Abstract
Therapeutic enzymes are often recognized as foreign by the immune system of patients undergoing enzyme replacement therapy. The antibodies that develop may alter pharmacokinetics and biodistribution of the therapeutic protein, may be able to neutralize the activity of the enzyme, or may cause immune reactions in certain patients. We have explored treatment regimens to reduce the antibody response to human α-galactosidase A (r-hαGAL) in Fabry (αGAL knock-out) and normal BALB/c mice. A wide variety of treatment modalities were tested, including high dose tolerance induction, increased frequency of therapeutic doses and immunosuppressive drugs in combination with administration of enzyme. The most substantial effects were observed in mice injected intravenously with r-hαGAL in combination with methotrexate (MTX), which significantly lowered r-hαGAL-specific serum antibody levels. A short course of treatment with MTX was able to reduce antibody and spleen cell proliferative responses to long-term r-hαGAL treatment. MTX was able to suppress the development of r-hαGAL-specific IgG in antigen-primed mice. However, MTX was not effective in dampening robust ongoing antibody responses. These experiments provide a framework for the design of clinical protocols to prevent the drug-specific antibody responses of patients undergoing enzyme replacement therapy.
Keywords: Fabry disease, enzyme replacement therapy, methotrexate, lysosomal storage disease, antibody, mouse model
INTRODUCTION
Therapeutic proteins used as treatments for human diseases are often recognized as foreign by the immune system of the treated patient. The antibodies that develop may be able to neutralize the activity of the therapeutic protein or alter its biodistribution, thereby decreasing its efficacy, or may cause immune reactions resulting in adverse events in certain patients. Such responses have been observed most notably in Factor VIII therapy for haemophilia [1], but they have also been observed in clinical trials of enzyme replacement therapy (ERT) for lysosomal storage diseases (LSD) [2].
Clinical studies of ERT for LSDs have shown that patients can seroconvert after receiving several biweekly infusions of recombinant human glucocerebrosidase for the treatment of Gaucher disease [3–5] or of recombinant human α-galactosidase (r-hαGAL) for the treatment of Fabry disease [6]. Mild infusion reactions have been observed in some of these seropositive patients. These reactions can generally be managed by use of antihistamines and, in some cases, steroids. In some studies of chronic ERT in Gaucher patients, antibody levels decrease over time in many patients and become undetectable after several months of ERT, a process that has been termed natural tolerance [5]. However, because ERT is lifelong and is the treatment of choice for a large number of LSDs [7], it is important to investigate methods to modulate these antibody responses.
Fabry disease is an X-linked lysosomal storage disease resulting from α-galactosidase A deficiency and the accumulation of its substrate, globotriaosylceramide (GL-3) in lysosomes of vascular endothelial cells of the heart, kidney, liver, skin and brain [7]. Patients with Fabry disease exhibit episodic pain, fever, impaired sweating and rashes. Progressive accumulation of GL-3 leads eventually to the premature demise of these patients due to vascular disease of the heart, kidney or brain. Until recently treatment options were limited, and without treatment patients often require renal dialysis or kidney transplantation later in life. Current therapy now includes the approved use of ERT for Fabry disease [8].
As a model of this disease, we have chosen to study Fabry mice, which lack a functional α-galactosidase A gene through targeted disruption by homologous recombination [9]. With time these mice accumulate GL-3 in tissues including plasma, liver, heart and kidney, although they have a normal lifespan and exhibit no clinical symptoms of disease. Treatment of Fabry mice with r-hαGAL has been shown to result in dose-dependent clearance of GL-3 [10]. In our studies, several approaches were explored to identify methods to prevent or reduce antibody responses to r-hαGAL in Fabry mice with focus on use of the enzyme itself to tolerize the immune response or through the use of drugs already approved for use in humans. The approaches studied included high dose tolerance induction, increased frequency of therapeutic doses, nasal tolerance induction and use of the immunosuppressive drug methotrexate (MTX) to reduce specific antibody responses in mice.
