Abstract
The objective of this study was to determine the diagnostic and prognostic values of antiglucose-6-phosphate isomerase (GPI) antibodies in patients with very early arthritis. Anti-GPI antibodies were measured by ELISA using purified GPI from rabbit muscle in: (i) 383 sera from healthy blood donors (n = 120), well-established rheumatoid arthritis (RA) (n = 99) and non-RA differentiated arthritis (NRADA) (n = 164) patients; (ii) 195 sera obtained from community-recruited patients with very early inflammatory arthritis (VErA cohort) that were studied for 1 year and classified as having RA (n = 116), NRADA (n = 41), and undifferentiated arthritis (UA) (n = 38) after the follow-up period. The criterion for severity was the progression of radiographic damage. Prevalence of anti-GPI antibodies was significantly higher in well-established RA patients (45·4%) compared to healthy subjects (2·5%). Anti-GPI antibodies were also present in sera from NRADA: systemic lupus erythematosus 53%, polymyositis 45·4%, adult-onset Still's disease 44%, systemic sclerosis 42·8%, spondylarthropathies 25% and primary Sjögren’s syndrome 5·8%. No significant association was found between the presence of anti-GPI antibodies and the 3 diagnostic groups from the VErA cohort. No correlation was observed between anti-GPI and autoantibodies usually associated with RA. Anti-GPI antibodies were not predictive of radiological progression in patients with very early arthritis. Thus, anti-GPI antibodies are not useful for discriminating RA from non-RA rheumatic diseases and do not constitute a predictive factor of structural damage.
Keywords: rheumatoid arthritis, anti-GPI, diagnosis, prognosis
INTRODUCTION
Rheumatoid arthritis (RA) is the most frequent chronic rheumatic disease thought to be of both inflammatory and autoimmune origin. A large number of autoantibodies (autoAbs) is associated with RA and some of them constitute useful markers for RA diagnosis and/or prognosis. Rheumatoid factor (RF) is the unique serological marker of the ACR criteria [1] and is also considered to have bad prognostic value [2–4]. AutoAbs recognizing citrullinated antigenic determinants of filaggrin [5], and particularly anticyclic citrullinated peptide (CCP) and antirat citrullinated recombinant filaggrin antibodies, are another interesting serological marker because of their early appearance in the course of the disease [6], their high diagnostic value [7–13], their ability to predict the persistence of the disease and particularly to distinguish erosive from non erosive RA [14–18]. However, anticitrullinated protein/peptide autoAbs do not allow to diagnose all RA. Thus, new serological markers are highly required.
Recently, a new animal model of RA has been described by Matsumoto et al. [19] which is characterized by the production of arthritogenic autoAbs. In this model, K/BxN T cell receptor transgenic mice spontaneously develop a destructive and chronic polyarthritis sharing features with human RA. These mice produce autoAbs directed against the ubiquitous cytoplasmic enzyme glucose-6-phosphate isomerase (GPI) that were shown to induce arthritis when injected into normal recipients. The pathogenic property of anti-GPI antibodies was demonstrated to be due to their interaction with GPI deposits present at the cartilage surface and the subsequent activation of complement alternative pathway [20–22] in the absence of membrane-bound complement regulatory proteins. Thus, the unique molecular characteristics of cartilage with the involvement of innate immunity can explain a tissue specific disease mediated by autoAbs directed to an ubiquitous molecule [23–25].
This model revived the role of autoAbs in RA and prompted several investigators to look for the presence of anti-GPI antibodies in RA and other rheumatic diseases. While the original study reported the presence of autoAbs to GPI in RA [26], recent data [27–31] suggest that they do not contribute to the diagnostic of RA. However, the clinical significance of these autoAbs has not yet been assessed in a well-documented cohort of community cases of very early arthritis, particularly in terms of prognostic value. Thus, the aim of this present study was to evaluate the diagnostic and prognostic significance of anti-GPI autoAbs in community-recruited patients with very early arthritis.
