Abstract
Human genital infection caused by Chlamydia trachomatis is thought to be immunologically mediated, resulting in local recruitment of lymphocyte subsets and inducing the production of cytokines. Little information is available about the role of lymphocyte recruitment and the regulation of cytokine production in the genital tract of C. trachomatis positive infertile women. We have evaluated the recruitment of lymphocyte subsets in the genital tract and production of Th1/Th2 cytokines in cervical secretions and laparoscopic specimens from the fallopian tubes of C. trachomatis positive infertile women (n = 17) and compared them with controls, viz. C. trachomatis negative infertile women (n = 20) using ELISA and flow cytometry. None of these patients were found to be infected either with Candida sps., bacterial vaginosis, Trichomonas vaginalis, Neisseria gonorrhoeae, Mycoplasma hominis or Ureaplasma urealyticum in the cervix. Flow cytometric analysis of cervical secretions in Chlamydia positive women revealed recruitment of both CD4 and CD8 lymphocytes to the genital tract was up-regulated and a variation in the production rates of different cytokines in cervical secretions and fallopian tube was observed. We found that the immune responses in cervical secretions were of Th0 type, since all the analysed cytokines, viz. IFN-γ, TNF-α, IL-10 and IL-12 were up-regulated. As, both CD4 and CD8 cells contribute to the production of IFN-γ and IL-10, these results suggest that along with CD4 cells, CD8 lymphocytes also may be important for local regulation of Th1/Th2 responses in the genital tract during C. trachomatis infection.
Keywords: cytokine expression, genital chlamydiasis, infertile women, ELISA, flow cytometry
INTRODUCTION
Chlamydia trachomatis is a major cause of sexually transmitted diseases in humans in developed as well as in developing countries [1]. A high prevalence of genital C. trachomatis infection has been reported in our country [2]. Left untreated, chlamydial infection can lead to pelvic inflammatory disease, fallopian tube injury and infertility in women. Although the growth of C. trachomatis is restricted to the mucosa, the consequences of infection, namely tubal sterility, pelvic inflammatory disease, etc. [3]. can be devastating. Protective immunity to C. trachomatis infection is limited and repeated episodes of infection are common. How the organism induces the inflammatory changes that lead to fibrosis has been the subject of study in recent years. It is largely believed that the inflammation associated with genital tract disease is immunologically mediated. Persistent antigen synthesis and an ineffective immune response largely contribute to chronic inflammation, tissue damage and immunopathology associated with salpingitis/infertility [4]. Treatment of chlamydial infection does not always prevent progressive tubal damage and little is known about the inflammatory process that leads to the damage of genital tract. Previous exposure to C. trachomatis offers only limited protection against reinfection because, the protection is mostly serovar specific and is due to antibody specific for MOMP, which defines the serovar primarily [5]. So, cell mediated immunity has to offer most of the protection against C. trachomatis infection. Therefore, eliciting protective immunity against C. trachomatis infection might be the most effective approach in either reducing or preventing the sequelae of events leading to the infertile state in women of child bearing age [6,7].
Genital infection with C. trachomatis and the resulting cytokine environment together with route of antigen presentation largely determine the outcome of infection and disease [8,9]. The expression of cytokines within the tissue regulates the recruitment of specific subsets of lymphocytes to distinct parts of genital tract [10,11]. Ultimately, the outcome of genital chlamydial infection depends on both the species as well as host immunological responses to infection and the balance between the pathogen specific Th1 and Th2 cell responses [12]. While critical role of cytokines and lymphocyte subsets recruitment in infection has been reported in different animal models, there is little information available in humans about the nature of immunological events occurring in the female genital tract following infection with C. trachomatis [6,7]. Further, the appropriate design of immunological intervention in disease requires that the source and role of individual cytokines and the relative contributors of individual cell populations to the overall cytokine environment during immune response be determined [13,14]. Local regulation of CD4+ and CD8 + lymphocytes and the role of Th1/Th2 responses in the genital tract during Chlamydia infection are considered to be crucial for controlling the duration of infection and subsequent tubal pathology. Therefore, the present study aims to determine the relative contributions of lymphocyte populations to the cytokine profile in genital C. trachomatis infection at the cellular level in infertile women.
