table 1.
Table 1.
Patient characteristics including serum immunoglobulin levels, response to vaccination and SAP screening findings and relevant history
| Immunoglobulin levels (g/l) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UPN | Age | IgG | IgA | IgM | Vaccine response n/a, L, UD, N H–T–P | CVID diagnosis Probable, possible hypogamma | SAP protein +/– | Mutation | Family history y-CVID, y-XLP, no | EBV disease |
| 1 | 2 | N | L | L | H- UD | prob | +* | ND | no | yes |
| 2 | 57 | L | UD | L | H-L | prob | + | ND | no | no |
| 3 | 12 | L | N | H | T-N, P-L | pos | + | ns | no | no |
| 4 | 64 | L | L | L | H-L | prob | + | ND | no | no |
| 5 | 48 | L | L | L | T-L | prob | + | ND | no | yes |
| 6 | 19 | L | L | L | T-N, H-N, P-L | prob | + | ND | no | yes |
| 7 | 14 | L | UD | L | T-L, P-UD | prob | + | ns | no | no |
| 8 | 15 | L | L | L | n/a | hypo | + | ns | no | no |
| 9 | 6 | N | L | L | H, T, P-UD | prob | + | ns | no | no |
| 10 | 5 | L | L | H | H-N, T-L | prob | + | ns | no | no |
| 11 | 25 | L | L | L | n/a | hypo | + | ND | no | no |
| 12 | 16 | L | L | N | P-L | prob | + | ND | y-CVID | no |
| 13 | 11 | L | L | N | P-L | prob | + | ND | y-CVID | no |
| 14 | 6 | L | L | L | T–N, H-N, P-L | prob | + | ns | no | no |
| 15 | 7 | L | N | L | T–UD, P-L | prob | + | ns | no | no |
| 16 | 52 | L | L | L | n/a | hypo | + | ND | no | no |
| 17 | 13 | L | L | L | T-N, H-UD, P-L | prob | + | ns | no | no |
| 18 | 21 | R | L | L | n/a | hypo | + | ns | no | no |
| 19 | 19 | N | L | L | P-L | prob | + | ND | y-CVID | yes |
| 20 | 2 | N | L | L | H-UD | prob | + | ns | no | no |
| 21 | 7 | L | L | L | T-L, H-UD, P-UD | prob | + | ns | no | no |
| 22 | 9 | L | L | L | T-L, H-UD, P-UD | prob | + | ns | no | no |
| 23 | 3 | L | L | L | n/a | hypo | + | ns | no | no |
| 24 | 6 | N | L | L | T-N, P–L | prob | + | ND | no | yes |
| 25 | 8 | H | L | L | T-L | prob | + | ns | no | no |
| 26 | 12 | N | L | L | T-N, H-UD, P-N | prob | + | ns | no | no |
| 27 | 47 | L | L | L | n/a | hypo | + | ND | no | no |
| 28 | 8 | L | N | L | T–N, P-L | prob | + | ns | no | no |
| 29 | 46 | L | L | L | n/a | hypo | + | ND | no | no |
| 30 | 8 | L | L | L | T-N, P-L | prob | + | ns | no | no |
| 31 | 25 | N | L | L | n/a | hypo | + | ns | no | no |
| 32 | 13 | L | N | L | T–N, P-L | prob | + | ns | no | no |
| 33 | 24 | L | L | H | n/a | hypo | +* | ND | no | no |
| 34 | 7 | L | L | L | T, H, P-UD | prob | + | ND | no | no |
| 35 | 21 | N | L | L | n/a | hypo | + | ns | no | no |
| 36 | 46 | R | L | H | n/a | hypo | + | ND | no | no |
