Abstract
Patients with chronic hepatitis C (CHC) are unable to prime and maintain vigorous T cell responses that are initiated during the acute phase of hepatitis C virus (HCV) infection. As dendritic cells (DCs) induce and regulate both innate and adaptive immune responses, the aim of this study was to analyse two critical functions of DCs: firstly, production of interferon (IFN)-α and, secondly, polarization of T helper 1 lymphocytes. The frequencies of plasmacytoid DC (PDC) and myeloid DC (MDC) were estimated in 63 patients with CHC and 34 normal controls using four-colour flow cytometry. Circulating DCs were isolated from peripheral blood of CHC patients (n = 10) and normal controls (n = 10). These DCs were cultured with herpes simplex virus-1 to evaluate their capacity to produce IFN-α. The capacity of DCs to induce polarization of autologous naive CD4+ T lymphocytes to IFN-γ-producing effector T lymphocytes was also assessed. The frequencies of PDCs producing intracellular IFN-α (P < 0·01) and the levels of IFN-α in culture supernatant of PDCs (P < 0·01) were significantly lower in patients with CHC compared to those of normal controls. The numbers of MDC were significantly lower in patients with CHC (8·2 (6·0)/µl, median (interquartile range), n = 63) compared to normal control (11·7 (7·8)/µl, n = 34) (P < 0·01). Moreover, DCs from patients with CHC induced significantly lower numbers of IFN-γ-producing effector T lymphocytes compared to that of controls (P < 0·01). This study indicates that the low IFN-α-producing capacity and impaired T helper 1 polarization ability of DCs from patients with CHC might be responsible for the typical low anti-HCV immune responses in these patients.
Keywords: plasmacytoid dendritic cells, myeloid dendritic cells, chronic hepatitis C, interferon-α, T cell polarization
INTRODUCTION
Hepatitis C virus (HCV), a single-stranded RNA virus of Flaviviridae family, is notorious for causing a high rate (70%-85%) of chronicity that, in 20% of cases, eventually leads to severe complications like liver cirrhosis and hepatocellular carcinoma [1,2]. A vigorous T-cell response encompassing a large numbers of HCV epitopes that are rapid, strong and maintained are required for clearance of HCV in infected humans and chimpanzees [3,4]. Unfortunately, patients with chronic hepatitis C (CHC) either mount an early and vigorous T cell response that temporarily controls viral replication but eventually wanes or do not develop detectable CD4+ and CD8+ T cell-mediated responses during acute infection [3–5]. Moreover, the ability of HCV to outpace the T cell response may explain its tendency to persist [6,7]. These features suggest that some defects in priming and maintenance of the HCV-specific immune responses occur in patients with CHC.
Induction and maintenance of HCV-specific immune responses requires active participations of host antigen-presenting cells, the most potent of which are dendritic cells (DCs) because of their professional competency to recognize, capture, internalization, processing and presentation of viruses to induce both innate and adaptive immune responses against the viruses [8,9]. Some studies, conducted to have insights about DC/HCV interactions, have documented HCV RNA in monocyte-derived DCs and impaired allostimulatory capacity of those DCs from patients with CHC [10–13]. They have suggested that infection of DCs by HCV might be responsible for impaired HCV-specific immune responses in these patients. However, recent advances in DC research indicate that this is an oversimplification of a very complex issue. Mere presence of HCV RNA does not explain the impaired HCV-specific immune responses in these patients because DCs are committed to capture and internalize HCV as professional antigen-presenting cell. Accordingly, it is natural that HCV RNA would be detected in DCs. Moreover, DCs infected with measles virus and influenza do not cause chronic and persistent infections [14,15]. This indicates that it is important to evaluate the functional consequences that arise from infection of DCs with HCV in patients with CHC.
Among different subpopulation of circulating DCs, plasmacytoid DCs (PDCs) produce abundant amounts of type-1 interferons (IFNs) in response to microbial agents [16,17] and are critical for antiviral immune responses. PDCs perform two major functions during their life time:
DCs also capture microbial agents and present those to naïve T cells for production of antigen-specific effector T cells [20].
In this study, we evaluated the capacities of circulating PDCs to produce IFN-α and circulating DCs to polarize naïve CD4+ T cells to IFN-γ-producing effector T cells from patients with CHC.