MATERIALS AND METHODS
Study population
With hospital Ethical Board approval, female infertility patients presenting at Safdarjang Hospital, New Delhi, India for gynecology investigations or else, referred for diagnostic laparoscopy were recruited for the study. A total of 37 infertile women (age group = 20–38 years) were enrolled after obtaining their informed oral consent.
At recruitment, a detailed clinical questionnaire was administered to each patient for collecting information on reasons for referral, gynecology history including menstruation, symptoms of genital and urinary tract infection, obstetric and medical histories including recent history of treated sexually transmitted disease (STD) infections, etc. The findings of routine haematological tests (ESR, WBC count, haemoglobin), chest X-ray diagnosis and endometrial biopsy were also recorded. Finding at diagnostic laparoscopy/HSG, viz. tubal patency, adhesions, hydrosalpinx formation, endometriosis, etc. were further noted.
The exclusion criteria were:
A positive urine pregnancy test;
Recent antibiotic therapy;
Presence of tubo-ovarian abscess, or any other pelvic or abdominal process requiring surgery (i.e. appendicitis and torsion of adnexae);
Infertility cases related to a male factor;
History of recent treated STD infections;
Presence of genital tuberculosis.
Collection of samples
The vulva was examined for lesions and vaginal/cervical discharge if any. The cervix was inspected for ulcers, warts, ectopy, erythema, discharge, etc. After cleaning the exocervix with a cotton swab (Hi Media, Mumbai, India), 4 cervical specimens (3 cotton swabs and 1 cytobrush) were collected individually in sterile vials containing 1·0 ml PBS each [15]. Cells along with secreted cytokines were extracted into PBS from swab/cytobrush after thorough vortexing, and pooled into one vial. After pelleting out cells by centrifugation at 400 g for 10 min, the supernatant was separated and made upto 4·0 ml volume and used for measurement of cytokines by ELISA. The pelleted cells were resuspended into 1·0 ml PBS. After performing cell count, an aliquot of 105 cells each was distributed into different vials for antibody staining by flow cytometry. Also, an additional endocervical cotton swab was collected for making smears on slides for the diagnosis of STD pathogens, viz. Candida sps., bacterial vaginosis, Trichomonas vaginalis and C. trachomatis.
Two laparoscopic specimens in 1·0 ml sterile PBS each, were collected from the fimbrial end of fallopian tubes using sterile spongostan (Johnson & Johnson Medical Limited, Skipton, UK). The samples were stored at −70oC until assayed. 1 ml nonheparinized blood was collected in sterilized vials. Serum was separated and stored at −20oC until use.
Diagnosis of endocervical C. trachomatis infection by Direct Fluorescent Assay (DFA) and polymerase chain reaction (PCR)
Spots were made on glass slides from cervical swabs/fallopian tube samples. These were stained with fluorescein isothiocyanate conjugated monoclonal antibodies to C. trachomatis major outer membrane protein using Chlamydia trachomatis Direct Specimen Test kit (Microtrak, Syva Corporation, Palo Alto, California, USA) according to the manufacturer's instructions. A sample was considered to be positive when at least 10 elementary bodies were detected. Cervical samples positive/negative by DFA were further confirmed by doing PCR analysis using C. trachomatis-specific 517 bp plasmid primers [16].
Ruling out other cervical STD pathogens, viz. Candida sps., bacterial vaginosis and T. vaginalis by microscopy
Gram stained cervical smears were examined for the presence of yeast cells (indicative of candidiasis) and clue cells for the diagnosis of bacterial vaginosis. Gram stain showing (a) predominance of the Lactobacillus morphotype (large Gram-positive rods) were interpreted as normal; (b) when showing Gardnerella morphotype (small Gram-variable rods) or mixed flora and the Lactobacillus morphotype (decreased or absent) were interpreted as consistent with bacterial vaginosis. Wet mount microscopy was done for the diagnosis of T. vaginalis.
Ruling out other cervical STD pathogens, viz. Neisseria gonorrhoeae, Mycoplasma hominis and Ureaplasma urealyticum by culture
For detection of N. gonorrhoeae, cervical specimens were incubated at 35°C in humidified CO2 incubator for 48 h on Thayer-Martin medium. Thereafter, colony growth was noted and N. gonorrhoeae was identified on the basis of Gram stained smears.