| 37 | 10 | L | L | L | H–L, T-L | prob | + | ns | no | no |
| 38 | 2 | L | L | L | T-L | prob | + | ns | no | no |
| 39 | 16 | L | UD | N | P-L | prob | + | ND | no | no |
| 40 | 51 | L | L | L | n/a | hypo | + | ND | no | no |
| 41 | 54 | L | L | L | n/a | hypo | + | ND | no | no |
| 42 | 13 | L | L | N | n/a | hypo | + | ns | no | no |
| 43 | 12 | L | L | L | n/a | hypo | +* | ND | y-CVID | no |
| 44 | 78 | L | L | L | H, T, P-UD | prob | + | ND | y-CVID | no |
| 45 | 11 | N | L | L | n/a | hypo | + | ND | no | no |
| 46 | 41 | L | L | L | H-UD | prob | + | ns | no | no |
| 47 | 9 | N | L | L | n/a | hypo | + | ns | no | no |
| 48 | 39 | L | UD | L | n/a | hypo | + | ND | no | no |
| 49 | 20 | R | L | L | n/a | hypo | + | ns | no | no |
| 50 | 7 | L | H | L | H, T, P-UD | prob | + | ns | no | no |
| 51 | 10 | L | L | L | T-L | prob | + | ns | no | no |
| 52 | 13 | L | L | L | T-UD | prob | +* | ND | no | no |
| 53 | 12 | N | N | L | H-UD, T-L | pos | + | ND | y-CVID | no |
| 54 | 4 | L | L | N | n/a | hypo | + | ND | y-CVID | no |
| 55 | 4 | L | L | N | H-L, T-N | prob | + | ND | y-CVID | no |
| 56 | 33 | L | L | N | n/a | hypo | +* | YES | y-XLP | yes |
| 57 | 4 | N | N | L | H-L, T-L, P-N | pos | + | ND | y-CVID | no |
| 58 | 5 | N | L | N | n/a | hypo | + | ND | no | no |
| 59 | 9 | L | L | L | H-L | prob | +* | ND | no | no |
| 60 | 19 | L | N | L | T-L | prob | + | ND | no | yes |
UD: undetectable; L: low; n/a: not available; N: normal; H: Haemophilus influenzae (Hib); H: high;T: tetanus; R: replacement; P: pneumococcal.
+: low SAP protein expression. ND: no mutation detected on genomic sequencing of SAP gene; ns: not sequenced; y-CVID: family history of CVID; y-XLP: family history of individuals with XLP-like symptoms; no: no history of note. EBV disease: defined as patients with EBV-related complications such as severe infectious mononucleosis, EBV infection prior to onset of hypogammaglobulinaemia or EBV-related lymphoma.
SAP protein expression
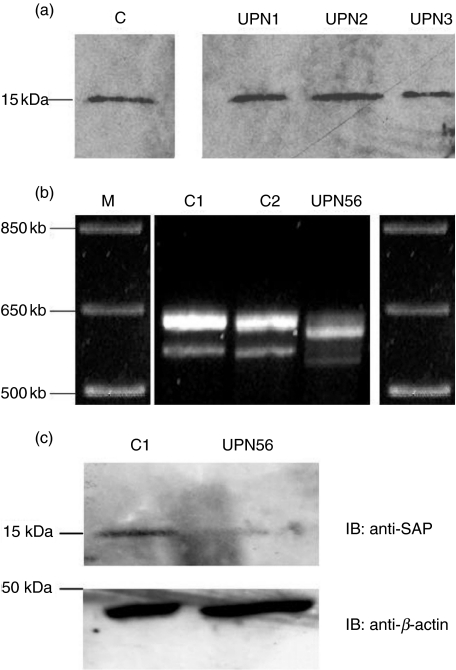
SAP protein expression has been established previously as a reliable diagnostic tool for XLP by consistent detection of SAP expression in PBMCs from healthy controls [21]. To exclude XLP as a diagnosis within our cohort, we initially screened PBMCs from each patient for SAP expression using immunoblot analysis. Within the total of 60 patients tested for SAP expression, all three ‘possible’, 35 ‘probable’ CVID and 22 ‘hypogammaglobulinaemia’ patients were shown to express SAP protein (examples shown in Fig. 1a).
Fig. 1.