PATIENTS AND METHODS
Group 1: patients studied to determine the characteristics of the ELISA used for detection of anti-GPI autoAb
From 1989 to 2002, sera from patients with classified rheumatic diseases referred to the departments of Rheumatology and Immunology were collected and stored at −80°C. A total of 383 disease-associated and control sera was tested. This group included 120 blood donors as the healthy control group, 99 patients with established RA (median duration, 6 years) and 164 patients with non-RA rheumatic diseases defined according to international criteria [1,32–37] i.e. 85 with systemic lupus erythematosus (SLE), 28 with spondylarthropathies (SPA), 17 with primary Sjögren syndrome (pSS), 14 with systemic sclerosis (SSc), 11 with polymyositis (PM) and 9 with adult-onset Still's disease (AOSD).
Group 2: community-recruited patients with very early arthritis (VErA cohort) to evaluate the diagnostic and prognostic values of anti-GPI autoAbs
At the time of anti-GPI autoAb evaluation, this cohort included 195 patients (mean age 51 ± 14·4 years, range 19–84 years; F/M ratio: 2·3) recruited prospectively in two French regions, i.e. the entire province of Haute-Normandie (1·8 million people) and the metropolitan area of Amiens (300 000 people). They are primarily European Caucasian. General practitioners, private and hospital rheumatologists of these areas recruited new cases of inflammatory arthritis. To contact a maximum of patients, so as to obtain a representative sample of those regions, a large information campaign was conducted yearly through the news, radio and TV medias. Patients were required to have swelling of at least 2 joints persisting for ≥4 weeks, evolving for < 6months (4·5 ± 2·4 month, range 2–6 months), and no local or systemic corticotherapy or disease-modifying antirheumatic drugs (DMRADs) before inclusion. Exclusion criteria were: inflammatory back pain, pregnant or nursing woman. At the end of the follow-up, i.e. 1 years after the first symptoms, patients were classified as having RA (n = 116) according to ACR criteria, non-RA differentiated arthritis (NRADA) (n = 41) (Table 1), and undifferentiated arthritis (UA) (n = 38). Patients with well defined non-RA rheumatic diseases were not followed-up. At baseline, several clinical and biological parameters were collected: Ritchie articular index, disease activity score (DAS), ESR, CRP and autoantibodies including RF isotypes, AKA, APF, anti-CCP, antiα-enolase and anti-GPI autoAbs (see below). Radiographs of the hands and feet were carried out at inclusion and at the end of follow-up. They were read chronologically by two experienced rheumatologists (OM and PF), using the van der Heijde-modified Sharp method. The criterion for severity of the disease was defined by progression of radiological damage during the follow-up period. The study was approved by the local Ethical Committee. Prior to entry into the protocol, each patient was informed of the nature, duration and purpose of the study and gave written consent.
Table 1.
Prevalence of anti-GPI autoantibodies in the different populations studied
| Anti-GPI | |||
|---|---|---|---|
| Patients n | n | (%) | |
| Group 1 | |||
| Healthy controls | 120 | 3 | (2·5) |
| Rheumatoid arthritis | 99 | 45 | (45·4) |
| Systemic lupus erythematosus | 85 | 45 | (52·9) |
| Spondylarthropathies | 28 | 7 | (25) |
| Primary Sjögren syndrome | 17 | 1 | (5·9) |
