Abstract
Natural killer (NK) cell interactions with macrophages have been shown to be important during bacterial sepsis in activating macrophages to improve bacterial clearance. The mechanism for this increased activation, however, is unclear. This study determines the relative roles of interferon (IFN)-γ and CD40/CD154 direct cell interactions on macrophage and NK cell activation in an experimental model of sepsis. Splenic NK cells and peritoneal macrophages were isolated and cultured alone or in coculture, with and without LPS. CD69 expression on NK cells, phagocytosis ability of macrophages, and cell cytokine production was assessed at 24 and 48 h. Coculture of NK cells and macrophages significantly increased activation levels of both cell types, and through experiments culturing NK cells with supernatants from stimulated macrophages and macrophages with supernatants from stimulated NK cells, this activation was determined to be cell-contact-dependent. Similar experiments were conducted using NK cells from IFN-γ deficient (–/–) mice, as well as anti-IFN-γ neutralizing antibody. These experiments determined that IFN-γ is not required for NK or macrophage activation, although it did augment activation levels. Experiments were again repeated using peritoneal macrophages from CD40-/– mice or splenic NK cells from CD154-/– mice. CD40/CD154 interactions were important in the ingestion of bacteria by macrophages, but did not affect NK cell activation at 24 h. There was, however, a protective effect of CD40/CD154 interactions on NK cell activation-induced cell death that occurred at 48 h. CD40/CD154 interactions between macrophages and NK cells are therefore important in macrophage phagocytosis, and are not dependent on IFN-γ.
Keywords: Phagocytosis, lipopolysaccharide, CD40/CD154, IFN-γ, cell activation
INTRODUCTION
Natural killer (NK) and natural killer T (NKT) cells are subsets of lymphocytes shown to be important in cytotoxic activity against tumours [1,2], in viral infections [3], and in autoimmunity [4,5]. The role of NK cells in bacterial infection is less well known, and has been less well studied, although they are major producers of interferon (IFN)-γ during bacterial peritonitis in mice [6]. IFN-γ is a pro-inflammatory cytokine important in macrophage activation and maintenance of T-helper (Th)1 responses during infection [7,8]. Macrophages are also vital antigen presenting cells and phagocytes during bacterial infection. There is also evidence that NK cells are the primary target of activation for bacterial-primed macrophages [9].
In a recent study, we showed that activated NK cells prime macrophages for a subsequent bacterial infection [10]. There are many potential mechanisms by which this priming could be achieved, and these fall into two main categories: activation through soluble mediators such as IFN-γ and activation secondary to direct cell-to-cell contact. IFN-γ, produced by NK cells and T cells, activates macrophages and other antigen presenting cells [7,8], with a reciprocal activation of NK cells via the production of interleukin (IL)-12 from these activated macrophages [11]. Interactions between cell surface CD40 on antigen presenting cells and its ligand CD154 on NK cells and T cells also confers bidirectional activation [12]. This cell-contact mechanism is important in interactions between dendritic cells and NK cells [13,14], and increases NK cell toxicity [15], and the production of IL-12 during intracellular bacterial infection [16].
This study was undertaken to establish the relative importance of IFN-γ and CD40/CD154 interactions on NK cell and macrophage reciprocal activation in an in vitro model of sepsis. We show that there is reciprocal activation between NK cells and macrophages enhanced by lipopolysaccharide (LPS), and that this activation is cell-contact-dependent. IFN-γ was not required for increased macrophage activation, although it did enhance activation levels. CD40/CD154 interactions were important in macrophage phagocytosis ability, but did not directly influence NK cell activation. Collectively the data indicate that cell-to-cell contact via CD40/CD154 interactions is important in macrophage priming by NK cells and plays a larger role than IFN-γ in this situation. Understanding the mechanism of NK cell action and interactions is important as these cells are increasingly found to be regulators of immune responses.
MATERIALS AND METHODS
Animals
C57BL/6 mice [wild type (WT)], CD40–/–, CD154–/–, and IFN-γ–/– mice (all having a C57BL/6 genetic background), aged 6–8 weeks, were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Animals were housed in a facility approved by the American Association for Accreditation of Laboratory Animal Care and provided with food and water ad libitum. Studies were approved by the Institutional Animal Care and Use Committee, and carried out according to the National Institutes of Health guidelines under the supervision of a veterinarian.
Experimental design
Peritoneal macrophages and splenic NK cells were cultured in a cell culture incubator in polypropylene cell culture tubes in single cell-type cultures or in coculture for 24 or 48 h to identify contact-dependent and cytokine-driven effects. Ratios of 1 : 10 NK cells to macrophages were used in cocultures. Similar proportions of cells to final volume of supplemented media were used for single cell-type culture. LPS (1 µg/ml) (Sigma, St. Louis, MO, USA) was added to some cultures as appropriate. Peritoneal macrophages were also cultured with supernatant from 48 h culture of WT NK cells stimulated with 10 ng/ml phorbol myristic acetate (PMA) (Sigma), and 500 ng/ml ionomycin (Sigma), to identify effects from soluble factors produced by NK cells. Macrophages were also cultured with NK cells and 1 µg/ml of neutralizing anti-IFN-γ monoclonal antibody (BD Pharmingen, San Diego, CA, USA), or with NK cells isolated from IFN-γ–/– mice, to elucidate the role of NK derived IFN-γ on macrophage activation. Control isotype matched antibodies (BD Pharmingen) were used in similar sets of coculture experiments and compared with results from antibody neutralizing experiments. Macrophages from CD40-/– mice were cultured with NK cells from WT mice, and NK cells from CD154–/– mice were cultured with macrophages from WT mice, to identify the role of CD40/CD154 interactions in contact-dependent macrophage and NK activation. NK cells from WT mice were also cultured with supernatant from 48 h culture of WT macrophages with LPS. CD69 expression on NK cells was determined by flow cytometry as a marker of activation of these cells. Macrophage phagocytosis of heat killed fluorescent E.coli was used to determine macrophage activation. Levels of interleukin (IL)-6, 12, and 18, and tumour necrosis factor (TNF)-α from macrophages, and IFN-γ from NK cells were also determined in culture supernatant by ELISA.