Analysis of SAP protein and cDNA expression in controls, CVID patients and UPN56. (a) SAP expression from PBMC lysates obtained from one control (C1) and three patients (UPN1-3), blotted with a human anti-SAP rabbit polyclonal antibody. Control and patient samples express a normal-sized SAP protein at 15 kDa. (b) cDNA from two controls (C1 and C2) and UPN56 was amplified using SAP-specific primers. The control sample shows two bands, a predominant species of 629 bp and a second smaller band of 574 bp. The SAP PCR products from UPN56 show altered mobility when compared to normal control PCR products. Marker bands (M) are shown on either side. (c) Quantitative analysis of SAP protein expression from a control (C1) and UPN56 shows markedly decreased SAP expression in the patient. Equal loading of protein by analysis of β-actin expression is seen in the lower panel.
The protein expression assay alone does not necessarily exclude a diagnosis of XLP, as a small proportion of patients with SAP mutations may express a stable SAP protein. Furthermore, in six patients (including UPN56) the level of SAP protein expressed was markedly decreased compared with the control sample (data not shown). The initial immunoblot analysis was not performed in a quantitative manner, and thus the significance of low protein expression was unclear. For these reasons further, more detailed investigation by sequencing of the SAP gene for genetic defects was performed on 32 patients (15 of ‘probable’ CVID, one of ‘possible’ CVID and 16 of hypogammaglobuliaemia patients), including those with a relevant family history or history of EBV disease and all those with decreased SAP protein expression.
SAP gene analysis
Direct genomic DNA sequencing of all four exons including intron/exon boundaries was performed on 32 patients (53%) in our cohort. No abnormalities were found in 31 patients. In one patient (UPN56) who had been shown previously to have low levels of SAP protein expression, sequencing of exon 1 revealed a c.117 c(r)t change which would be predicted to affect exon splicing. Analysis of SAP cDNA from control individuals shows a 629 base pair band and a smaller second band of 574 bp, which arises as a result of an infrequently used splicing acceptor site within exon 3. However, analysis of SAP cDNA from UPN56 revealed a predominant SAP PCR product which was significantly smaller than the 629 bp predominant control SAP band (Fig. 1b). Direct sequencing of the predominant SAP cDNA band from this patient confirmed a deletion mutation of 21 base pairs (c.116–137), which arises as a direct consequence of the splice site abnormality detected at the intron/exon boundary of exon 1. These findings confirm a diagnosis of XLP in UPN56.
In view of these cDNA abnormalities SAP protein expression from UPN56 was re-analysed in a quantitative manner. This showed a marked decrease in the amount of SAP protein (which was normal in size) expressed in comparison to the control, while β-actin expression was equivalent, suggesting that equal amounts of total protein were loaded in patient and control samples (Fig. 1c). These data suggest that UPN56 is capable of generating both a normal-sized transcript (which was not visible on cDNA analysis, Fig. 1b) and a normal-sized protein, albeit at very low levels. The protein species arising from the mutated mRNA was not visible, probably as a result of unstable protein degradation.
The diagnosis of CVID remains problematic and requires exclusion of other known genetic abnormalities [3]. This includes XLP which can present with dys- or hypogammaglobulinaemia and thus mimic CVID. In this study we analysed prospectively patients who fulfilled the existing diagnostic criteria of CVID for defects in SAP using both protein- and genetic-based analysis. Our findings show that the prevalence of XLP within an unselected but well-defined male CVID and hypogammaglobulinaemic population is low.
In this study, only 1/60 patients was found to have a defect, but we note that not all patients were sequenced and so a figure of 1/32 may be more accurate. It is possible that of the 28 unsequenced patients with normal protein expression and no suggestive history, some may harbour mutations in the SAP gene, but we believe this to be unlikely.
Previous reports of SAP defects in CVID patients prompted the present study. However, careful analysis of these reports demonstrates that in the majority of these cases [19,20], there was a history of affected males in the family or the onset of symptoms after EBV infection. Only in the report of Nistala et al. [18] were SAP mutations found in two sporadic cases without evidence of EBV infection. In the one individual (UPN56) identified in our study as having XLP, it was learned subsequently that a brother had died in early childhood from probable fulminant infectious mononucleosis. Thus the majority of cases of CVID or hypogammaglobulinaemic patients with SAP defects appear to have a positive family history or evidence of EBV infection prior to the onset of symptoms. This is supported by evidence from Sumegi et al. [23], where it was shown that even in patients presenting with typical XLP, mutations were only found in those with an X-linked pedigree and no mutations were found in sporadic cases and atypical cases.