| Systemic sclerosis | 14 | 6 | (42·8) |
| Polymyositis | 11 | 5 | (45·4) |
| Adult-onset Still's disease | 9 | 4 | (44·4) |
| Group 2 | |||
| Rheumatoid arthritis | 116 | 33 | (28·4) |
| Unclassified arthritis | 38 | 8 | (21·1) |
| Non-RA differentiated arthritis | 41 | 11 | (26·8) |
| Spondylarthropathies | 14 | 3 | (21·4) |
| Crystal-induced arthritis | 5 | 0 | (0) |
| Osteoarthritis | 11 | 1 | (9·1) |
| Paramalignant arthritis | 3 | 1 | (33·3) |
| Parvovirus B19 arthritis | 1 | 1 | (100) |
| Connective tissue diseases | |||
| Primary Sjögren syndrome | 2 | 2 | (100) |
| Mixed connective tissue disease | 1 | 1 | (100) |
| Polymyositis | 1 | 0 | (0) |
| Behcet's disease | 1 | 1 | (100) |
| Wegener's granulomatosis | 1 | 1 | (100) |
| Unclassified | 1 | 0 | (0) |
ELISA for the detection of anti-GPI antoAbs (anti-GPI ELISA)
Microtitre plates (96 wells, CEB, Angers, France) were coated with purified rabbit muscle GPI (Sigma, Saint-Quentin Fallavier, France), at 10 µg/ml in carbonate/bicarbonate buffer for 2 h at 37°C. A unique batch of GPI was used for all the study. Free sites were blocked with PBS containing Tween 0·05% (V/V) (PBST) and bovine serum albumin 1% (W/V) (PBSTBSA) for 1 h at 37°C. Serum samples were diluted at 1 : 100 in PBSTBSA and 100 µl were added to each well for 2 h at 37°C. Each serum sample was assayed in duplicate on wells coated with rabbit GPI or coating buffer alone. After 6 washes in PBST, F(ab′)2 goat antihuman IgG Fc labelled with peroxydase (Jackson Immunology, Baltimore, PA, USA) were added for 1 h at 37°C. After 3 washes, O-phenylenediamine substrate (Sigma) was used to detect enzymatic activity and reaction was stopped with 3 N HCl. Plates were read at 492 nm. A standard curve was established using dilutions of a serum known to have a high anti-GPI Ab titre. AutoAb titres were expressed in arbitrary units (AU).
Western blotting
Purified rabbit muscle GPI was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a nitrocellulose filter using NuPage system (Novex, Invitrogen, Cergy Pontoise, France). The membrane was air dried overnight and incubated with anti-GPI Ab positive sera diluted at 1 : 80. IgG were revealed using goat antihuman IgG labelled with phosphatase alkaline (Caltag Laboratories, Burlingame, CA, USA). Revelation was performed with 5-Bromo-4-Chloro-3-Indolyl phosphate/Nitro Blue Tetrazolium substrate (Sigma).
Indirect immunofluorescence detection of AKA/APF
APF was detected on freshly prepared human buccal mucosa cells and AKA by a semiquantitative indirect immunofluorescence assay on sections of the middle third of rat oesophagus, as previously reported [38,39].
ELISA for the detection of anti-CCP autoAbs
The second generation CCP2 ELISA from Euroimmun (Groß Grönau, Germany) was used for the detection of anti-CCP autoAbs.
ELISA for the detection of IgG, IgA and IgM rheumatoid factor(RF) and antiα-enolase autoAbs
RFs of the different isotypes and antihuman recombinant α-enolase autoAbs were detected using in-house ELISAs as previously described [40,41].
Statistical analysis
Comparisons of anti-GPI autoAb titres and percentages were performed using Kruskal–Wallis One-Way anova Rank-Test. χ2-test or Fisher's exact test were used for comparison of percentages in different groups of patients. Correlations between anti-GPI antibodies and other biological markers were performed using the non parametric Spearman Rank-Test. A P < 0·05 was considered significant.