Cell culture
Mice were euthanized followed by collection of peritoneal lavage and spleens. Peritoneal macrophages were recovered by peritoneal lavage with 4 ml of ice-cold, heparinized, RPMI 1640 medium (Gibco/BRL, Bethesda, MD, USA) and were counted manually using a haemocytometer, centrifuged to pellet the cells and then resuspended to 1 × 106 cells/ml in RPMI 1640 media supplemented with 10% heat inactivated fetal bovine serum (Sigma), 1% antibiotic/antimycotic (Sigma), 1%l-glutamine (Sigma) and 0·1%β-mercaptoethanol (Sigma). Flow cytometric analysis revealed greater than 90% purity of macrophages. Spleen cells were separated through sterile 70 µm filters in RPMI 1640 medium. NK and NKT cells were enriched from the spleen cells as described below, and resuspended in supplemented RPMI 1640 media to 1 × 106 cells/ml. Cells were cultured in a cell-culture incubator in round bottomed polypropylene culture tubes. As appropriate, 1 µg/ml LPS (Sigma) was added to culture. For experiments neutralizing IFN-γ, 1 µg/ml of anti-IFN-γ monoclonal antibody (BD Pharmingen) was preincubated for 1 h with culture supernatant obtained from 24 h culture of NK cells stimulated with 10 ng/ml PMA, and 500 ng/ml ionomycin (Sigma). For experiments neutralizing IL-12, 1 µg/ml of anti-IL-12 antibody (R & D Systems) was preincubated for 1 h with supernatant obtained from 48 h culture of peritoneal macrophages with 1 µg/ml LPS. Cultures were for 24 h and 48 h as described, at 37°C/5%CO2.
Separation and enrichment of NK and NKT cells from spleen
NK and NKT cells were isolated from spleen cells using a negative selection density centrifugation technique (SpinSep©, StemCell Technologies, Vancouver, BC, Canada). Briefly, 5 × 107 spleen cells were incubated on ice for 30 min with 10 µl of negative selection antibody cocktail, containing anti-CD5, anti-CD19, anti-F4/80, anti-Gr-1, and anti-Ter119 antibodies. 400 µl of dense particles were then added to the cell suspension for a further 10 min on ice. The dense particles crosslink the antibodies attached to unwanted cells. The cell suspension was then layered onto supplied density media. Following centrifugation, enriched NK and NKT cells were collected at the interface and counted manually using a haemocytometer. Cells were surface stained with anti-NK1·1-PE and anti-CD3-PerCP and analysed by flow cytometry, as above, to determine purity of the population. Typically, approximately 80% purity of NK and NKT cells was obtained, with NKT comprising approximately 5% of total cell number.
Cytokine assays
Concentrations of IL-6, 12, 18, tumour necrosis factor (TNF)-α and IFN-γ in culture supernatants were determined by enzyme-linked immunosorbent assays (ELISA) (Biosource International, Camarillo, CA, USA), performed according to the manufacturer's instructions.
Analysis of CD69 on NK and NKT cells
NK cells from cultures were identified using anti-NK1·1 monoclonal antibody (NK1·1-PE), and anti-CD3-PerCP (both from Pharmingen, San Diego, CA, USA). NK and NKT cell activation was identified according to expression of the early activation marker, CD69 using FITC-labelled anti-CD69 (Pharmingen). Isotype-matched control antibodies were also used. To prevent nonspecific binding of PE to mouse B cells via Fc receptors, cells were pre-incubated at 4°C for 5 min with Mouse Fc Block™ (Pharmingen). Samples were stained simultaneously with the anti-NK1·1, anti-CD3, and anti-CD69 or control IgG antibodies. Red cells were hypotonically lysed with ammonium chloride, and samples were then washed with phosphate buffered saline (PBS), and fixed in 1% paraformaldehyde (Polysciences, Warrington, PA, USA). Ten thousand cells were acquired and the data analysed on a FACScan flow cytometer (Becton Dickinson) equipped with CellQuest software. NK cells were identified as NK1·1+/CD3- lymphocytes and NKT cells as NK1·1+/CD3+ lymphocytes. Percentages of CD69+ NK or NKT cells were obtained by gating around the appropriate cell populations.
Macrophage phagocytosis assay
Heat-killed fluorescein isothiocyanate (FITC)-labelled Escherichia coli (Molecular Probes, Eugene, OR, USA) were opsonized by incubation with 5% pooled mouse serum in a shaking water bath at 37°C for 30 min. The bacteria were then washed twice in PBS prior to the addition of macrophages in ratios of 1:100 peritoneal macrophages to bacteria, and incubated for a further 30 min in the water bath. The red blood cells were then lysed, and cells and bacteria washed twice in PBS, followed by fixation with 1% paraformaldehyde. Fluorescence was measured in the macrophage population unquenched, and quenched with trypan blue (Sigma) using a FACScan flow cytometer (Becton Dickinson) equipped with CellQuest software. Quenching prevents fluorescence of bacteria attached to the outside of the cell, allowing determination of ingested bacterial levels. Results are expressed as mean channel fluorescence (MCF).