MATERIALS AND METHODS
Mice
A colony of α-galactosidase A knock-out mice (Fabry mice) was generated from breeding pairs kindly provided by Dr Robert Desnick (Mount Sinai School of Medicine, NY, USA) and was maintained at Genzyme Corporation. These Fabry mice were derived by Desnick and colleagues [9] by targeted disruption of the α-galactosidase A gene by homologous recombination in ES cells using a neomycin resistance gene for positive selection. The genetic background of the resulting knock-out mice is a mixture of C57BL/6 and 129/Sv. Female BALB/c (H-2d) were purchased from Taconic Laboratories (Germantown, NY, USA). All mice were housed and maintained in accordance with the Guide for Care and Use of Laboratory Animals and under AAALAC-I accreditation and all animal protocols used in these studies were approved by the institutional IACUC review committee. Male and female mice between 6 and 16 weeks of age were used for all Fabry mouse experiments. Groups of animals were age- and sex-matched for each experiment.
Culture medium and reagents
The culture medium used was RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 15 m m HEPES and 10% fetal bovine serum (FBS), all from Gibco. Medium containing all supplements is referred to as complete medium. Recombinant human α-galactosidase A (r-hαGAL) and recombinant human acid α-glucosidase (r-hGAA) were produced in CHO cells at Genzyme and were used as formulated drug product [11]. Mice were treated with r-hαGAL (1 or 3 mg/kg) by bolus tail vein injections at designated times. Methotrexate (Sigma, St Louis, MO, USA) was dissolved in sterile, endotoxin-free Dulbecco's phosphate buffered saline (PBS) (Gibco) and was administered by tail vein injection 48 h following r-hαGAL treatments. In some experiments, r-hGAA (1 mg/kg) was administered by intraperitoneal (i.p.) injection as a control antigen.
Measurement of α-galactosidase A-specific IgG or acid α-glucosidase-specific IgG
Mice were bled 5–9 days following r-hαGAL or r-hGAA injections and specific IgG was measured by r-hαGAL or r-hGAA specific ELISA. Briefly, 96-well plates (Corning Inc., Corning, NY, USA) were coated overnight with 1 µg/ml of r-hαGAL in sodium phosphate buffer (pH 7·0) or r-hGAA in sodium acetate buffer (pH 5·0). Following washing and blocking, serial dilutions of sera were added and allowed to incubate at 37°C for 1·5 h. The plates were subsequently incubated at 37°C with HRP-conjugated goat antimouse IgG (Southern Biotechnology Associates, Birmingham, AL, USA) for 60 min. Following additional washes, o-phenylenediamine substrate was added and the plates were allowed to develop for 30 min at room temperature. The reaction was stopped and optical density was measured at 490 nm/650 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader (Molecular Devices, Sunnyvale, CA, USA). End-point titres were defined as the reciprocal of the last sample dilution resulting in an absorbance value of greater than 0·10 for r-hαGAL antibody responses and 0·20 for r-hGAA antibody responses (Softmax 2·35, Molecular Devices). Serum samples from individual mice were tested and the mean and standard error of end-point titres are reported for each experimental group (four to eight mice/group).
In vitro proliferation assays
Ten days after intravenous injection of Fabry mice with r-hαGAL (3 mg/kg), spleens were removed and spleen cell suspensions were prepared by disruption with sterile frosted glass microscope slides. Spleen cells from individual mice were resuspended in complete medium and pooled spleen cells from each group were seeded in quadruplicate at 1 × 105 cells/well in 0·2 ml into 96-well flat-bottomed plates in the presence of dilutions of test antigen (r-hαGAL). Cells were incubated for 5 days at 37°C with 5%CO2, with addition of 1 µCi of tritiated thymidine (NEN, Boston, MA, USA) to each well for the last 4 h of culture. Cells were harvested with a cell harvester (Tomtec, Hamden, CT, USA) onto filter mats and thymidine incorporation was measured using a BetaPlate counter (Wallac, Turku, Finland). Stimulation indices were calculated by dividing counts per minute (cpm) incorporated by cells in the presence of antigen by cpm incorporated by cells cultured in complete medium alone. Concanavalin A (Con A, Sigma) at 1 µg/ml was used as a positive control. Standard errors of quadruplicate cultures were generally less than 25% of the mean.