PPLO broth was used for the isolation of M. hominis and U. urealyticum by diluting the cervical samples in arginine-containing and urea-containing liquid media, respectively, and thereafter, incubating the media at 37°C until the development of colour change after 5–7 days and 2 days for M. hominis and U. urealyticum, respectively.
Estimation of cytokines by ELISA
Cytokines, viz IFN-γ, IL-1, IL-2, IL-6, IL-8, IL-10 and IL-12 were assayed in cervical secretions and fallopian tube samples of patients by commercially available ELISA kits (Opt EIA, Pharmingen, CA, USA) according to the manufacturer's instructions. This test is a solid phase sandwich ELISA utilizing a monoclonal antibody (specific for the cytokine to be assayed) coated on a 96-well plate. Briefly, standards and samples were added to the wells, washed and a mixture of biotinylated antihuman cytokine antibody and avidin horseradish peroxidase was further added, thereby producing an antibody-antigen-antibody ‘sandwich’. The wells were washed and the substrate solution was added that produced a blue colour in direct proportion to the amount of cytokine present in the sample. After adding the stop solution, the wells were read at A450. Further, total protein levels were also estimated in cervical secretions and fallopian tube samples using protein estimation kit based on Lowry's method (Bangalore Genei, Bangalore, India) according to the manufacturer's instructions. Cytokines were expressed as pg cytokine/mg protein.
Dual staining for measurement of different T-lymphocyte subsets and expression of cytokines by flow cytometry
Quantification of different T-cell subsets and various intracellular cytokines was performed in cervix by standard flow cytometric methodology [18]. Cells were pelleted and 2 × 105 cells were distributed in 96-well round-bottom plates and were stimulated for 72 h with Chlamydia recombinant antigen (L2 serovar C. trachomatis). Cells were then collected and stained for T-cell surface markers with predetermined dilutions (10 µl) of fluorescein isothiocyanate (FITC) conjugated anti CD-4 and anti CD-8 antibodies (Becton Dickinson, San Jose, CA, USA) for 25 min. After surface staining, preparations were washed with PBS containing 0·1% sodium azide. Cells were then permeabilized with Cytoperm solution (Becton Dickinson) and stained for 30 min using phycoerythrin (PE) conjugated anticytokine antibodies, viz. anti IFN-γ, anti TNF-α, anti IL-10 and anti IL-12 immunoglobulins (IgG1 monoclonal antibodies) (Becton Dickinson). Preparations were then washed twice with Pharmingen stain buffer (BD Biosciences, San Diego, CA, USA) containing buffered balanced salt solution, 0·09% NaN3 and 2% FBS and acquired using FACS caliber (BD Biosciences, San Jose, CA, USA). In all cases, a total of 10 000 events were acquired for analysis. Appropriate isotype matched control antibodies (FITC & PE conjugated) were also used to rule out any nonspecific fluorescence at the time of acquisition.
Analysis of FACS data
T-lymphocyte subsets were analysed for their intracellular cytokine expression patterns by dot blot analysis on a logarithmic scale using Cell Quest software. The frequency of positive cells was analysed in two regions for each staining: region 1 (R1) lymphocyte gate and region 2 (R2), a cytokine gate. Limits for quadrant markers were always set based on negative population and isotype controls.
Statistical evaluation
Data was analysed using Student's ‘t’ test.
RESULTS
Diagnosis of STD pathogens in the cervix of infertility patients
Cervical C. trachomatis infection was diagnosed by DFA/PCR in 17 infertile patients. None of these patients was found to be infected either with Candida sps., bacterial vaginosis, T. vaginalis, M. hominis, U. urealyticum or N. gonorrhoeae in the cervix. Based on the diagnostic findings, patients were categorized into Group I (C. trachomatis positive) and Group II (C. trachomatis negative). The clinical profile of these patients has been summarized in Table 1.
Table 1.