Statistical analysis
Concentrations of cytokines, and MCF levels were compared by anova. Percentages were compared nonparametrically using the Kruskal–Wallis test. Cytokine levels, mean channel fluorescence and cell percentages are expressed as means ± SEM. A P-value of 0·05 or less was considered significant.
RESULTS
Increased NK cell and macrophage activation during coculture
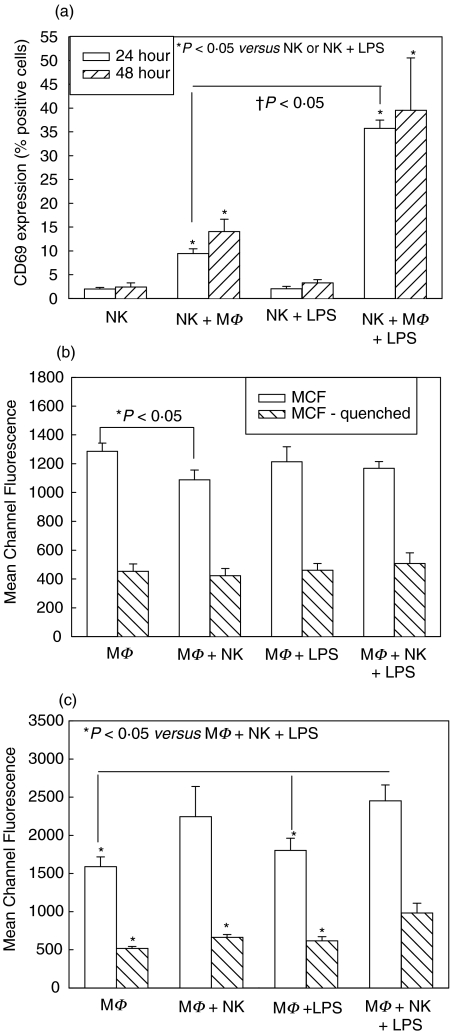
Splenic NK cell activation, measured by cell surface expression of CD69, and peritoneal macrophage activation, measured by ability to phagocytose fluorescent-labelled E. coli, were determined in cells from wild type (WT) C57BL/6 mice. NK cells were cultured with macrophages in a ratio of 1 : 10, respectively, to reproduce as closely as possible the in vivo situation [5]. LPS was used to represent sepsis, as well as being a known macrophage activator. Phagocytosis levels are expressed as mean channel fluorescence (MCF), which corresponds with numbers of attached and ingested fluorescent bacteria. MCF was also determined following quenching of fluorescence with trypan blue. This quenches the fluorescence of bacteria attached to the outside of the macrophages allowing determination of intracellular bacterial levels.
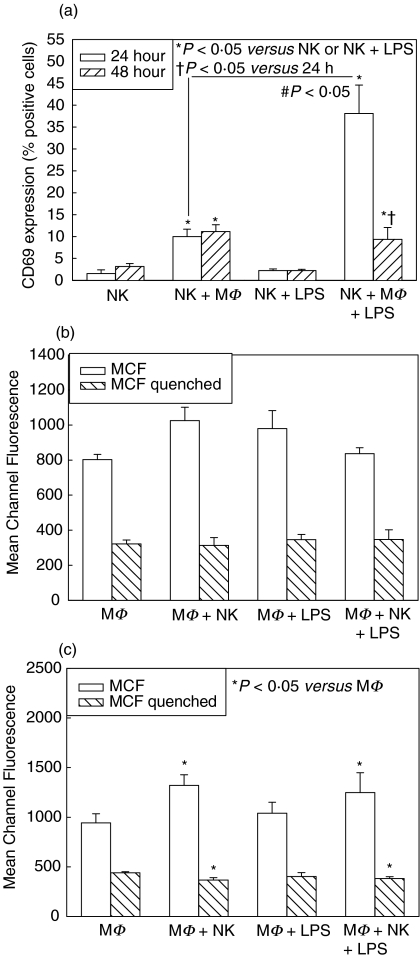
There was significantly increased CD69 expression on NK cells cultured with macrophages, compared with NK cell culture alone, at both 24 and 48 h, and the level of activation was increased with addition of LPS to the coculture (Fig. 1a). LPS by itself, however, had no effect on the level of NK cell activation, indicating that increases in NK cell activation in LPS-cocultures were secondary to increased LPS-induced activation of macrophages. Activated macrophages are therefore more able to activate NK cells. There were no significant differences between NK cell activation at 24 h and 48 h.
Fig. 1.
Splenic NK cell activation at 24 and 48 h (a) and peritoneal macrophage (MΦ) phagocytosis at 24 h (b) and 48 h (c) in single cell-type and mixed-cell cocultures from wild type C57BL/6 mice. Isolated splenic NK cells were cultured alone and with MΦ with and without LPS. CD69 expression was determined after 24 and 48 h culture, and levels were significantly increased in cocultures with MΦ (NK + MΦ, NK + MΦ+ LPS) (a). MΦ were similarly cultured alone or with NK cells, with and without LPS, and levels of phagocytosis of fluorescent-labeled E. coli determined at 24 h and 48 h. No differences were seen in phagocytosis after 24 h (b). Phagocytosis mean channel fluorescence (MCF) was significantly increased in cocultures of MΦ+NK cells +LPS at 48 h compared with other groups (c). n = 6 per experimental group and data are representative of separate repeated experiments. Data presented as mean ± s.e.m. * or †P < 0.05, Kruskal–Wallis (CD69 percentages), anova (MCF).