RESULTS
Anti-GPI autoAbs in healthy subjects, RA and non-RA patients (group 1)
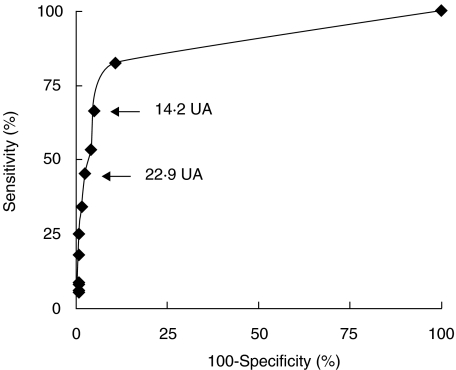
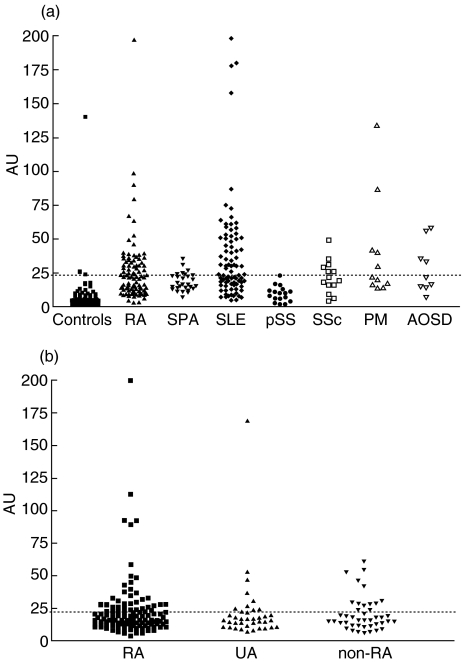
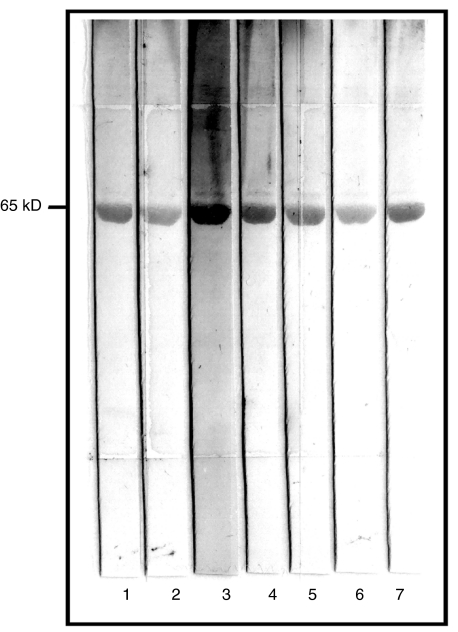
One hundred and twenty sera from healthy controls and 99 patients with well-established RA were tested in anti-GPI ELISA. Receiver Operating Characteristic (ROC) Curve (Fig. 1) indicated that this serological marker discriminated RA patients from healthy subjects. Using cut-off values of 14·2 and 22·9 AU, the sensitivity was 66·7% and 45·4% and specificity 95% and 97·5%, respectively. However, the analysis of 166 sera from patients with various well-established rheumatic diseases (Fig. 2a and Table 1) indicated that anti-GPI autoAbs were not specific for RA. Indeed, using a cut-off value of 22·9 UA, 52·9% of SLE, 45·4% of PM, 44% of AOSD, 42·7% of SSc, 25% of SPA and 5·9% of pSS sera were positive for this marker. The GPI-binding activity detected in these sera was not due to recognition of potential contaminants present in the commercial GPI preparation as demonstrated by the immunoblotting assay of the rabbit muscle GPI used as antigen. Indeed, all ELISA-anti-GPI positive sera tested had a unique reactivity with a 65 kD molecule corresponding to GPI (Fig. 3).
Fig. 1.
Receiver operating characteristic curve of anti-GPI ELISA: 120 blood donor and 99 rheumatoid arthritis sera were used to establish the curve.
Fig. 2.
Anti-GPI antibody levels in sera from (2a) group 1 including NC (normal controls, n = 120), RA (rheumatoid arthritis, n = 99), SLE (systemic lupus erythematosus, n = 85), SPA (spondylarthropathies, n = 28), pSS (primary Sjögren syndrome, n = 17), SSc (systemic sclerosis, n = 14), PM (dermatopolymyositis, n = 11), AOSD (adult-onset Still's disease, n = 9); and (2b) group 2 corresponding to the 195 patients of the VErA cohort classified as RA (n = 116) according to ACR criteria, non-RA differentiated arthritis (n = 41) and undifferentiated arthritis (UA; n = 38).
Fig. 3.