MATERIALS AND METHODS
Clinical characteristics of patients
Sixty-three patients with CHC were enrolled in this study. These patients were attending our hospital for periodic follow up and management. Various data regarding the clinical background of these patients is supplied in Table 1. All patients enrolled in this study did not receive any antiviral therapy during last six months. Thirty-four HCV-seronegative age and sex matched normal volunteers were also included as control subjects. The numbers of circulating DCs, PDCs and MDCs were estimated in these patients by flow cytometry.
Table 1.
Clinical profile of the patients and the normal controls
| Patients with chronic hepatitis C | Normal controls | |
|---|---|---|
| Numbers | 63 | 34 |
| Age (years) | 56 ± 12 | 50 ± 18 |
| Sex (male: female) | 36 : 27 | 21 : 13 |
| Total bilirubin (0·1–1·1 mg/dl)* | 0·7 ± 0·3 | 0·7 ± 0·2 |
| Alanine aminotransferase (9–37 U/l) * | 63·4 ± 60·9 | 28·8 ± 14·9 |
| Asparate aminotransferase (3–49 U/l) * | 47·0 ± 31·2 | 27·4 ± 8·9 |
| γ-glutamyl transpeptidase (6–71 IU/l) * | 41·4 ± 32·8 | 32·2 ± 14·0 |
| HCV RNA serotype (1: 2: unknown) | 32 : 17 : 14 | N.T. |
Normal values are shown in the parenthesis. N.T., not tested.
The production of IFN-α by PDCs and the capacity of DCs to polarize naïve autologous CD4+ T cells to IFN-γ-producing effector T cells were studied in 10 patients with CHC (Table 2). In these studies, 10 normal volunteers with no evidence of liver diseases served as controls.
Table 2.
Clinical characteristics of patients with chronic hepatitis C
| No. | Sex/age (year) | ALT (IU/l) | Viral load (KIU/ml) | HCV RNA serotype | Prior therapy |
|---|---|---|---|---|---|
| 1. | M/56 | 141 | 240 | 1 + 2 | None |
| 2. | M/53 | 59 | >850 | 1 | None |
| 3. | M/60 | 59 | >850 | 1 | None |
| 4. | M/50 | 55 | 6 | 1 + 2 | None |
| 5. | M/45 | 46 | >850 | 1 | IFN-α |
| 6. | M/54 | 29 | 1 | 2 | IFN-α |
| 7. | M/48 | 47 | 2·4 | 2 | None |
| 8. | M/46 | 72 | 140 | 2 | None |
| 9. | M/30 | 92 | 130 | 2 | None |
| 10. | M/51 | 55 | 94 | 2 | None |
Production of interferon-α by plasmacytoid DCs due to stimulation with herpes simplex virus-1 and the capacity of DCs to polarize autologous naïve CD4+ T lymphocytes to IFN-γ-producing effector T lymphocytes were evaluated in these patients. M, male; ALT, alanine aminotransferase.
Informed consent was obtained from each patient included in the study and the study protocol was approved by the Human Research Committee of Ehime University.
The frequencies of DCs, PDCs and MDCs in the peripheral blood
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood by density gradient separation method using Ficoll-Conray (Pharmacia, San Jose, CA, USA) and suspended in RPMI 1640 (Iwaki, Chiba, Japan) plus 10% heat-inactivated fetal calf serum (Filtron Pty. Ltd, Brooklyn, Australia).
The frequencies of DCs, PDCs and myeloid DCs (MDCs) in the peripheral blood were calculated by incubating PBMCs with a cocktail of fluorescein isothiocyanate-conjugated (FITC) monoclonal antibodies to CD3, CD14, CD16, CD19, CD20 and CD56, peridinin chlorophyll protein (PerCP)-conjugated antibody to CD4 (clone SK3), phycoerythrin (PE)-conjugated antibody to CD123 (IL-2Rα) (clone 9F5) and allophycocyanin (APC)-conjugated antibody to CD11c (clone S-HCL-3) (all from Becton Dickinson, San Jose, CA, USA). Isotype-matched antibodies were used as controls. The cells were incubated on ice for 30 min, and were then washed with phosphate-buffered saline. Four-colour staining profiles were analysed on FACS Calibur (Becton Dickinson) after the removal of dead cells and debris. In each case, 5000 lineage–CD4+ DCs were counted by flow cytometry. The relative frequencies of PDCs and MDCs in blood were expressed as lineage–CD4+CD11c–CD123bright DCs and lineage–CD4+CD11c+CD123dim DCs, respectively. The absolute numbers of PDCs and MDCs were calculated from the following formula; absolute number of PDCs or MDCs = % of PDCs or MDCs × numbers of PBMCs in the blood.