There were no significant differences in phagocytosis ability of macrophages between groups cultured with and without NK cells and/or LPS at 24 h (Fig. 1b). However, by 48 h there were significant increases in attachment and ingestion of bacteria by macrophages cocultured with NK cells, compared with culture alone or with LPS alone (Fig. 1c). The increases reached statistical significance in macrophages cultured with NK cells and LPS (Fig. 1c). Again, the action of LPS alone was not sufficient to increase phagocytosis in macrophages, suggesting synergistic activation of macrophages by LPS and NK cells. It is unclear why increases in phagocytosis occurred only after 48 h of culture, as differences were expected to be observed more rapidly. It may be secondary to the isolated system consisting of only NK cells and macrophages, as, in vivo, other cells such as T cells, would also likely have some role to play.
Increased NK cell and macrophage activation is cell-contact-dependent
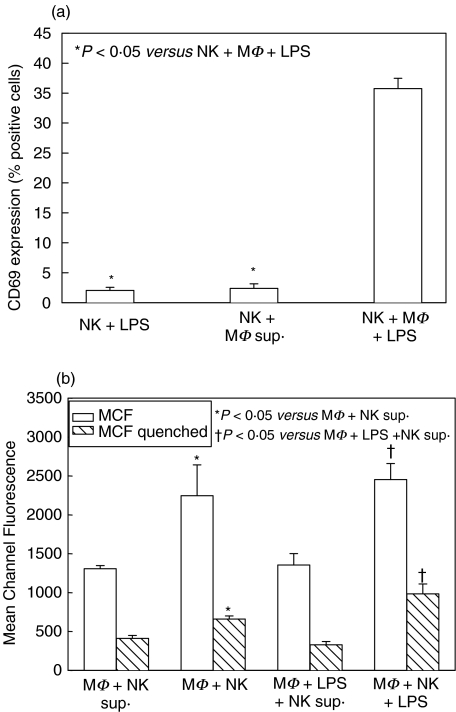
Similar experiments to those described above were performed using splenic NK cells or peritoneal macrophages cultured with supernatants from stimulated macrophages or NK cells, respectively. The supernatants contain soluble mediators, such as cytokines, produced by LPS-stimulated macrophages, or PMA and ionomycin-stimulated NK cells, during 48 h of cell culture. These experiments therefore allowed investigation of the requirements for direct cell contact between macrophages and NK cells to induce activation.
NK cell activation following 24 h culture with supernatant from LPS-stimulated macrophages only reached similar levels to NK cell culture with LPS alone, and was significantly decreased compared to NK cell activation in coculture with macrophages and LPS (Fig. 2a). Similarly, macrophage phagocytosis, after 48 h culture with supernatant from stimulated NK cells, was significantly decreased compared to phagocytosis of macrophages cocultured with NK cells (Fig. 2b). Addition of LPS did not affect these differences. These data show that reciprocal NK cell and macrophage activation is cell-contact-dependent.
Fig. 2.
NK cell activation after 24 h culture with supernatant from stimulated macrophage (MΦ) culture (a), and MΦ phagocytosis after 48 h culture with supernatant from stimulated NK cell culture (b) in single cell-type and mixed-cell cocultures from wild type C57BL/6 mice. CD69 cell surface expression was determined on NK cells after 24 h culture with supernatant collected after 48 h culture of MΦ + 1 µg/ml LPS. Soluble mediators in the supernatant were not sufficient to increase NK cell activation to the level of mixed-cell cocultures (a). Levels of macrophage phagocytosis of fluorescent-labelled E. coliwere determined at 48 h after culture with supernatant collected after 24 h culture of NK cells with 10 ng/ml PMA and 500 ng/ml ionomycin. Soluble mediators in the supernatant were not sufficient to increase phagocytosis to the level of mixed-cell cocultures (b). n = 6 per experimental group and data are representative of separate repeated experiments. Data presented as mean ± s.e.m. * or †P < 0·05, Kruskal–Wallis (CD69 percentages), anova (MCF).
NK cell and macrophage activation does not require IFN-γ
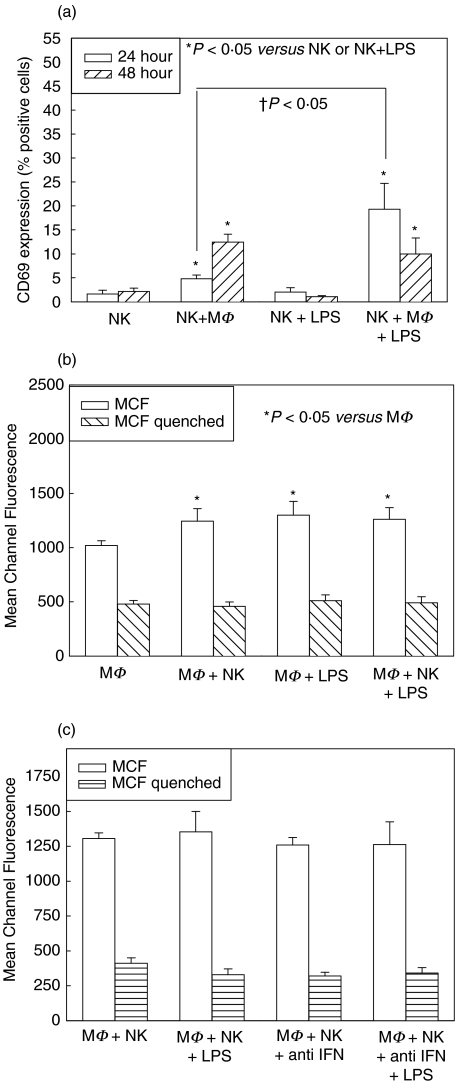
One likely interaction between NK cells and macrophages is through the production of IFN-γ by NK cells. IFN-γ alone, and synergistically with LPS, is well known to activate macrophages [8]. We therefore specifically investigated the role of NK cell-derived IFN-γ in this model system using splenic NK cells isolated from IFN-γ-deficient (IFN-γ–/–) mice cocultured with WT macrophages, as well as WT splenic NK cells and macrophages cultured with anti-IFN-γ neutralizing antibody.