Clinical characteristics of infertile women (n = 37)
| Clinical findings | Group I (n = 17) | Group II (n = 20) |
|---|---|---|
| Infertility | ||
| Primary | 9 | 14 |
| Secondary | 8 | 6 |
| Age (range in years) | 20–38 | 23–37 |
| Severity of disease (based on finding in HSG/laparoscopy) | ||
| Normal tubes (without tubal obstruction or peritubal adhesions) | 8 | 10 |
| Tubal pathology | 9 | 10 |
| Peritubal and/or periovarian adhesions | – | 1 |
| Unilateral/bilateral obstruction | 5 | 4 |
| Any other | 4* | 5† |
2 patients with obstruction & adhesion; 2 patients with hydrosalpinx & adhesion;
1 patient with obstruction, adhesion & hydrosalpinx; 1 patient with hydrosalpinx & obstruction; 3 patients with obstruction & adhesion
Estimation of cytokines in cervix and fallopian tube by ELISA
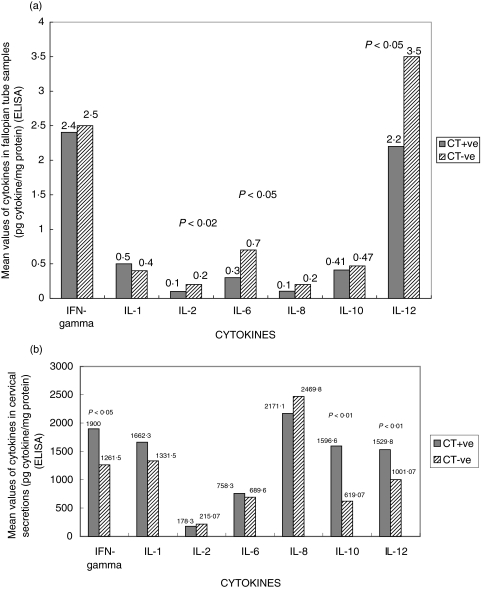
Total protein levels (mg/ml) (C. trachomatis positive versus negative patients) in cervical secretions and fallopian tube samples were 0·04 versus 0·05 and 32·8 versus 21·1, respectively. The decrease in IL-2, IL-6 and IL-12 cytokines was found to be statistically significant (P < 0·01, P < 0·05 and P < 0·05, respectively) in the fallopian tube of Group I patients. The levels of IFN-γ and IL-12 were up-regulated in the cervical secretions of Group I patients (statistically significant; P < 0·05 and P < 0·01, respectively). Also, the level of IL-10 cytokine was found to be up-regulated in the cervix of Group I patients (statistically significant; P < 0·01). These results are summarized in Fig. 1.
Fig. 1.
Cytokine profile in the genital tract of C. trachomatis positive versus C. trachomatis negative infertile women in (a) fallopian tube- decreased levels of IL-2, IL-6 and IL-12 were statistically significant in Chlamydia-positive women (P < 0·01, P < 0·05 and P < 0·05, respectively) and (b) cervix- up-regulated levels of IFN-γ, IL-10 and IL-12 were statistically significant in Chlamydia-positive women (P < 0·05, P < 0·01 and P < 0·01, respectively). Secreted cytokines were measured by ELISA and expressed as mean values (pg cytokine/mg protein) on Y- axis.
Differential recruitment of T-lymphocyte subsets in the cervix of C. trachomatis infected women
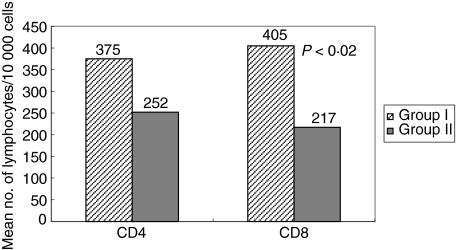
There was an increase in the mean number of CD4+ T-lymphocytes per 10 000 events in Group I patients as compared to Group II (375 versus 252). However this increase in the number of CD4 T-cells was statistically insignificant. The number of CD8 T-lymphocytes per 10 000 events was significantly increased in Group I in comparison to Group II patients (405 versus 217; P < 0·02). These results have been summarized in Fig. 2.
Fig. 2.
lymphocyte subsets (CD4+ and CD8+ T-cells) in cervical secretions of C. trachomatis positive versus C. trachomatis negative infertile women by flow cytometry and expressed as mean number of T-lymphocytes per 10 000 events on y-axis. Mean number of CD8+ T-cells/10, 000 events was significantly increased (P < 0·02) in cervix of Chlamydia-positive women.