Isolation of circulating DCs and naïve CD4+ T lymphocyte
Circulating DCs were isolated from the peripheral blood by immunomagnetic cells sorting using commercial kit (Blood Dendritic Cells Isolation Kit, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), exactly as described [21]. Briefly, T cells, monocytes and natural killer cells were depleted from PBMCs using magnetic beads coated with monoclonal antibodies against CD3 (clone BW264/56), CD11b (clone M1/70·15·11·5) and CD16 (clone VEP-13) by magnetic-activated cell sorter (MACS, Miltenyi Biotec GmbH). Circulating DCs were isolated from the depleted cell fractions by a positive selection step using a monoclonal antibody against CD4 (clone M-T321, Miltenyi Biotec GmbH). The purity of isolating DCs was more than 90%. The ratio of PDC and MDC in isolated DCs varied considerably among controls and patients with CHC. About 20–40% of the isolated DCs expressed CD123 and 60–80% DCs expressed CD11c.
Naïve CD4+ T lymphocytes were isolated using a commercial kit (CD4+ T cell isolation kit, Miltenyi Biotec GmbH), exactly according to the description of the manufacturer. In short, CD8+, CD11b+, CD16+, CD19+, CD36+ and CD56+ cells of the PBMCs were depleted by a cocktail of monoclonal antibodies supplied with the kit. Naïve CD4+ T lymphocytes were isolated by incubating CD4+ T lymphocytes with monoclonal antibody to CD45RA (clone L48)-coated magnetic beads. The purity of naïve T cells were about 95%.
Production of IFN-α by PDCs
To evaluate the production of IFN-α by PDCs, circulating DCs (5 × 105/ml) were cultured with 1 × 105, 5 × 105, 1 × 106 plaque forming units (PFU)/ml of herpes simplex virus (HSV) [22] (kindly provided by Dr Masaki Yasukawa, First Department of Internal Medicine, Ehime University School of Medicine, Japan) for 5 h, as described [22]. After 4 h of incubation, brefeldin A (10 µg/ml, Sigma, St. Louis, MO, USA) was added to the culture to block IFN-α secretion from PDCs. The cells were then incubated with PE-labelled monoclonal antibody to CD123 (IL-2Rα), fixed and permeabilized using a commercial kit (FIX and PERM kit; Caltag Laboratories, Burlingame, CA, USA). PDCs stimulated with HSV-1 were then stained with mouse antihuman IFN-α monoclonal antibody (MMHA-11, PBL Biomedical Laboratories, Piscataway, NJ, USA) as primary antibody and FITC-conjugated goat antimouse IgG F(ab′)2 (Jackson ImmunoReserach Laboratories, Inc, West Groove, PA, USA) as secondary antibody. The relative frequencies of PDCs expressing intracellular IFN-α were estimated by flow cytometry.
The levels of IFN-α in the culture supernatants of HSV-stimulated DCs were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Human IFN-α multi-Species ELISA kit, PBL Biomedical Laboratories, Piscataway, NJ, USA).
Differentiation of naïve CD4+T lymphocytes into IFN-γ-producing T lymphocytes by DCs
This was done according to a published study with some modifications [23]. Autologous CD4+CD45RA+ naïve T lymphocytes (1 × 106/ml) were cultured with graded doses of DCs in a 96-well plate in 200 µl of culture media. After 7 days culture, the T lymphocytes were washed and subsequently stimulated with phorbol 12-myristate 13-acetate (PMA) (40 ng/ml, Sigma) and ionomycin (2 µg/ml, Sigma) for 8 h. Brefeldin A (5 µg/ml, Sigma) was added to the cultures for the last 4 h. T lymphocytes were then stained with Per CP-labelled CD3 (SK7, Becton Dickinson) monoclonal antibody, and then fixed, permeabilized, and stained with FITC-conjugated mAbs to IFN-γ (clone 25723·11, Becton Dickinson) and PE-conjugated interleukin-4 (clone 3010·211, Becton Dickinson).