RESULTS
Studies were initiated in animal models to investigate methods to reduce antibody responses to r-hαGAL enzyme replacement, a recently approved therapy for Fabry disease [8]. Exploratory studies were first carried out in BALB/c mice, which possess a functional mouse α-galactosidase gene. It was determined in pilot experiments (data not shown) that BALB/c mice injected with r-hαGAL are capable of mounting an antibody response to the human protein when administered repeatedly at 1 or 3 mg/kg. This finding was expected, given that the human and murine enzymes are 22% different in protein sequence and that the mouse enzyme is 10 amino acids shorter at the C terminus than the human enzyme [12]. Thus, BALB/c mice were used to gather preliminary information on the dosing regimens or on the potential for immunosuppressive drugs to reduce serum antibody levels. Results from these studies were then used to design experiments in Fabry mice, which lack a functional α-galactosidase A gene and cannot produce functional enzyme.
Timing of r-hαGAL injection does not reduce long-term antibody responses to r-hαGAL in BALB/c mice
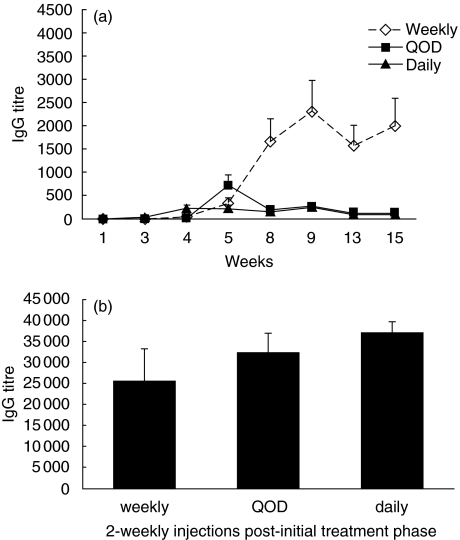
It has been reported that T cell tolerance can be induced by frequent injection of protein or peptide antigen [13,14]. To test the possibility that such a protocol might have a similar effect on antibody production through tolerization of helper T cells, BALB/c mice were injected i.v. with r-hαGAL (3 mg/kg) daily, every other day or weekly for a total of five injections. As shown in Fig. 1a, mice that received five weekly injections mounted a substantially higher IgG response than mice that received five daily injections or five injections given every other day. At week 15 all mice were challenged with two weekly injections of r-hαGAL to determine if the lower response seen in groups of mice injected daily or every other day was the result of tolerance induction or was simply the result of inefficient priming. As shown in Fig. 1b, all groups exhibited similar titres at week 17, indicating that frequent injection of r-hαGAL at this dosage did not result in tolerance. Thus, such a protocol is predicted to provide little benefit in the clinic. Other reports have suggested that systemic administration of high doses of protein antigen or of lower doses administered nasally is capable of inducing T cell tolerance [15–17]. Pilot experiments using these methods to induce tolerance to r-hαGAL in BALB/c mice were not effective in the reduction of specific antibody (data not shown). Therefore, these methods were not explored further.
Fig. 1.
Repeated injection of r-hαGAL does not tolerize the antibody response to r-hαGAL in BALB/c mice. (a) Groups of five female BALB/c mice received five i.v. injections of r-hαGAL (3 mg/kg) every day, every other day (QOD) or weekly for a total of 15 weeks. Serum was analysed for αGAL-specific IgG by ELISA at the timepoints indicated. Daily and QOD group means were significantly different (P < 0·01 by Student's t-test) from the weekly mean at 15 weeks. (b) Two weekly challenge doses of r-hαGAL (3 mg/kg) were administered i.v. to all mice beginning at week 16. At week 18 bleeds were taken and serum was analysed for αGAL-specific IgG by ELISA. All group means were not significantly different by Student's t-test.