Western blot analysis of commercially available rabbit muscle GPI with anti-GPI antibody positive sera. Lane 1: healthy control, lanes 2–4: rheumatoid arthritis sera, lanes 5–7: nonrheumatoid arthritis sera. All sera positive in the solid phase ELISA reacted exclusively with a 65 kD polypeptide corresponding to the molecular weight of GPI.
Diagnostic value of anti-GPI autoAbs for very early RA (group 2)
The clinical significance of anti-GPI autoAbs was determined in community-recruited patients with very early arthritis (VErA cohort). 195 sera obtained at entry in the study were tested (Tables 1 and 2) and 25·6% of them were found positive using a cut-off value of 22·9 AU (Fig. 2b). No difference in terms of anti-GPI autoAb levels (P = 0·8) or percentage of positive sera (P = 0·72) was found between RA, NRADA and UA groups. Moreover, no relationship was found between anti-GPI autoAbs and any other autoAb populations usually associated with RA, i.e. RF, AKA, APF and anti-CCP Abs (data not shown). Of note, no positive correlation with antiα-enolase autoAbs was observed. In the RA subgroup, a significant positive correlation was found with ESR and CRP (r = 0·25, P = 0·006 and r = 0·2, P = 0·03, respectively). However, anti-GPI autoAbs were not associated with DAS and Ritchie articular index (r= 0·1, P = 0·14 and r = 0·11, P = 0·1, respectively).
Table 2.
Demographic, clinical and biological characteristics of the 195 patients from the VErA cohort (group 2)
| Characteristics | Rheumatoid arthritis | Undifferentiated arthritis | Non-RA differentiated arthritis |
|---|---|---|---|
| Number | 116 | 38 | 41 |
| Age in years (mean ± SD (range)) | 52·2 ± 14·5 (24–84) | 50·4 ± 13·4 (19–79) | 51·4 ± 15·1 (23–84) |
| RA duration in months (mean ± SD (range)) | 4·2 ± 1·6 (0·9–6) | 4·5 ± 1·5 (1.5–6) | 4·1 ± 2·1 (1–6) |
| Ritchie index (/78) (mean ± SD (range)) | 10·3 ± 8·9 (0–58) | 5·6 ± 5·1 (0–15) | 6·5 ± 5·3 (0–21) |
| DAS (mean ± SD (range)) | 3·5 ± 1·1 (0·5–7·4) | 2·3 ± 1 (0·6–4·5) | 2·6 ± 0·9 (0·9–4·7) |
| CRP (mg/l) (mean ± SD (range)) | 20·9 ± 27 (2–164) | 13·8 ± 19 (1.5–108) | 17·3 ± 27·5 (3–128) |
| ESR (mm in 1 h) (mean ± SD (range)) | 26·9 ± 23 (2–105) | 17·2 ± 16·5 (2–68) | 24·2 ± 23·5 (2–110) |
| Antibody frequency (%) | |||
| RF-IgG | 38 | 10·5 | 7·3 |
| RF-IgA | 27·6 | 0 | 4·9 |
| RF-IgM | 35·3 | 5·3 | 9·8 |
| Latex test | 28·1 | 5·3 | 2·4 |
| Rose-Waaler test | 27·2 | 2·7 | 5 |
| AKA | 21·6 | 7·9 | 7·3 |
| APF | 37·1 | 10·5 | 7·3 |
| Anti-CCP | 40·5 | 2·6 | 7·3 |
| Anti-GPI | 28·4 | 21·1 | 26·8 |
| Anti-α-enolase | 20·6 | 7·8 | 4·8 |
| Presence of radiological progression (%) | 30·2 | 15·8 | ND |
DAS, Disease Activity Score; RF, rheumatoid factor; AKA, antikeratin antibodies; APF, antiperinuclear factor; ND, not done.
Prognostic value of anti-GPI autoAbs in the VErA cohort (group2)
The severity of the disease was assessed by the radiological progression during the 1-year follow-up. Among the 154 patients with RA or UA for whom radiographs were available both at onset of the study and at the end of follow-up, 41 were characterized by the presence of radiological progression. Since the pathogenicity of anti-GPI autoAbs has been demonstrated in the K/BxN model, their presence in RA patients may constitute a predictive marker of cartilage destruction. Statistical analysis showed that the presence (P= 0·77) or high titres (P = 0·7) of anti-GPI autoAbs at baseline were not predictive of radiological progression.