Detection of cytokines in the cervix by flow cytometry
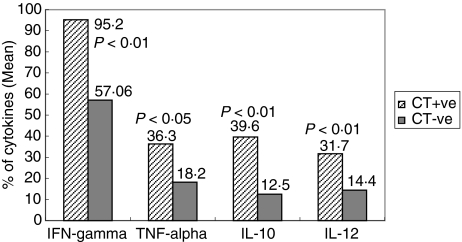
IFN-γ, TNF-α and IL-12 were significantly (P < 0·01,P < 0·05 and P < 0·01, respectively) more often detected in the cervix of Group I infertile patients in comparison to Group II (95·2%versus 57·06%, 36·3%versus 18·2%, 31·7%versus 14·4%, respectively). Also, the level of Th2 cytokine, IL-10 was significantly up-regulated in the cervix of Group I patients when compared to Group II infertile women (39·6%versus 12·5%; P < 0·01). These results are summarized in Figs 3 and 4.
Fig. 3.
Pattern of cytokines (IFN-γ, TNF-α, IL-10 and IL-12) in the cervix of C. trachomatis positive versus C. trachomatis negative infertile women. The cytokine producing cells (CD4+/CD8+) were detected by intracytoplasmic staining and mean percentage of cytokines was evaluated by gating 10 000 total events in flow cytometry. Statistically significant increase was found in levels of IFN-γ, TNF-α, IL-10 and IL-12 (P < 0·01, P < 0·05, P < 0·01 and P < 0·01, respectively).
Fig. 4.
Representative histograms showing frequency of (2) CD4+ T cells producing IFN-γ cytokine and (3) CD8+ T-cells producing IFN-γ, measured by dual staining in flow cytometry in C. trachomatis positive infertile women in comparison to negative/unstained controls (1). FL-1 height was assigned to surface marker (CD4 & CD8) staining using antibodies conjugated with FITC (X-axis) and FL-2 height was assigned to cytokine (IFN-γ) staining using antibodies conjugated with PE (x-axis).
DISCUSSION
In humans, the dichotomy of the T-cell response following C. trachomatis genital tract infection is not well established although there are reports in animal models stating the importance of CD4 cells in clearing the C. trachomatis infection and also the importance of CD8 cells in the resolution of C. trachomatis infection [17,18,22]. In mouse model, it was reported that more CD4 cells were recruited mainly to the upper genital tract compared to the lower genital tract during primary genital infection with Chlamydia [10]. It has been suggested that failure of eradication might induce a state of chlamydial persistence characterized by the presence of noncultivable but fully viable bacteria and development of aberrant inclusions [19]. Therefore the present study has explored certain aspects of local and systemic immunity in terms of T-lymphocyte subsets and the ensuing cytokine milieu against C. trachomatis infection in infertile women. We assessed the cytokine profile locally in lower and upper genital tracts (cervix and fallopian tubes) and lymphocyte recruitment in the lower genital tract after ruling out other STD pathogens, viz. Candida spp., bacterial vaginosis, T. vaginalis, N. gonorrhoeae, M. hominis and U. urealyticum as coinfection with these pathogens might also alter the immune profile [20,21].
The flow cytometry analysis of cervical secretions of Chlamydia infected patients on stimulation with Chlamydia recombinant antigens revealed that the mean number of CD4+ T-cells per 10 000 events in Group I patients was increased in comparison to Group II (375 versus 252), however, this finding was statistically insignificant. Earlier studies have also reported an increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases [22]. In our study, a significant increase was observed in the mean number of CD8+ T-lymphocytes per 10 000 events in Chlamydia-positive infertile patients in comparison to Chlamydia-negative patients (405 versus 217; P < 0·02). In cell-mediated immunity, antigen presenting cells present the C. trachomatis derived peptide antigens to CD4+ T-cells in conjunction with MHC-class II molecules, however, once the microorganism successfully enters the epithelial cells that typically don’t express MHC-class II, then recognition by CD4+ T-cells is unlikely and it may be possible that CD8+ T-cells come into play through MHC-class I molecule [23–25]. It was also reported that Mip protein, a protein of C. trachomatis inclusion body, traffic across inclusion membrane into cytoplasm [26], which makes the CD8+ T-cells activate and lysis of infected cells by MHC-class I molecule. Thus during chlamydial infection, the production of both CD8 and CD4 specific cells can and does indeed occur [27].