Chronic or short-term administration of methotrexate reduces r-hαGAL antibody responses in BALB/c and Fabry mice
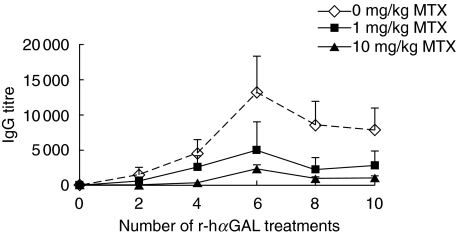
Several immunosuppressive drugs, including cyclophosphamide, rapamycin and methotrexate (MTX), were tested for the ability to prevent anti-r-hαGAL antibody production in BALB/c mice. Despite their known immunosuppressive effects, short courses of rapamycin or cyclophosphamide did not have a significant effect on the development of antibody responses in this model (data not shown). In contrast, MTX was shown to reproducibly prevent the development of r-hαGAL-specific antibody in BALB/c mice. Representative results (Fig. 2) demonstrate that MTX can significantly reduce anti-r-hαGAL levels when given throughout the course of r-hαGAL treatment. In the experiment shown, MTX was administered 48 h after each of nine injections of r-hαGAL (3 mg/kg biweekly). Biweekly administration was chosen because that regimen is used in treatment of human Fabry patients [6,8]. Antibody titres were reduced in the 10 mg/kg MTX-treated group by 95% (area under the curve) compared to the control group that received only r-hαGAL. Timing of MTX administration was explored in pilot studies. Results of these experiments (data not shown) indicated that MTX given 24 h before or 24 h after administration of r-hαGAL was not as effective as MTX given at 48 h.
Fig. 2.
Reduction of anti-r-hαGAL responses in BALB/c mice by chronic administration of methotrexate. Groups of five female BALB/c mice received 10 biweekly i.v. injections of r-hαGAL (3 mg/kg) with or without i.v. injections of MTX (0, 1·0 or 10 mg/kg) administered 48 h following the initial nine r-hαGAL treatments. Serum was collected and analysed for αGAL-specific IgG by ELISA at the time-points indicated. After 10 treatments, the mean of the 10 mg/kg MTX group was significantly different (P < 0·01 by Student's t-test) from the 0 mg/kg group.
Having demonstrated that repeated administration of MTX could reduce antibody levels, it was of interest to determine if this effect could be maintained after only a short course of MTX treatment. In these experiments, BALB/c mice were given MTX or PBS intravenously 48 h after each of the first 3 r-hαGAL injections (3 mg/kg biweekly), after which mice in all groups were treated with r-hαGAL without MTX for several weeks. Data shown in Fig. 3a demonstrate that a short course of MTX is capable of reducing the antibody response to continued treatment with r-hαGAL for at least 16 treatments (8 months treatment duration). Furthermore, a MTX dose of at least 10 mg/kg appears to be required for the prevention of antibody responses in BALB/c mice.
Fig. 3.