DISCUSSION
In this study we showed that autoAbs directed against GPI are produced in the course of RA and could be detected in 45% of patients with a well-established disease using a cut-off value corresponding to a specificity of 97·5% as determined by a ROC curve established from RA and healthy control sera. Our anti-GPI ELISA was very similar to that used by others [26–31] although 3 modifications were introduced which, in our hands, allowed a high reproducibility of the assay: serum samples were used at 1 : 100 dilution; OD values given by sera incubated on uncoated wells were subtracted from OD value on coated-wells and our conjugate was peroxydase-labelled. In addition, no immunoreactive contaminants were present in the unique batch of commercially available GPI used in the study, since none of the ELISA anti-GPI Ab positive sera tested recognized polypeptides with molecular weights corresponding to creatine kinase or phosphoglucomutase, two molecules previously reported to be present in GPI obtained from the same commercial source and bound by some RA sera [27,29]. The 45% prevalence of anti-GPI Abs observed in well-established RA is similar to that reported by Schaller et al. [31] but is higher than that reported by other groups using purified rabbit or human recombinant GPI as antigen [26,27]. Although the lack of standardization of the ELISA utilized, in particular the level of the cut-off value, may account for these differences, it is likely that other factors including the composition of the RA cohorts or groups also play a role [26]. In this regard, the duration of the disease may constitute one of these factors and participate in the appearance of anti-GPI Abs. Indeed, while the prevalence of these autoAbs was of 45% in the group of patients with RA having a median duration of 6 years, it was only of 28% in RA patients with a median duration of 4 months. Thus, it is tempting to consider that a sustained inflammation may favour the appearance of anti-GPI Abs, in as much as, in the present study, a significant positive correlation was observed between anti-GPI Ab titres and the levels of CRP and ESR.
The clinical significance of anti-GPI autoAbs was evaluated in two populations, including a cohort of community-recruited patients with very early arthritis. No diagnostic value could be assigned to anti-GPI autoAbs, which is in accordance with results previously reported by others [27–31]. Indeed, anti-GPI autoAbs were not specific for RA and could be detected with high prevalence in other chronic rheumatic diseases such SLE, pSS, SSc, PM, SPA and AOSD. A similar conclusion was reached when the diagnostic value of anti-GPI was assessed in a cohort of patients with recent onset arthritis. The demonstration that anti-GPI autoAbs are pathogenic in K/BxN mice prompted us to determine whether anti-GPI autoAbs were associated with disease progression in RA patients. In the VErA cohort of recent-onset arthritis, the presence of anti-GPI autoAbs at onset of the disease had no predictive value of radiological progression, suggesting that this autoAb population has no prognostic value.
Although anti-GPI autoAbs have no clinical significance, one may question why such autoAbs are present in sera of patients with autoimmune and/or inflammatory diseases. Two arguments suggest that anti-GPI autoAbs belong to natural autoantibody repertoire [42]. First, OD values observed in healthy subject sera ranged from 0·150 to 0·400 (data not shown and results reported in [26,30]), which may indicate the presence of IgG anti-GPI autoAbs in the normal B-cell repertoire. Second, SLE, a pathology in which polyclonal B cell activation is thought to participate in autoAb production, exhibits the highest anti-GPI AutoAb levels.
It is worth noting that anti-GPI autoAbs do not constitute the unique autoAb population direct against enzymes of the glycolytic cycle. Indeed, our group described antiα enolase autoAbs [41] in patients with very early arthritis, that have potential diagnostic (confirmed here, see Table 2) and prognostic values. We thus asked whether the presence of anti-GPI autoAbs was associated with antiα enolase autoAbs. No positive correlation was observed between these two autoAbs populations autoAbs, indicating that the breakage of B-cell tolerance to enzymes of the glycolytic pathway is dissociated. However, since the pathogenicity of RA in human is complex and the disease probably heterogeneous, autoAbs directed to glycolytic enzymes should be studied in selected subgroups of patients, such as those refractory to anti-TNFα therapy, the K/BxN model having demonstrated its absence of sensitivity to this drug [43].