Statistical analysis
The data were expressed as mean ± standard deviation. Means were compared with unpaired t-test. In case of differences (as assessed by an F-test), t-tests were adjusted for unequal variances (Mann–Whitney's U-test). P < 0·05 was considered to be statistically significant. Statistical calculations were performed using the Stat View (version 5·0) statistical program in Intel Computer (Pentium 4).
RESULTS
Characterization of DCs
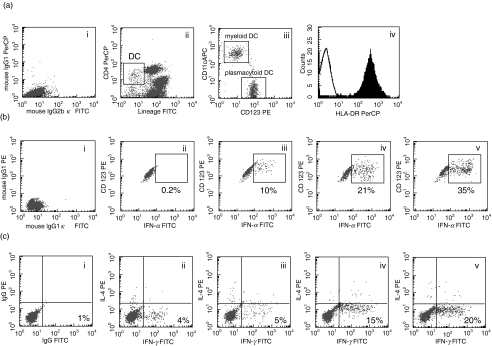
Circulating DCs were lineage– and CD4+(Fig. 1a(ii)). These DCs also expressed HLA DR (Fig. 1a(iv)) and either CD11c or CD123. PDCs and MDCs were detected from the gated DCs by staining with antibodies to CD123 and CD11c, respectively (Fig. 1a(iii)). Functionally, circulating DCs stimulated allogenic T lymphocytes in a dose dependent manner. On the other hand, PDCs expressed intracellular IFN-α in response to HSV-1 (data not shown).
Fig. 1.
Flow cytometric profile showing the method of detection of circulating dendritic cells (DCs), myeloid DC and plasmacytoid DC. The flow cytometric profiles of isotype controls have been shown in part (i) of (a), (b) and (c). (i) Circulating DCs were detected as lineage negative and CD4+ lymphocytes of peripheral blood mononuclear cells (ii). Plasmacytoid DCs and myeloid DCs were enumerated by the expression of CD123 and CD11c on circulating DCs (iii). Circulating DCs expressed high levels of HLA-DR (iv). (b) Plasmacytoid DCs were stimulated with no HSV-1 (ii), 1 × 105 (iii) 5 × 105 (iv) and 1 × 106 (v) plaque forming units/ml of HSV. DCs were then stained for intracellular IFN-α, as described in Method. Plasmacytoid DC expressing intracellular IFN-α showed a right shift in the histogram depending on the dose of HSV-1. (c) Intracellular expression of IFN-γ by CD4+ T lymphocytes due to stimulation of naïve CD4+ T cells with 1 × 103 (iii) 3 × 103 (iv) and 1 × 104 (v) of DC or without DCs (ii). The culture was done for 7 days to evaluate the polarization of naïve CD4+ T lymphocytes by DCs. The numbers of IFN-γ-producing T lymphocytes increased as the amounts of DCs were increased in the cultures.
Impaired production of IFN-α by PDC from patients with CHC
The capacity of PDCs to produce IFN-α was assessed by two methods [1]; counting the numbers of PDCs expressing intracellular IFN-α and [2] measuring the levels of IFN-α in the culture supernatants of HSV-stimulated DCs.
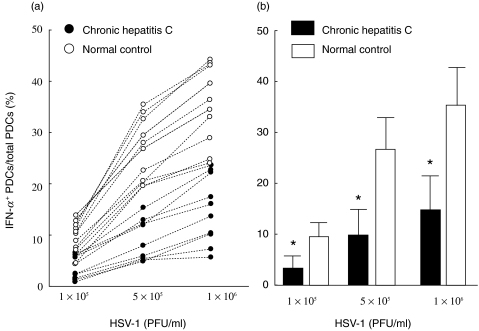
Stimulation of DCs with HSV-1 induced intracellular IFN-α in PDCs in a dose-dependent manner (Fig. 1b). As shown in Fig. 2a, 0·7%-5·7% PDCs from CHC patients expressed intracellular IFN-α those were stimulated with 1 × 105 PFU/ml of HSV-1. However, 4·2%-13·4% PDCs from normal controls expressed intracellular IFN-α under the similar conditions. This trend persisted when 5 × 105 or 1 × 106 PFU/ml of HSV-1 were used to stimulate DCs from patients with CHC or control subjects. The mean numbers of PDCs expressing intracellular IFN-α were significantly lower in patients with CHC compared to control subjects (P < 0·01) (Fig. 2b). Although the levels of ALT and HCV RNA varied considerably among patients (Table 2), there was no relationship between the IFN-α-producing capacities of DCs from CHC patients and these clinical parameters (data not shown).