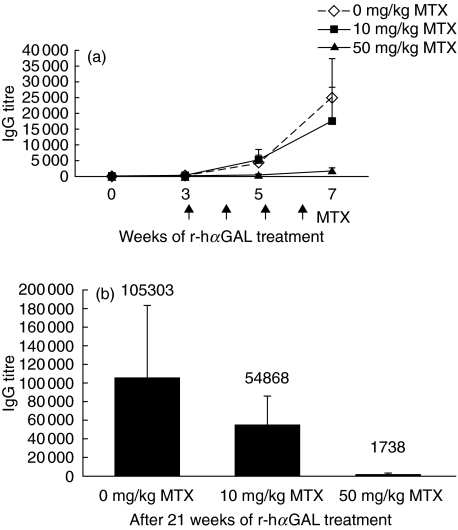
Long-term reduction of anti-r-hαGAL responses in BALB/c or Fabry mice after short-term treatment with methotrexate. (a) Groups of eight female BALB/c mice received 16 biweekly i.v. injections of r-hαGAL (3 mg/kg) with or without i.v. injections of MTX (0, 1·0 or 10 mg/kg) administered 48 h following the initial 3 r-hαGAL treatments. Serum was collected and analysed for αGAL-specific IgG by ELISA at the timepoints indicated. After 16 treatments, the mean of the 10 mg/kg MTX group was significantly different (P < 0·05 by Student's t-test) from the 0 mg/kg group. (b) Groups of eight Fabry mice received 12 biweekly i.v. injections of r-hαGAL (3 mg/kg) with or without i.v. injections of MTX (0, 10 or 50 mg/kg) administered 48 h following the initial three r-hαGAL treatments. Serum was collected and analysed for αGAL-specific and IgG by ELISA at the time-points indicated. After eight treatments, the means of both the 10 mg/kg and 50 mg/kg MTX groups were significantly different (P < 0·05 by Student's t-test) from the 0 mg/kg group.
Knockout mice generated by targeted disruption of the murine α-galactosidase A gene have proven to be a useful animal model of Fabry disease [10]. Because treatment of BALB/c mice with MTX was capable of reducing antibody levels (Fig. 3a), we next focused on studies of the dose and timing of MTX administration in the reduction of serum IgG to r-hαGAL in Fabry mice. As shown in Fig. 3b, anti-r-hαGAL IgG titres were significantly reduced in serum of Fabry mice that had been treated with 10 or 50 mg MTX/kg 48 h after each of the first 3 r-hαGAL injections. Because Fabry mice produce no αGAL and could potentially mount a more robust immune response to r-hαGAL than BALB/c mice, a higher dose (50 mg/kg) of MTX was included in this experiment. Titres reached a plateau and remained stable over the following 18 weeks of treatment in the absence of further MTX. There does not appear to be an advantage in using the higher MTX dose (50 mg/kg) in Fabry mice. Both the 10 mg/kg and the 50 mg MTX doses were similar in their ability to reduce antibody levels when given early in the course of r-hαGAL therapy (Fig. 3b).
Short-term MTX administration results in diminished in vitro r-hαGAL-specific spleen cell proliferative responses in Fabry mice
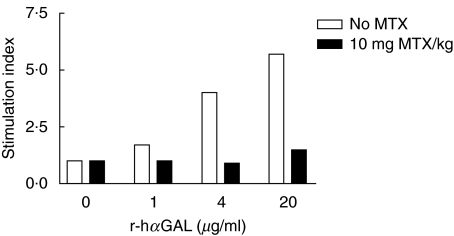
In order to determine if the reduction of antibody titres after a short course of MTX treatment is at least in part due to its effect on helper T cells, r-hαGAL-specific proliferation of spleen cells derived from r-hαGAL + MTX-treated mice was compared to control spleen cells from mice injected with r-hαGAL alone. In this experiment, Fabry mice were given three weekly injections of r-hαGAL with or without MTX 48 h after each r-hαGAL injection. A week later a fourth injection of r-hαGAL was given to all mice, and after another 10 days spleens were harvested for proliferation assays. In this particular experiment, administration of MTX resulted in a >90% reduction in antibody titre after the fourth injection of r-hαGAL in the MTX-treated mice relative to control mice that were given only r-hαGAL (data not shown). As shown in Fig. 4, proliferative responses to r-hαGAL were markedly lower in mice that had received MTX, providing support for the idea that MTX had induced the depletion or anergy of activated T cells. Spleen cells from both groups exhibited similar responses to Con A, which was used as a control for nonspecific suppression of proliferation (Fig. 4). These results are consistent with the idea that MTX induces apoptosis of T cells recently activated by r-hαGAL and that these T cells are incapable of responding to subsequent challenge with the antigen. However, it is also possible that MTX induces anergy of r-hαGAL-specific T cells. Experiments are in progress to determine more definitively the mechanism of action of MTX.