NK cell activation in IFN-γ–/– mice followed similar patterns to activation in WT mice at 24 and 48 h (Fig. 3a). Levels of activation were, however, decreased compared to NK activation in WT mice, and this reached statistical significance at both 24 and 48 h in groups with NK cell coculture with macrophages and LPS. This suggests either that NK cells from IFN-γ–/– mice are not capable of the same level of activation as WT mice, or that IFN-γproduction from NK cells themselves is important in increasing the level of NK cell activation, although it is not required for activation itself. Macrophage phagocytosis was increased after 48 h of coculture with NK cells from IFN-γ–/– mice, but this level was also decreased compared with culture with NK cells from WT mice (Fig. 3b). There were no differences in macrophage phagocytosis between macrophages cocultured for 48 h with NK cells, with and without LPS, and macrophages in similar cocultures with anti-IFN-γ neutralizing antibody (Fig. 3c). CD69 levels on NK cells in similar groups treated with anti-IFN-γ antibody were also not different (data not shown). Isotype-matched control antibodies were also used in separate experiments and no differences in activation level were determined through addition of control antibody alone (data not shown). This confirms that IFN-γ is not required for either NK cell or macrophage activation in this system.
Fig. 3.
Role of IFN-γ on NK cell and macrophage (MΦ) activation in single cell-type and mixed-cell coculture. CD69 expression was determined on splenic NK cells isolated from IFN-γ–/– mice after 24 h culture alone or with MΦ from wild type (WT) C57BL/6, with and without LPS (a). Cocultures of NK cells with MΦ (NK + MΦ, NK + MΦ + LPS) showed increases in CD69 expression compared to culture alone (NK, NK + LPS) both with and without LPS, although these levels were significantly decreased from CD69 levels in NK cells from WT mice (compare with Fig. 1a). Phagocytosis level was determined at 48 h in WT MΦ with and without NK cells from IFN-γ–/– mice and with and without LPS (b). There were significant increases in phagocytosis mean channel fluorescence (MCF) of MΦ cocultured with NK cells (MΦ + NK, MΦ + NK + LPS), although these levels were again lower than in WT mice (compare with Figs 1b,c). There were no differences in phagocytosis MCF between MΦ cocultured with NK cells with and without 1 µg/ml anti-IFN-γ neutralizing antibody (c). n = 6 per experimental group and data are representative of separate repeated experiments. Data presented as mean ± s.e.m. * or †P < 0·05, Kruskal–Wallis (CD69 percentages), anova (MCF).
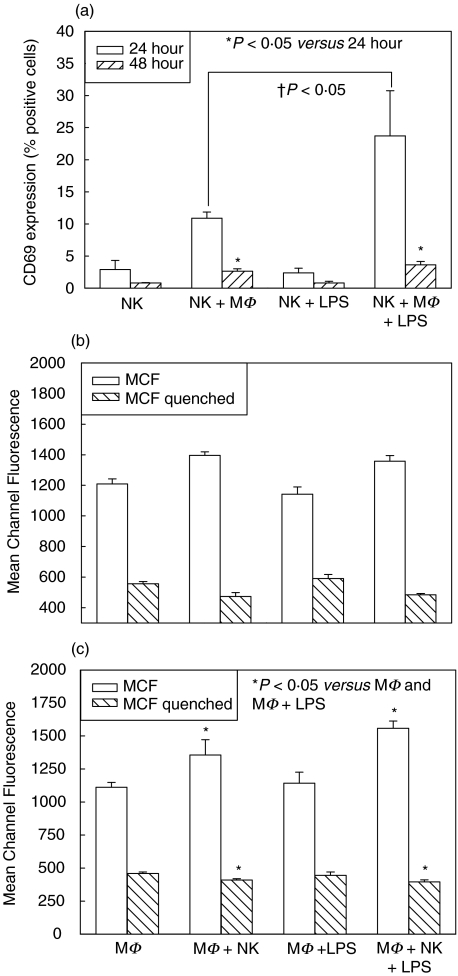
Ingestion of bacteria by macrophages is dependent on CD40/CD154 interactions
The interaction of CD40 on macrophages with its ligand CD154 on T cells is important in macrophage activation as well as polarization of T cell responses [14,17]. CD154 is also on NK cells, and NK cell interactions with CD40 on B cells have induced B cell maturation and immunoglobulin class switching in vitro [14]. The effects of interactions between NK cell CD154 and CD40 on macrophages are less well known. We therefore performed similar experiments to those described above using WT splenic NK cells cultured with peritoneal macrophages from CD40–/– mice (Figs 4a–c), and splenic NK cells from CD154–/– mice cultured with WT macrophages (Figs 5a–c). In both cases, NK cell CD69 expression was increased to levels observed in WT cocultures at 24 h (Fig. 4a and 5a). However, this increase was not maintained at 48 h in coculture either with CD40–/– macrophages (Fig. 4a), or CD154–/– NK cells (Fig. 5a), and was actually significantly reduced from activation at 24 h. The reasons for this difference are not clear, although as CD69 expression is maintained on cells for up to 48 h, the data suggest NK cell death. Indeed, overall cell numbers in 48 h cultures were decreased compared with WT (data not shown). This would be consistent with reports that CD40 interactions rescue cells from apoptosis [18]. Macrophage activation at 24 h again showed no differences between the experimental groups in cultures with either CD40–/– (Fig. 4b) or CD154–/– cells (Fig. 5b). At 48 h, however, there were differences in macrophage phagocytosis, with significant increases in MCF (represents numbers of attached and ingested bacteria), and significant decreases in quenched levels of MCF (represents numbers of ingested bacteria), of macrophages cocultured with NK cells in both CD40–/– (Fig. 4c) and CD154–/– (Fig. 5c) experimental groups. These data indicate that macrophages cocultured with NK cells were activated to attach bacteria, the first step in phagocytosis, although without CD40/CD154 interactions these macrophages were less able to ingest bacteria. Taken together, these results suggest that CD40/CD154-dependent cell contact is important in macrophage phagocytosis, but not in NK cell activation, and may be important in prevention of NK cell activation- induced cell death.