Fig. 2.
Impaired production of intracellular IFN-α in plasmacytoid DCs from patients with chronic hepatitis C due to stimulation with various doses of herpes simplex virus-1. The numbers of plasmacytoid DCs expressing IFN-α from individual normal control (○) and patient with chronic hepatitis C (•) are plotted in (A). Mean and standard deviation of the data of Figure in (a) is shown in (b). *P < 0·01 compared to normal controls.
The levels of IFN-α in culture supernatants of HSV-1-stimulated DCs were significantly lower in patients with CHC (72·3 ± 74·1 pg/ml, n = 10) compared to control subjects (520·7 ± 297·1 pg/ml, n = 10) (P < 0·01) (Table 3).
Table 3.
Expression of intracellular interferon-α in plasmacytoid DCs due to stimulation with herpes simplex virus-1
| Diagnosis | Numbers | Dose of HSV-1 (PFU/ml) | IFN-α+ cells in plasmacytoid DCs (%) | Mean fluorescence intensity | Levels of IFN-α (pg/ml) |
|---|---|---|---|---|---|
| Patients with chronic hepatitis C | 10 | 1 × 105 | 3·4 ± 2·2* | 339 ± 67 | N.T. |
| 5 × 105 | 9·9 ± 5·0* | 420 ± 69* | N.T. | ||
| 1 × 106 | 14·7 ± 6·5* | 460 ± 67* | 72·3 ± 74·1* | ||
| Normal controls | 10 | 1 × 105 | 9·5 ± 2·8 | 432 ± 96 | N.T. |
| 5 × 105 | 26·7 ± 6·0 | 578 ± 109 | N.T. | ||
| 1 × 106 | 35·0 ± 7·6 | 610 ± 109 | 520·7 ± 297·1 |
DCs were stimulated with various doses of plaque forming units (PFU) of herpes simplex virus (HSV)-1 for 5 h and the levels of expression of intracellular IFN-α in plasmacytoid DC were estimated from flow cytometric profiles. The levels of IFN-α in the culture supernatants were estimated by enzyme-linked immunosorbent assay. The results were expressed as percentage of IFN-α+ cells in plasmacytoid DCs, its mean fluorescence intensity and the levels of IFN-α in the culture supernatants. Data are shown as mean+-standard deviation.
P < 0·01 compared to normal controls of the same dose of HSV-1. N.T., not tested.
DCs from patients with CHC had decreased capacity to induce Th1 polarization
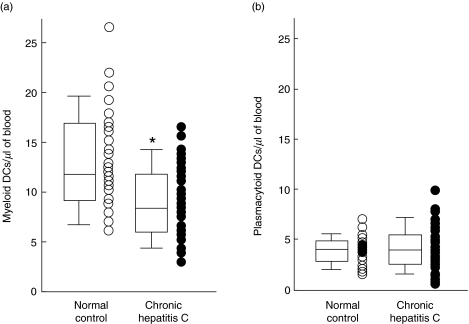
DCs induced polarization of naïve CD4+ T cells into IFN-γ-producing T cells. In this study, to evaluate the T cell polarization capacity of DCs, we cultured naïve CD4+ T cells and DCs in an autologous mixed leucocyte reaction. As shown in Fig. 1c, the polarization of naïve CD4+ T cells was dependent on the numbers of DCs in the culture. As shown in Fig. 3a, the numbers of MDCs varied considerably among individual patients with CHC (2·9–16·1 MDCs/µl of blood) and individual normal controls (6·1–26·6 MDCs/µl of blood). The median (interquartile range) ratio of MDCs to total PBMC was significantly decreased in patients with CHC (0·38 (0·20)%, n = 63) compared to normal controls (0·49 (0·27)%, n = 34) (P < 0·01). The absolute numbers of MDCs/µl of blood were also significantly lower in patients with CHC (8·2 (6·0)/µl, n = 63) compared to normal controls (11·7 (7.8)/µl, n = 34) (P < 0·01, Fig. 3). However, the numbers of PDCs in the blood did not vary considerably among patients with CHC (4·0 (2.8)/µl, n = 64) and normal controls (4·0 (2·0)/µl, n = 34) (P = 0·55).
Fig. 3.
Decreased numbers of myeloid DC from patients with chronic hepatitis C. The numbers of myeloid DC and plasmacytoid DC from normal control (○) and patient with chronic hepatitis C (•) are plotted from the data of flow cytometric analysis. *P < 0·01.