In response to C. trachomatis infection, variation in detection of different cytokines was observed in cervix and fallopian tube. Flow cytometry showed that, all the major cytokines analysed viz; IFN-γ, IL-10, IL-12 and TNF-α were up-regulated in the cervical region of genital tract during C. trachomatis infection. We also analysed secreted cytokines, viz: IFN-γ, IL-1, IL-2, IL-6, IL-8, IL-10 and IL-12 in cervix and fallopian tube samples by ELISA. IFN-γ levels were significantly high in the cervix of Chlamydia-positive infertile women when compared to uninfected controls. Increased level of IFN-γ has been reported in the endocervical secretions of C. trachomatis positive women [28]. Further, the antichlamydial activity of CD4+ and CD8+ T-cells is primarily associated with the production of high levels of IFN-γ [29–31]. IFN-γ reportedly promotes the destruction of Chlamydia [32] and also triggers macrophage release of inflammatory mediators that cause fibroblast proliferation, thereby enhancing the synthesis of collagen. In addition, IFN-γ delays the developmental cycle of Chlamydia so that chlamydial reticulate bodies persist longer, which might result in persistent unapparent infection and also, play a role in immunopathogenesis by promoting inflammatory damage and fibrosis [33].
In our study, C. trachomatis infection also up-regulated the cervical production of TNF-α which plays an important role in the initiation of inflammatory response. It is reported that TNF–α displays antichlamydial properties [34] and infertility associated with endometriosis is related to the production of TNF-α in the mouse genital tract [35]. Marginal increase in IL-1 was observed in the cervix in this study. It is reported that IL-1 is important both for the recovery process and also, for causing inflammatory response [36].
Increased levels of IL-12 were observed in cervical secretions of C. trachomatis-positive infertile women, whereas low level of IL-12 was observed in the upper genital tract. It was suggested that IL-12 is important for the initial clearance of bacteria [2,6,9,10]. Further, IL-12 is required for promoting IFN-γ production by NK cells [37]. In our laboratory, preliminary studies on enumerating dendritic cell (DC) population in cervical secretions of infertile women revealed a high percentage of DC(s) [38] using CD83 monoclonal antibody. It may also be possible that anti-CD4 and CD8 antibodies are detecting CD4 or CD8 antigen on non T-cells such as DCs. Phagocytosis of chlamydiae induces DC(s) to produce IL-12, which in turn promotes Th-1 response and induces the presentation of chlamydial antigen to CD4+ T-cells [39]. There was an increase in IL-6 production in the cervix of C. trachomatis infected infertile women and vice versa in the fallopian tube. Increased IL-6 levels in cervical secretions have been associated with pelvic inflammatory disease in women [40]. However, levels of IL-8 cytokine were decreased in both cervix and fallopian tube in our study.
Levels of Th-2 cytokine, IL-10 were found to be high in cervical secretions, thereby showing that the immune response in C. trachomatis infected infertile women is of a mixed type, with both IFN-γ and IL-10 being up-regulated. Earlier studies in mice revealed that at the onset of C. trachomatis infection, both IFN-γ and IL-10 are up-regulated and later, IL-10 levels go up and down depending on the immune status [41] and hormonal balance [42]. Also, IL-10 is not always an inflammatory/inhibitory cytokine; instead high levels of IL-10 probably prevent the pathological effects of the inflammatory cytokines like, IL-1, IFN-γ, TNF-α, etc. [43] and play a role in limiting the host-induced damage to the upper genital tract.