Fig. 4.
CD69 expression and macrophage phagocytosis in single cell-type and mixed-cell cocultures with macrophages (MΦ) from CD40-/– mice and splenic NK cells isolated from WT mice. CD69 expression was determined on NK cells at 24 and 48 h after culture with and without MΦ and LPS (a). There were significant increases in CD69 expression at 24 h in cocultured cells (NK + MΦ, NK + MΦ + LPS), although this increase was not maintained to 48 h (a). There were no differences in MΦ activation, as determined by phagocytosis ability, after 24 h culture with and without NK cells and LPS (b). There were significant increases mean channel fluorescence (MCF) (attached and ingested bacteria) between MΦ cocultures (MΦ + NK, MΦ + NK + LPS) and MΦ cultured alone (MΦ, MΦ + LPS) or with LPS for 48 h (c). However, there were significant decreases in quenched MCF (ingested bacteria alone) in the cocultured MΦ (MΦ + NK, MΦ + NK + LPS) compared with MΦ cultured alone (MΦ, MΦ + LPS) or with LPS at 48 h (c). n = 6 per experimental group and data are representative of repeated experiments. Data presented as mean ± s.e.m. * or †P < 0·05, Kruskal–Wallis (CD69 percentages), anova (MCF).
Fig. 5.
CD69 expression and macrophage phagocytosis in single cell-type and mixed-cell cocultures with macrophages (MΦ) from wild type (WT) mice and splenic NK cells isolated from CD154–/– mice. Cell surface CD69 expression was determined on NK cells at 24 and 48 h after culture with and without MΦ and LPS (a). There were significant increases in CD69 expression at 24 h in cocultured cells (NK + MΦ, NK + MΦ + LPS), although this increase was not maintained to 48 h (a). There were no differences in MΦ activation, as determined by phagocytosis ability, after 24 h culture with and without NK cells and LPS (b). There were significant increases mean channel fluorescence (MCF) (attached and ingested bacteria) between cocultured MΦ (MΦ + NK, MΦ + NK + LPS) and MΦ cultured alone (MΦ, MΦ + LPS) or with LPS for 48 h (c). However, there were significant decreases in quenched MCF (ingested bacteria alone) in the cocultured MΦ (MΦ + NK, MΦ + NK + LPS) compared with MΦ cultured alone (MΦ, MΦ + LPS) or with LPS at 48 h (c). n = 6 per experimental group and data are representative of separate repeated experiments. Data presented as mean ± s.e.m. * or † or ♯P < 0·05, Kruskal–Wallis (CD69 percentages), anova (MCF).
Cytokine production from macrophage and NK cell coculture
Levels of IL-6, IL-12, IL-18, and TNF-α from cultures containing macrophages, and levels of IFN-γ from cultures containing NK cells were obtained by ELISA of cell culture supernatants from each experimental group at 48 h after culture. Levels of IFN-γ and IL-18 were low, with levels around the limit of detection (data not shown). No useful analysis of these cytokine levels could therefore be made. In cultures with LPS, levels of IL-6 (Table 1) and TNF-α (Table 2) were significantly increased, as expected in the supernatants from stimulated macrophages. Statistical analysis of cytokine production between single cell-type cultures (MΦ, MΦ + LPS) and cocultures (MΦ + NK, MΦ + NK + LPS) showed very few differences between the groups, mainly secondary to large standard errors, and this makes any interpretation of results difficult. There was a significant increase in IL-6 levels produced by macrophages cultured alone, compared with culture with NK cells, as well as a significant increase in IL-6 production from macrophages cultured with LPS compared with culture together with NK cells in CD154–/– mice. This could represent potential regulation of IL-6 production from macrophages by NK cells, although further experiments would be required to confirm this. There were also no differences in IL-12 production (Table 3) between single cell-type cultures (MΦ, MΦ + LPS) and mixed-cell coculture (MΦ + NK, MΦ + NK + LPS). There were statistical differences between the groups using cells derived from either WT or genetic knockout mice (Table 3), although these were not consistent, and interpretation of these results was, again, difficult. Overall there were very few differences between any of the groups, and cytokine levels did not follow similar patterns to either CD69 expression or phagocytosis.
Table 1.