The most important function of DCs is to induce polarization of naïve autologous CD4+ T lymphocytes to IFN-γ-producing effector CD4+ T lymphocytes. These effector lymphocytes possess various types of antiviral properties. In order to assess the capacity of DCs from CHC to induce IFN-γ-producing T lymphocytes, we cultured naïve autologous CD4+ T lymphocytes with DCs from patients with CHC.
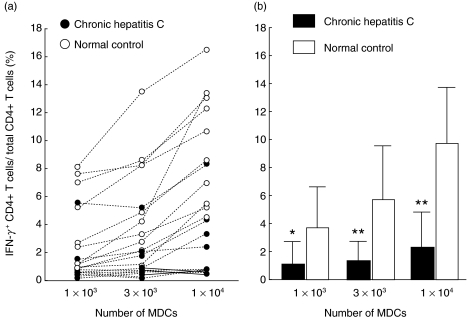
As shown in Fig. 4, DCs from patients with CHC induced significantly lower numbers of IFN-γ-producing CD4+ T lymphocytes compared to that of DCs from normal controls. The data shown in Fig. 4a was adjusted according to the numbers of MDCs in each patient. The mean numbers of IFN-γ-producing CD4+ T lymphocytes were significantly lower in patients with CHC compared to control subjects irrespective of the numbers of MDCs used to induce T helper polarization (Numbers of MDCs; 1 × 103, P < 0·05, No. of MDCs; 3 × 103, P < 0·01; numbers of MDCs; 1 × 104, P < 0·01) (Fig. 4b).
Fig. 4.
Decreased capacity of myeloid DCs from patients with chronic hepatitis C to induce Th1 polarization of naïve autologous CD4+ T cells. The ratio of IFN-γ-expressing CD4+ T lymphocytes to total CD4+ T lymphocytes represents the polarization of naïve CD4+ T lymphocytes to effector T lymphocytes due to stimulation with myeloid DC for 7 days. The frequencies of IFN-γ-expressing CD4+ T lymphocytes to total CD4+ T lymphocytes was adjusted according to the numbers of myeloid DCs (MDCs) in the culture. Individual data are shown in (a) and mean and standard deviation are shown in (b). *P < 0·05. **P < 0·01.
DISCUSSION
The nature of immune response of HCV-infected patients is poorly understood. Patients who spontaneously clear the virus mount vigorous T cell responses encompassing large numbers of viral epitopes and these responses are maintained after the clearance of HCV infection in these patients. On the contrary, vigorous T cell responses that were developed during acute phase of HCV infection wane in patients with CHC in spite of presence of high levels of HCV genomes in the sera and the liver [3–7]. In general, virus particles activate innate as well as adaptive immune responses. Thus, it is elusive why patients with CHC are unable to maintain or initiate proper anti-HCV immune responses in situ.
To address these issues, we decided to study the functions of DCs in patients with CHC because DCs play critical roles during induction and maintenance of antiviral immune responses. This study is completely different from previously described investigations about DC/HCV interactions in CHC patients [10–13]. First, we used circulating DCs, not monocyte-derived DCs, because monocytes seldom convert to DCs and very high levels of selective cytokines that are required for conversion of monocytes to DCs in vitro are not usually available in vivo. Circulating DCs were used because these DCs represent the functional DCs in vivo. They mobilize to the sites of microbial infection, capture those and induce microbial agent-specific immune responses [24,25]. The next, although most of the investigators have checked the allostimulatory capacity of DCs from CHC patients [10–13], we analysed two critical functions of DCs: firstly,IFN-α-producing capacity of PDCs and secondly, IFN-γ-polarization capacity of DCs. These two antiviral capacities of DCs have not been investigated in patients with CHC by any investigators till now [8–10]
PDCs from patients with CHC did not produce IFN-α spontaneously although they were stimulated by HCV in vivo indicating that HCV is a poor inducer of IFN-α. When stimulated with HSV-1, PDCs from CHC patients produced significantly lower levels of IFN-α compared to that of control subjects. Low IFN-α-producing capacity of PDCs from patients with CHC was confirmed by two methods: checking the numbers of PDCs expressing intracellular IFN-α and measuring the levels of IFN-α in supernatants of cultures containing PDCs and HSV-1.