In conclusion, this study confirmed the lack of diagnosis value of anti-GPI autoAbs and their inability to predict radiological progression in early arthritis.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche médicale (INSERM), the Fondation de la Recherche Médicale (FRM), the Programme Hospitalier de Recherche Clinique (PHRC), the association de recherche sur la polyarthrite (ARP) and Pharmacia-Pfizer France. We would like to thank Mrs Annick Valaunay and Marlène Thomas for technical assistance.
REFERENCES
- 1.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:314–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 2.Vittecoq O, Pouplin S, Krzanowska K, et al. Rheumatoid factor is the strongest predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatol. 2003;42:939–46. doi: 10.1093/rheumatology/keg257. [DOI] [PubMed] [Google Scholar]
- 3.Bas S, Perneger TV, Seitz M, et al. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatol. 2002;41:809–14. doi: 10.1093/rheumatology/41.7.809. [DOI] [PubMed] [Google Scholar]
- 4.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girbal-Neuhauser E, Durieux JJ, Arnaux M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro) filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–94. [PubMed] [Google Scholar]
- 6.Rantappa-Dahlqvist S, De Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 7.Schellekens GA, Visser H, De Jong BAW, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Vincent C, Nogueira L, Sebbag M, et al. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay. A high effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2002;46:2051–8. doi: 10.1002/art.10436. [DOI] [PubMed] [Google Scholar]
- 9.Bas S, Genevay S, Meyer O, et al. Anti-cyclic citullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatol. 2003;42:677–80. doi: 10.1093/rheumatology/keg184. [DOI] [PubMed] [Google Scholar]
- 10.Bas S, Perneger TV, Seitz M, et al. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factor. Rheumatol. 2002;41:809–14. doi: 10.1093/rheumatology/41.7.809. [DOI] [PubMed] [Google Scholar]
- 11.Susuki K, Sawada T, Murakami A, et al. high diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol. 2003;32:197–204. doi: 10.1080/03009740310003677. [DOI] [PubMed] [Google Scholar]
- 12.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62:870–4. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bizzaro N, Mazzanti G, Tonutti E, et al. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. Clin Chem. 2001;47:1089–93. [PubMed] [Google Scholar]
- 14.Visser H, Le Cessie S, Vos K, et al. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–65. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 15.Vencovsky J, Machacek S, Sedova L, et al. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis. 2003;62:427–30. doi: 10.1136/ard.62.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forslin K, Vincent C, Serre G, et al. Antifilaggrin antibodies in early rheumatoid arthritis may predict radiological progression. Scand J Rheumatol. 2001;30:221–4. doi: 10.1080/030097401316909567. [DOI] [PubMed] [Google Scholar]
- 17.Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–6. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroot EJ, De Jong BA, Van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–5. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto I, Staub A, Benoist C, et al. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–5. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 20.Solomon S, Kolb C, Mohanty S, et al. Transmission of antibody-induced arthritis is independent of complement component 4 (C4) and the complement receptor 1 and 2 (CD21/35) Eur J Immunol. 2002;32:644–51. doi: 10.1002/1521-4141(200203)32:3<644::AID-IMMU644>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ji H, Ohmura K, Mahmood U, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Gauguier D, Ohmura K, Gonzalez A, et al. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194:321–30. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto I, Maccioni M, Lee DM, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nature Immunol. 2002;3:360–5. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 24.Wipke BT, Wang Z, Kim J, et al. Dynamic visualization of a joint-specific autoimmune response through positron emission tomography. Nature Immunol. 2002;3:342–4. doi: 10.1038/ni775. [DOI] [PubMed] [Google Scholar]