Levels of IL-6 measured by ELISA in culture supernatant from peritoneal macrophages cultured for 48 h with and without splenic NK cells and with and without 1 µg/ml LPS. Peritoneal macrophages from CD40−/– mice were also used, as well as splenic NK cells from CD154−/– and IFN-γ−/– mice
| MΦ (IL-6 pg/ml) | MΦ + ΝΚ (IL-6 pg/ml) | MΦ + LPS (IL-6 pg/ml) | MΦ + NK + LPS (IL-6 pg/ml) | |
|---|---|---|---|---|
| WT | 260 ± 60 | 87 ± 17 | 14100 ± 1800* | 8500 ± 300* |
| CD40–/– | 230 ± 40 | 370 ± 100 | 9800 ± 800* | 9500 ± 100* |
| CD154–/– | 290 ± 90 | 220 ± 60 | 10600 ± 1100* | 7100 ± 1300* |
| IFNγ–/– | 460 ± 210 | 400 ± 140 | 15900 ± 1600* | 13400 ± 1700* |
MΦ, peritoneal macrophages; NK, splenic NK cells; LPS, lipopolysaccharide; WT, wild type C57BL/6 mice; n = 6 per group, averaged from separate repeated experiments
P < 0·05 LPS versus no LPS.
Table 2.
Levels of TNF-α measured by ELISA in culture supernatant from peritoneal macrophages cultured for 48 h with and without splenic NK cells and with and without 1 µg/ml LPS. Peritoneal macrophages from CD40–/– mice were also used, as well as splenic NK cells from CD154–/– and IFN-γ–/– mice
| MΦ (TNF-α pg/ml) | MΦ + ΝΚ (TNF-α pg/ml) | MΦ + LPS (TNF-α pg/ml) | MΦ + NK + LPS (TNF-α pg/ml) | |
|---|---|---|---|---|
| WT | 34 ± 15 | 46 ± 31 | 340 ± 160* | 240 ± 60* |
| CD40–/– | 48 ± 15 | 55 ± 16 | 500 ± 150* | 420 ± 140* |
| CD154–/– | 5 ± 2 | 4 ± 3 | 292 ± 62* | 180 ± 18* |
| IFNγ–/– | 14 ± 2 | 12 ± 3 | 381 ± 54* | 338 ± 54* |
MΦ, peritoneal macrophages; NK, splenic NK cells; LPS, lipopolysaccharide; WT, wild type C57BL/6 mice; n = 6 per group, averaged repeated experiments
P < 0·05 LPS versus no LPS.
Table 3.
Levels of IL-12 measured by ELISA in culture supernatant from peritoneal macrophages cultured for 48 h with and without splenic NK cells and with and without 1 µg/ml LPS. Peritoneal macrophages from CD40−/– mice were also used, as well as splenic NK cells from CD154–/– and IFN-γ–/– mice
| MΦ (IL-12 pg/ml) | MΦ + ΝΚ (IL-12 pg/ml) | MΦ + LPS (IL-12 pg/ml) | MΦ + NK + LPS (IL-12 pg/ml) | |
|---|---|---|---|---|
| WT | 7·7 ± 1·0 | 7·7 ± 1·0 | 9·2 ± 0·9 | 6·0 ± 1·0 |
| CD40–/–* | 18·7 ± 3·0 | 15·2 ± 1·2 | 19·2 ± 1·9 | 15·0 ± 2·3 |
| CD154–/– | 13·0 ± 2·1 | 10·6 ± 0·4 | 11·8 ± 1·3 | 10·0 ± 2·6 |
| IFNγ–/– | 8·2 ± 2·5 | 10·2 ± 2·5 | 8·8 ± 2·2 | 3·0 ± 0·9¶ |
MΦ, peritoneal macrophages; NK, splenic NK cells; LPS, lipopolysaccharide; WT, wild type C57BL/6 mice; n = 6 per group, averaged repeated experiments
P < 0·05 WT versus CD40–/–.
P < 0·05 MΦ + NK + LPS (IFN-γ–/–) versus MΦ or MΦ + NK or MΦ + LPS (IFN-γ–/–).
DISCUSSION
NK cell interactions with other cells of the immune system are important in regulating a wide range of immune responses. Previous work from our laboratory showed important interactions between NK cells and macrophages during bacterial sepsis to improve bacterial clearance after peritonitis [10,19]. In this study we have determined that CD40/CD154 direct cell contact interaction is a main activating mechanism for increasing macrophage phagocytosis during sepsis, although it does not appear to be the main mechanism providing reciprocal NK cell activation. Our experimental system compares interactions between peritoneal macrophages and splenic NK cells. The possibility exists that different interactions between macrophages and NK cells may occur at different sites.
CD40/CD154 interactions have previously been identified as being important in macrophage activation, after interactions with T cells [14,17], as well as B cell maturation and immunoglobulin class switching, after interactions with NK cells [14]. Recent evidence has strengthened the role of CD40/CD154 interactions between NK cells and dendritic cells in producing dendritic cell maturation and activation, and this provides a mechanism for a genuine link between the innate and adaptive immune response following sepsis [12,13,20,21]. CD40/CD154 interaction between macrophages and NK cells was therefore a prime candidate as the mechanism of reciprocal activation of these cells during bacterial peritonitis. Despite this, the in vivo significance of NK-expressed CD154 remains unknown, and has not been investigated widely in bacterial models of sepsis. We have provided evidence in this report that NK cell CD154 interaction with macrophage CD40 is important in macrophage activation and phagocytosis with LPS stimulation. Interestingly, however, NK cell activation does not appear to be regulated through interactions via CD154, although these interactions may be important in maintaining NK cell activation, by protecting against activation-induced cell death [18]. Interactions between NK cells and macrophages therefore have the potential to be extremely important in the duration of responses during sepsis, and may play a role in the imbalance of pro- and anti-inflammatory responses believed to be crucial in the development of multiple organ dysfunction following sepsis [22,23].