The differences in cytokine levels in cervix and fallopian tubes detected in our study might also be due to the anatomical and functional differences between the two sites. Also, there is a substantial difference in the number of control subjects with primary infertility that might have influenced the results obtained. C. trachomatis infects squamous epithelial cells in the cervix, often inducing an acute inflammatory reaction, followed by lymphocytic infiltration and the development of lymphoid follicles. From the cervix, the bacteria ascend and establish infection in columnar cells of the fallopian tubes [14]. Experimental data in mice also suggest that the upper and lower regions of the genital tract respond differently to C. trachomatis infection [10]. Further, considering the extensive surface area of the genital tract mucosa, CD8+ T-cells might be important together with CD4+ T-cells for local regulation of Th1/Th2 responses during C. trachomatis infection. Thus both, CD4+ and CD8+ T cell responses are interdependent and are required for optimal immunity to C. trachomatis infection. However, the presence of numerous other cell types responsible for the production of cytokines in cervical secretions and fallopian tube cannot be completely ruled out in this study. Further studies to define the individual contribution of CD4+ and CD8+ T-cells in determining the T-helper cell response (Th1, Th2 or Th0) during chlamydial pathogenesis in the human female need to be undertaken. This may eventually lead to new approaches, viz. designing effective vaccines for preventing and treating C. trachomatis induced sequelae of events leading to infertility in women.
Acknowledgments
We thank Mrs Madhu Badhwar and Mrs Asha Rani for their technical assistance. This study was supported by a grant from Department of Science and Technology (DST), Government of India (Award No. DST/SP/SO/B-69/2000).
REFERENCES
- 1.Schachter J. Infection and Epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis and Immunity. Washington DC: ASM Press; 1999. pp. 391–405. [Google Scholar]
- 2.Mittal A, Kapur S, Gupta S. Host Immune response in chlamydial cervicitis. Br J Biomed Sci. 1996;53:941–7. [PubMed] [Google Scholar]
- 3.Arora M, Malhotra S, Sharma M. Role of Chlamydia trachomatis in pelvic inflammatory disease. Indian J Med Res. 1992;95:41–2. [PubMed] [Google Scholar]
- 4.Pal S, Hui W, Peterson EM, de la Maza LM. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol. 1998;47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 5.Brunham RC. Human immunity to chlamydiae. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis and Immunity. Washington DC: ASM Press; 1999. pp. 211–38. [Google Scholar]
- 6.Kelly KA. Cellular immunity and Chlamydia genital infection: Induction, recruitment and effector mechanisms. Int Rev Immunol. 2003;22:3–41. doi: 10.1080/08830180305229. [DOI] [PubMed] [Google Scholar]
- 7.Rank RG, Bavoil PM. Prospects for a vaccine against Chlamydia genital disease. 2 Immunity and vaccine development. Bull Inst Pasteur. 1996;94:55–82. [Google Scholar]
- 8.Igietseme JU, Uriri IM, Kumar SN, Anaqnaba GA, Ojior OO, Momodu IA, Caudal DH, Black CM. Route of infection that induces a high intensity of gamma interferon-secreting T-cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–5. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly KA, Robinson E, Rank RG. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–83. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly KA, Walker JC, Jameel SH, Gray HL, Rank RG. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis infection. Infect Immun. 2000;68:1519–28. doi: 10.1128/iai.68.3.1519-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rank RG, Bowlin AK, Kelly KA. Characterization of lymphocyte response in the female genital tract during ascending chlamydial genital infection in the guinea pig model. Infect Immun. 2000;68:5293–8. doi: 10.1128/iai.68.9.5293-5298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Openshaw P, Murphy EE, Hosken NA, et al. Heterogeneity of intracellular cytokine synthesis at the single cell level in polarized T helper-1 and T helper-2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morell V. Attacking the causes of ‘silent’ infertility. Science. 1995;269:775–7. doi: 10.1126/science.7638588. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen S, Eckmann J, Quayle AJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal A, Kapur S, Gupta S. Infertility due to Chlamydia trachomatis infection. What is appropriate site for obtaining sample? Genitourin Med. 1995;71:267. doi: 10.1136/sti.71.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh V, Rastogi S, Garg S, Kapur S, Kumar A, Salhan S, Mittal A. Polymerase chain reaction for detection of endocervical C. trachomatis infection in women attending a gynecology outpatient department in India. Acta Cytol. 2002;46:540–4. doi: 10.1159/000326874. [DOI] [PubMed] [Google Scholar]