- 25.Maccioni M, Zeder-Lutz G, Huang H, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–7. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaller M, Burton DR, Ditzel HJ. Autoantibodies to GPI in rheumatoid arthritis: linkage between an animal model and human disease. Nature Immunol. 2002;2:746–53. doi: 10.1038/90696. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto I, Lee DM, Goldbach-Mansky R, et al. Low prevalence of antibodies to glucose-6-phosphate isomerase in patients with rheumatoid arthritis and a spectrum of other chronic autoimmune disorders. Arthritis Rheum. 2003;48:944–54. doi: 10.1002/art.10898. [DOI] [PubMed] [Google Scholar]
- 28.Herve CA, Wait R, Venables PJ. Glucose-6-phosphate isomerase is not a specific autoantigen in rheumatoid arthritis. Rheumatology. 2003;42:986–8. doi: 10.1093/rheumatology/keg271. [DOI] [PubMed] [Google Scholar]
- 29.Schubert D, Schmidt M, Zaiss D, et al. Autoantibodies to GPI and creatine kinase in RA. Nature Immunol. 2002;3:411. doi: 10.1038/ni0502-411a. [DOI] [PubMed] [Google Scholar]
- 30.Kassahn D, Kolb C, Solomon S, et al. Few human autoimmune sera detect GPI. Nature Immunol. 2002;3:411–2. doi: 10.1038/ni0502-411b. [DOI] [PubMed] [Google Scholar]
- 31.Schaller M, Benoit VM, Ditzel HJ. Response. Nature Immunol. 2002;3:412–3. [Google Scholar]
- 32.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 33.Dougados M, Van Der Linden S, Juhlin R, et al. The european spondylarthropathy Study Group, preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 34.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren Syndrom. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 35.Masi AT, Rodnan GP, Medsger TA, et al. Preliminary criteria for the classification of systemic sclerosis (sclerodermia) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 36.Tanimoto K, Nakano K, Kano S, et al. Classification criteria for polymyositis and dermatomyositis. J Rheumatol. 1995;22:668–74. [PubMed] [Google Scholar]
- 37.Cassidy JT, Levinson JE, Bass JC, et al. A study of classification criteria for the diagnosis of juvenil rheumatoid arthritis. Arthritis Rheum. 1986;29:274–81. doi: 10.1002/art.1780290216. [DOI] [PubMed] [Google Scholar]
- 38.Youinou P, Le Goff P, Dumay A, et al. The antiperinuclear factor 1. Clinical and serological associations. Clin Exp Rheumatol. 1990;8:259–64. [PubMed] [Google Scholar]
- 39.Vincent C, Serre G, Lapeyre F, et al. High diagnostic value in rheumatoid arthritis of antibodies to the stratum corneum of rat oesophogus epithelium, so-called ‘antikeratin antibodies’. Ann Rheum Dis. 1989;48:712–22. doi: 10.1136/ard.48.9.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vittecoq O, Jouen-Beades F, Krzanowska K, et al. Rheumatoid factors, anti-filaggrin antibodies and low in vitro interleukin-2 and interferon-gamma production are useful immunological markers for early diagnosis of community cases of rheumatoid arthritis. Preliminary study. Joint Bone Spine. 2001;68:144–53. doi: 10.1016/s1297-319x(00)00244-x. [DOI] [PubMed] [Google Scholar]
- 41.Saulot V, Vittecoq O, Charlionet R, et al. Presence of autoantibodies to the glycolytic enzyme α-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002;46:1196–9. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- 42.Mouthon L, Haury M, Lacroix-Desmazes S, et al. Analysis of the normal human IgG antibody repertoire. Evidence that IgG autoantibodies of healthy adults recognize a limited and conserved set of protein antigens in homologous tissues. J Immunol. 1995;154:5769–78. [PubMed] [Google Scholar]
- 43.Kyburz D, Carson DA, Corr M. The role of CD40 ligand and tumor necrosis factor alpha signaling in the transgenic K/BxN mouse model of rheumatoid arthritis. Arthritis Rheum. 2000;43:2571–7. doi: 10.1002/1529-0131(200011)43:11<2571::AID-ANR26>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]