As most of the capsulated virus such as HSV, Sendai virus, cytomegalovirus and influenza virus induces IFN-α from PDCs [10], it is elusive why HCV could not induce IFN-α in PDCs from patients with CHC. It is also unknown why PDCs from patients with CHC were refractive to the stimulation of HSV-1. We could not study the capacity of PDCs to produce IFN-α due to direct stimulation with HCV due to non availability of HCV virus particles, however, the low IFN-α-producing capacity of PDCs from CHC patients should be evaluated using other viruses. However, it appears that the levels of ALT and HCV RNA did not have any significant role in this regard. PDC represents a unique cell lineage within immune system, which performs two major functions during their life time [1]: killing of viruses and [2] initiation and dictation of adaptive immune response [10,17–19]. Moreover, IFN-α enhances the cytotoxic effect of natural killer cells and macrophages, induce T cell activation and play a vital role in the survival of activated T cells. The incapability of PDCs from patients with CHC to induce adequate IFN-α partly explains why these patients were unable to destroy HCV completely and why vigorous T cell responses that are initiated during acute phase of HCV infection are not maintained in patients with CHC.
Impaired ability of DCs from patients with CHC to induce polarization of naïve T lymphocytes to IFN-γ-producing effector T lymphocytes would hinder their ability to maintain HCV-specific immune responses in vivo.
In general three mechanisms are proposed to justify the viral persistence in a host:
the integration of the virus into the genome of the host cells;
the alteration of replication of the virus;
the disruption of the immune system.
The possibility of integration of HCV into the genome of the host is not so evident in HCV infection. We have provided two important evidences which explain why patients with CHC are unable to trigger and maintain efficient antiviral immune responses.
Patients with CHC are treated with antiviral drugs like type-1 IFN and ribavirin. This study indicates that manipulation of function of PDCs in vivo in patients with CHC might represent a unique therapeutic approach for these patients. Vaccines and antigen-pulsed DCs are now used to activate DCs and to treat patients with chronic viral infection such as chronic hepatitis B [26] and malignancies [27].
In summary, IFN-α-producing capacity and T helper 1 polarization ability of DCs were impaired in patients with CHC, which might account for weak and waning T cells responses of these patients. Further studies are warranted to investigate the methods of up-regulating the functions of these DCs in vivo for developing novel therapeutic strategies for treating these patients.
REFERENCES
- 1.Miller RM, Purcell RH. Hepatitis C virus shares amino acid sequence similarity with pestivirus and flavivirus as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–61. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang TJ, Seeff L, Hoofnagle JH. Pathogenesis, natural history, treatment and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Diepolder HM, Gerlach JT, Zachoval R, et al. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J Virol. 1997;71:6011–9. doi: 10.1128/jvi.71.8.6011-6019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper S, Erickson AL, Adams EJ, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–49. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 5.Gerlach JT, Diepolder HM, Jung M-C, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 6.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechner F, Wong DKH, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;108:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 11.Auffermann-Gretzinger S, Keefe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–8. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 12.Bain C, Fatmi A, Zoulim F, Zarski J-P, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 13.Tsubouchi E, Akbar SMF, Horiike N, Onji M. Infection and dysfunction of circulating blood dendritic cells and their subsets in chronic hepatitis C virus infection. J Gastroenterol. 2004 doi: 10.1007/s00535-003-1385-3. in press. [DOI] [PubMed] [Google Scholar]
- 14.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:901–1012. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–44. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–12. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type 1 interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 19.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principle type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 20.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;108:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 21.Murakami H, Akbar SMF, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504–10. doi: 10.1046/j.1365-2249.2002.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasukawa M, Inatsuki A, Horiuchi T, Kobayashi Y. Functional heterogeneity among herpes simplex virus-specific human CD4+ T cells. J Immunol. 1991;146:1341–7. [PubMed] [Google Scholar]
- 23.Uehira K, Amakawa R, Ito T, et al. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin Immunol. 2002;105:296–303. doi: 10.1006/clim.2002.5287. [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama H, Matsuno K, Zhang Y, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Medical. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T–lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 26.Horiike N, Akbar SMF, Ninomiya T, Abe M, Michitaka K, Onji M. Activation and maturation of antigen-presenting dendritic cells during vaccine therapy in patients with chronic hepatitis due to hepatitis B virus. Hepatol Res. 2002;23:38–47. doi: 10.1016/s1386-6346(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–4. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]