Fig. 4.
T cell tolerance to r-hαGAL in Fabry mice induced by short-term methotrexate treatment. Groups of four Fabry mice received four biweekly i.v. injections of r-hαGAL (3 mg/kg) with or without i.v. injections of MTX (10 mg/kg) administered 48 h following the initial three r-hαGAL treatments. Spleens were taken 10 days after the fourth r-hαGAL injection and pooled spleen cells from each group were cultured in quadruplicate for 5 days in vitro for assay of proliferative responses to r-hαGAL at the doses indicated. Results are presented as stimulation index (cpm incorporated by cells in the presence of antigen divided by cpm incorporated by cells cultured in medium only). Background proliferation (cpm in complete medium) was 414 cpm for the r-hαGAL group and 352 cpm for the r-hαGAL + MTX group. The Con A (1 µg/ml) response was 11 931 cpm for the r-hαGAL group and 14 253 for the r-hαGAL + MTX group. Standard errors of quadruplicate cultures were generally less than 25% of the mean.
Table 1 provides further evidence that this lower responsiveness is specific. In this experiment, Fabry mice with reduced r-hαGAL-specific antibody titres after treatment with MTX during the first 6 weeks were also injected with r-hGAA as a control antigen during the last 4 weeks of the experiment (in the absence of MTX). As shown in Table 1, MTX-treated mice were able to mount an immune response to GAA similar to control mice that had not received MTX.
Table 1.
Short-term methotrexate treatment does not result in generalized immunosuppression.
| Number of r-hαGAL treatments | r-hαGAL-specific IgG titres | |
| 0 mg/kg methotrexate | 50 mg/kg methotrexate | |
| 8 | 6201 ± 2125* | 626 ± 299 |
| Number of r-hGAA treatments | r-hGAA-specific IgG titres | |
| 0 mg/kg methotrexate | 50 mg/kg methotrexate | |
| 2 (post 16th r-hαGAL) | 8456 ± 5780 | 9412 ± 3204 |
Groups of five Fabry mice were given 8 biweekly i.v. injections (3 mg/kg r-h αGAL). For the first 6 weeks mice received MTX (i.v., 10 mg/kg) or vehicle 48 h after each r-h αGAL injection (three administrations of MTX or vehicle). For the last 4 weeks the same mice were also injected i.p. with an irrelevant enzyme (1 mg/kg r-hGAA). Serum samples were collected at weeks 17 and 37 and were analysed for GAA-specific IgG or αGAL-specific IgG by ELISA. Results shown represent mean and standard error of end-point titres.
P < 0·05 compared to MTX group.
Short-term MTX treatment reduces anti r-hαGAL antibody responses in Fabry mice previously primed with r-hαGAL but not in mice with robust pre-existing anti-r-hαGAL titres
To investigate the possibility that MTX might be capable of down-regulating ongoing antibody responses, Fabry mice were given three weekly injections of r-hαGAL to initiate an immune response. After the third r-hαGAL injection, MTX treatment was initiated using the standard 10 mg/kg dose or at a higher dose of 50 mg/kg, anticipating the possibility that a higher dose of MTX may be required to reduce antibody levels in antigen-primed mice than in naive mice. As shown in Fig. 5a, the 10 mg/kg MTX dose was relatively ineffective in reducing anti-r-hαGAL antibody levels in this experiment, but the higher dose of 50 mg/kg was capable of substantially reducing titres. This effect was maintained even after 16 weeks of treatment with r-hαGAL (eight injections) in the absence of MTX (Fig. 5b). Thus, MTX is capable of reducing antibody levels in mice primed with antigen. However, it appears that a higher dose of MTX is necessary to dampen r-hαGAL antibody responses in antigen-primed mice (50 mg/kg) compared to naive mice (10 mg/kg).
Fig. 5.