- 17.Su H, Caldwell HD. CD4+ T-cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;65:3302–8. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starnbach MN, Bevan MJ, Lampe MF. Protective Cytotoxic T lymphocytes as induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;53:5183–9. [PubMed] [Google Scholar]
- 19.Kunimoto D, Burnham RC. Human immune response and Chlamydia trachomatis infection. Rev Infect Dis. 1985;7:665–73. doi: 10.1093/clinids/7.5.665. [DOI] [PubMed] [Google Scholar]
- 20.Hedges SR, Sibley DA, Mayo MS, Hook EW, Russell MW. Cytokine and antibody response in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–51. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 21.Messmer TO, Black CM, Tkacker WL. Mycoplasma contamination of chlamydiae isolated from clinical specimens. APMIS. 1994;102:793–6. doi: 10.1111/j.1699-0463.1994.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine WC, Pope V, Bhoomkar A, et al. Increase in endocervical CD4 lymphocytes among women with non-ulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–74. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 23.Magee DM, William DM, Smith JG, Bleicker CA, Grubbs BG, Schachter J, Rank RG. Role of CD8 T-cells in primary Chlamydia infection. Infect Immun. 1995;63:516–21. doi: 10.1128/iai.63.2.516-521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomis WP, Starnbach MN. T cell responses to Chlamydia trachomatis. Curr Opin Micro. 2002;5:87–91. doi: 10.1016/s1369-5274(02)00291-6. [DOI] [PubMed] [Google Scholar]
- 26.Lundemose AG, Kay JE, Pearce JH. Chlamydia trachomatis Mip-like protein has peptidyl-prolyl cis/trans isomerase activity that is inhibited by FK506 and rapamycin and is implicated in initiation of chlamydial infection. Mol Microbiol. 1993;7:777. doi: 10.1111/j.1365-2958.1993.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 27.Thoma-Uszynski S, Simnacher U, Marro R, Essig A. Clearance of Chlamydia trachomatis induced polyserositis in SCID mice requires both CD4+ and CD8+ cells. Microbiol Immunol. 1998;187:71–8. doi: 10.1007/s004300050076. [DOI] [PubMed] [Google Scholar]
- 28.Arno JN, Ricker VA, Batteiger BE. Interferon-γ in endocervical secretions of women infected with Chlamydia trachomatis. J Infect Dis. 1990;162:1385–9. doi: 10.1093/infdis/162.6.1385. [DOI] [PubMed] [Google Scholar]
- 29.Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–9. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong G, Peterson EM, Czarniecki CW, Schreiber RD, de la Maza LM. Role of endogenous gamma interferon in host defence against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–7. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T-helper 1 cells through IFN-gamma-dependent and independent pathways. J Immunol. 1997;158:3344–52. [PubMed] [Google Scholar]
- 32.Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon- gamma mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. The role of IFN-gamma in the outcome of chlamydial infection. Curr Opin Immunol. 2002;14:444–51. doi: 10.1016/s0952-7915(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 34.Shemer-Avni Y, Wallach D, Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumour necrosis factor. Infect Immun. 1988;56:2503–6. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darville T, Andrews CW, Rank RG., Jr Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect Immun. 2000;68:5299–305. doi: 10.1128/iai.68.9.5299-5305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durum SK, Schimidt JA, Oppenheim JJ. Interleukin-1: An immunological perspective. Ann Rev Immunol. 1985;3:263–87. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- 37.Tseng CK, Rank RG. Role of NK cells in early host immune response to chlamydial genital infection. Infect Immun. 1998;66:5867–75. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittal A, Rastogi S, Reddy BS, Verma S, Salhan S, Gupta E. Enhanced immunocompetent cells in chlamydial cervicitis. J Reprod Med. 2004;49:1–7. [PubMed] [Google Scholar]
- 39.Stagg AJ, Elsley WAJ, Holland MJ, et al. Proceedings Of European Society for Chlamydia Research. Town: Publisher; 1992. Dendritic cells (DC) in the initiation of immune responses to Chlamydia; pp. 77–80. [Google Scholar]
- 40.Richter HE, Holley RL, Andrews WW, Owen J, Miller KB. The association of interleukin 6 with clinical and laboratory parameters of acute pelvic inflammatory disease. Am J Obstet Gynecol. 1999;181:940–4. doi: 10.1016/s0002-9378(99)70329-7. [DOI] [PubMed] [Google Scholar]
- 41.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T-helper cells producing Th2-type cytokines and promotes both IL-4 production, membrane CD30 expression in established Th 1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 43.Conti P, Kempuraj D, Kandere K, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–9. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]