We would therefore suggest that, given the low prevalence of XLP within the CVID population, routine SAP protein and gene screening of all male CVID patients is not indicated unless there are other clinical features such as a positive family history and/or a history of EBV related onset of symptoms.
Acknowledgments
We would like to thank the members of the Great Ormond Street clinical immunology and molecular genetics laboratories for their help in processing these samples, and we would like to thank the PiA (primary immunodeficiency association) for funding this work.
References
- 1.Cunningham-Rundles C. Common variable immunodeficiency. Curr Allergy Asthma Rep. 2001;1:421–9. doi: 10.1007/s11882-001-0027-1. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 3.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 4.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 5.Yel L, Minegishi Y, Coustan-Smith E, et al. Mutations in the mu heavy-chain gene in patients with agammaglobulinemia. N Engl J Med. 1996;335:1486–93. doi: 10.1056/NEJM199611143352003. [DOI] [PubMed] [Google Scholar]
- 6.Minegishi Y, Coustan-Smith E, Wang YH, et al. Mutations in the human lambda5/14.1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–7. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minegishi Y, Coustan-Smith E, Rapalus L, et al. Mutations in Igalpha (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999;104:1115–21. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minegishi Y, Rohrer J, Coustan-Smith E, et al. An essential role for BLNK in human B cell development. Science. 1999;286:1954–7. doi: 10.1126/science.286.5446.1954. [DOI] [PubMed] [Google Scholar]
- 9.Korthauer U, Graf D, Mages HW, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–41. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Giliani S, Insalaco A, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci USA. 2001;98:12614–9. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–75. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 12.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–9. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 13.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–35. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 14.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci USA. 1998;95:13765–70. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seemayer TA, Gross TG, Egeler RM, et al. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr Res. 1995;38:471–8. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Sharifi R, Sinclair JC, Gilmour KC, et al. SAP mediates specific cytotoxic T cell functions in X-linked lymphoproliferative disease. Blood. 2004 doi: 10.1182/blood-2003-09-3359. [DOI] [PubMed] [Google Scholar]
- 17.Bottino C, Falco M, Parolini S, et al. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein–Barr virus-infected B cells in X-linked lymphoproliferative disease. J Exp Med. 2001;194:235–46. doi: 10.1084/jem.194.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nistala K, Gilmour KC, Cranston T, et al. X-linked lymphoproliferative disease: three atypical cases. Clin Exp Immunol. 2001;126:126–30. doi: 10.1046/j.1365-2249.2001.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morra M, Silander O, Calpe S, et al. Alterations of the X-linked lymphoproliferative disease gene SH2D1A in common variable immunodeficiency syndrome. Blood. 2001;98:1321–5. doi: 10.1182/blood.v98.5.1321. [DOI] [PubMed] [Google Scholar]
- 20.Soresina A, Lougaris V, Giliani S, et al. Mutations of the X-linked lymphoproliferative disease gene SH2D1A mimicking common variable immunodeficiency. Eur J Pediatr. 2002;161:656–9. doi: 10.1007/s00431-002-1083-9. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour KC, Cranston T, Jones A, et al. Diagnosis of X-linked lymphoproliferative disease by analysis of SLAM-associated protein expression. Eur J Immunol. 2000;30:1691–7. doi: 10.1002/1521-4141(200006)30:6<1691::AID-IMMU1691>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Eastwood D, Gilmour KC, Gaspar HB. Molecular diagnosis of congenital immunodeficiency. Meth Mol Med. 2004;91:91–108. doi: 10.1385/1-59259-433-6:91. [DOI] [PubMed] [Google Scholar]
- 23.Sumegi J, Huang D, Lanyi A, et al. Correlation of mutations of the SH2D1A gene and Epstein-Barr virus infection with clinical phenotype and outcome in X-Linked lymphoproliferative disease. Blood. 2000;96:3118–25. [PubMed] [Google Scholar]