Long-term reduction of primed antibody responses in Fabry mice to r-hαGAL induced by acute methotrexate treatment. (a) Groups of five Fabry mice received two weekly injections of r-hαGAL (1 mg/kg) to induce αGAL specific IgG production. These primed mice were given four additional weekly r-hαGAL injections (1 mg/kg) with or without i.v. MTX (0, 10 or 50 mg/kg) 48 h following each r-hαGAL treatment. Serum was collected at the time-points indicated and analysed for αGAL-specific IgG by ELISA. After seven treatments, the mean of the 50 mg/kg MTX group was significantly different (P < 0·05 by Student's t-test) from the 0 mg/kg group. (b) At week 15, weekly r-hαGAL (1 mg/kg) injections were resumed without MTX treatment. At 21 weeks, serum was collected and analysed for αGAL-specific IgG by ELISA. After 21 treatments, the mean of the 50 mg/kg MTX group was significantly different (P < 0·05 by Student's t-test) from the 0 mg/kg group.
Additional experiments were performed to determine if MTX treatment is capable of halting or reducing antibody production in Fabry mice with measurable, significant titres. In these experiments, Fabry mice were injected weekly with r-hαGAL until plateau antibody titres were observed (about 6 weeks), at which time MTX treatment was initiated in combination with r-hαGAL. Results of several experiments (data not shown) demonstrated that even a high dose of MTX (50 mg/kg) was unable to halt a robust ongoing anti-r-hαGAL antibody response in Fabry mice. These results suggest that clinical use of MTX for reduction of antibody responses to therapeutic proteins may be limited to patients that are just beginning ERT, because once antibody titres have reached significant levels, MTX cannot prevent further antibody production. The data are consistent with the hypothesis that MTX acts on T cells in the early stages of their activation resulting in lower levels of T cell help. There appear to be no effects on antibody production by B cells or plasma cells once activated.
DISCUSSION
Fabry disease, an X-linked lysosomal storage disease caused by a deficiency in α-galactosidase A, can be treated with infusions of r-hαGAL to replace the defective enzyme [6]. A majority of treated patients (∼90%) exhibit dramatic clearance of GL-3, the substrate for r-hαGAL, from plasma and from the capillary endothelium of the kidneys, heart and skin. Most patients develop circulating anti-r-hαGAL IgG over the course of r-hαGAL treatment [6]. However, patients continue to respond to therapy despite the presence of circulating antibody, and infusion reactions are generally limited and controlled clinically by antihistamines or steroids. In similar clinical trials of ERT for another LSD (Gaucher disease), a substantial number of patients undergoing long-term treatment with Alglucerase exhibited decreased antibody titres over time, a result of apparent tolerization of the immune response after many months of therapy [5]. A similar trend has been observed in patients who have received Fabrazyme for Fabry disease for more than 1 year [6]. Thus, at the present time, seroconversion has not been viewed as a serious obstacle for ERT in Fabry disease. However, antibody responses to other protein therapies can be problematic [1] and methods to accelerate natural tolerance or prevent antibody formation altogether may be needed for other ERTs [18].
In this report we have investigated methods for the reduction of antibody responses to r-hαGAL in normal BALB/c mice and in Fabry mice, which lack a functional α-galactosidase gene. With increasing age, Fabry mice accumulate GL-3 in tissues including plasma, liver, heart and kidney. Treatment of these mice with r-hαGAL results in decreased accumulation of GL-3 in plasma and tissues [10]. As in human patients, repeated injection of Fabry mice with r-hαGAL results in IgG responses to the drug, making this an appropriate model for study of methods to dampen these responses. In these mice, relatively few adverse events were noted, consistent with the patient response.