Potential activators of NK cell function also include IL-12 from activated macrophages [11]. We also investigated the role of IL-12 on NK cell activation in our in vitro model of sepsis, using neutralizing anti-IL-12 antibody in cocultures. Methods were similar to those in experiments using anti-IFN-γantibodies. We found that there was no difference in activation of NK cells or macrophages after 48 h with or without anti-IL-12 (data not shown). This is surprising as IL-12 is a widely recognized activator of NK cells through a STAT4-dependent mechanism [11,24]. The results in this study, however, are consistent with results from other experiments we have undertaken that indicate IL-12 and IL-18 do not play a major role in NK cell activation during bacterial peritonitis (unpublished observation). Altogether, these data strengthen the evidence for direct cell-contact mechanisms to play larger role in NK cell activation during sepsis, with other costimulatory molecules apart from CD154, such as CD80, CD86 [25], and inducible costimulator (ICOS) [26] also having potential importance. Further investigations will be required to identify the relative roles of these costimulatory molecules.
IFN-γ is undoubtedly important in activating macrophages, and also in maintaining Th1/Th2 responses [8], and although we didn’t determine a requirement for IFN-γ in macrophage and NK cell reciprocal activation in this present study, it appears to play a role in regulating the magnitude of the cell activation and inflammatory response during sepsis, IFN-γ is important in a good outcome during human sepsis and this relates directly to increased antigen presenting capacity by up-regulation of human leucocyte antigen (HLA)-DR on macrophages and monocytes [27]. There is also a well known synergism of IFN-γ with LPS to activate macrophages, and this is used widely to stimulate macrophages prior to assessment of activation. It has been discovered recently that macrophages are capable of producing their own IFN-γ in response to stimulation by IL-12 and IL-18 [28], which may allow them to be sensitized to further IFN-γ produced by NK cells and T cells following sepsis. One postulated role for NK cells in vivo is through their role in this IFN-γ sensitization of macrophages [29], which may allow IFN-γfrom T cells, present in much larger numbers compared with NK cells, to have greater effects. Within our study there is also the potential for contaminating T cells in our macrophage population contributed to IFN-γ production, and despite the small number of cells involved this may influence the results obtained.
Interactions between NK cells and macrophages during sepsis are important, and future research in this area may elucidate regulatory mechanisms that can be manipulated to control immune responses during sepsis. Further elucidation of the function of these interactions in vivo is required, however, before such manipulation can take place, but may hold the key to novel treatments and prevention of the consequences of unregulated immune responses following sepsis, such as multiple organ failure.
Acknowledgments
We thank Dr Sam Wellhausen and the flow cytometry facility at the James Graham Brown Cancer Center for technical assistance and support. This material was based on work supported by the Office of Research and Development, Department of Veterans Affairs, and by fellowship awards to MJS from the Surgical Infection Society.
Data presented at EB2004, Washington D.C., April 2004 – AAI Block Symposium
REFERENCES
- 1.Kiessling R, Klein E, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 2.Brutkiewicz RR, Sriram V. Natural killer T (NKT) cells and their role in antitumor immunity. Crit Rev Oncol Hematol. 2002;41:287–98. doi: 10.1016/s1040-8428(01)00198-6. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 4.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 5.Seaman WE. Natural killer cells and natural killer T cells. Arthritis Rheum. 2000;43:1204–17. doi: 10.1002/1529-0131(200006)43:6<1204::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Seki S, Osada S, Ono S, et al. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect Immun. 1998;66:5286–94. doi: 10.1128/iai.66.11.5286-5294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancroft GJ, Schreiber RD, Bosma GC, Bosma MJ, Unanue ER. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987;139:1104–7. [PubMed] [Google Scholar]
- 8.Lieberman LA, Hunter CA. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 2002;4:1531–8. doi: 10.1016/s1286-4579(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 9.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro. evidence of NK cells as primary targets. Infect Immun. 2000;68:752–9. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott MJ, Hoth JJ, Gardner SA, Peyton JC, Cheadle WG. Natural killer cell activation primes macrophages to clear bacterial infection. Am Surg. 2003;69:679–86. [PubMed] [Google Scholar]
- 11.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–78. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 12.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amakata Y, Fujiyama Y, Andoh A, Hodohara K, Bamba T. Mechanism of NK cell activation induced by coculture with dendritic cells derived from peripheral blood monocytes. Clin Exp Immunol. 2001;124:214–22. doi: 10.1046/j.1365-2249.2001.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 15.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J Exp Med. 1997;185:2053–60. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaussabel D, Jacobs F, de Jonge J, et al. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect Immun. 1999;67:1929–34. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–92. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 18.Poe JC, Wagner DH, Jr, Miller RW, Stout RD, Suttles J. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1beta synthesis and rescue from apoptosis. J Immunol. 1997;159:846–52. [PubMed] [Google Scholar]
- 19.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–9. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–8. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 21.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Scott MJ, Godshall CJ, Cheadle WG. Jaks, STATs, Cytokines, and Sepsis. Clin Diagn Laboratory Immunol. 2002;9:1153–9. doi: 10.1128/CDLI.9.6.1153-1159.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thierfelder WE, van Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–4. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–8. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 26.Ogasawara K, Yoshinaga SK, Lanier LL. Inducible costimulator costimulates cytotoxic activity and IFN-gamma production in activated murine NK cells. J Immunol. 2002;169:3676–85. doi: 10.4049/jimmunol.169.7.3676. [DOI] [PubMed] [Google Scholar]
- 27.Hershman MJ, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC., Jr Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clin Exp Immunol. 1989;77:67–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–8. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Herrero C, Li WP, et al. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–66. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]