Several experimental approaches for tolerance induction were considered, including blockade of adhesion molecules, interference with co-stimulation or antagonism of cytokines that are required for antibody production. For the present studies we chose to focus on drugs currently approved for use in humans or on the use the recombinant enzyme itself to tolerize the immune response by varying the dose and timing of enzyme administration. Several studies have shown that high doses of Factor VIII are capable of tolerizing inhibitory antibody responses in haemophilia patients [19]. Similarly, experiments in mice have shown that T cell responses can be down-regulated by frequent injection of protein or peptide antigens [13,15,16] or by nasal administration of protein antigen [17]. In our experiments frequent systemic administration and nasal administration of enzyme were not successful in reducing the antibody response to r-hαGAL in either BALB/c or Fabry mice. The reasons for these results are unclear, but may be due to the fact that most of the work in this field has focused on methods to tolerize T cell proliferative responses or cytokine production, not on methods to antagonize T cell-dependent antibody production by B cells or plasma cells.
Short-term and chronic administration of the immunosuppressive folate antagonist methotrexate (MTX) was studied in an effort to modulate antibody responses to r-hαGAL. It was reported as early as 1975 that acute treatment with a relatively high dose of MTX (30 mg/kg) can specifically reduce the antibody response to synthetic nucleic acid polymers in mice [20]. In our experiments, antibody responses by naive Fabry mice in response to chronic administration of r-hαGAL were reduced by 95% by a short course of MTX treatment at 10 mg/kg. Higher doses of MTX (50 mg/kg) were capable of reducing antibody responses in recently primed mice, but even at this high dose MTX was not capable of halting robust ongoing antibody responses. These results indicate that MTX would need to be administered early in the course of ERT, preferably at the onset of therapy. Further studies will need to be conducted in order to identify methods for down-regulating ongoing antibody responses in patients that have already seroconverted.
One proposed mechanism for the induction of tolerance by MTX is through the apoptosis of T cells that have been recently activated by r-hαGAL. Studies have demonstrated that MTX induces apoptosis of Con A-activated T cells [21,22]. Our data show that MTX down-regulates spleen cell proliferative responses to r-hαGAL, perhaps through a similar mechanism, and suggest that disruption of T cell help may be responsible for lower antibody production. In similar studies, preliminary ELISPOT data indicate that MTX does not directly affect enzyme-specific IgM production by B cells or plasma cells in a mouse model of Pompe disease, an LSD whose primary defect is the enzyme GAA (unpublished data). However, enzyme-specific IgG production was reduced in these experiments, consistent with down-regulation of helper T cell responses by MTX. Experiments to more fully characterize the mechanism (anergy or deletion of r-hαGAL-specific T cells) responsible for the diminished antibody response to r-hαGAL are under way and will be reported elsewhere.
Our experiments show that MTX can prevent specific antibody production to r-hαGAL when administered 48 h after each r-hαGAL injection. Antibody responses to another enzyme (r-hGAA) were unaffected by MTX when r-hGAA was administered many weeks after the last MTX injection. These results indicate that the decreased antibody responses to r-hαGAL induced by MTX are due to antigen-specific mechanisms, and that lower antibody responses are not simply due to general immunosuppression.
MTX is widely used clinically for the treatment of rheumatoid arthritis (RA) at doses similar to those used in our experiments in mice [23]. Recently, anti-tumour necrosis factor (TNF)-α monoclonal antibodies have been used in conjunction with MTX for the treatment of RA [24]. In several clinical trials it was shown that chronic administration of MTX results in the reduction of human antichimeric antibody (HACA) responses in RA patients [25,26]. Our experiments in mice suggest that MTX may similarly be useful for the reduction of antibody titres to therapeutic proteins (such as ERT) but that chronic administration of MTX may not be necessary. The data presented and from several other unpublished experiments have shown that 3–6 weeks of MTX treatment in Fabry mice undergoing chronic r-hαGAL therapy is capable of reducing and maintaining diminished enzyme-specific antibody levels for at least 10 months. Thus, long-lasting attenuation of the immune response to r-hαGAL can be induced by a relatively short course of MTX treatment. However, our data suggest that MTX treatment of patients undergoing ERT would need to be initiated at the onset of ERT, because MTX was not able to reduce robust, ongoing antibody responses. Although it appears that seroconversion is not a problem in the treatment of Fabry patients with r-hαGAL [2], recent experiences in the treatment of Pompe patients with r-hGAA suggest that prevention of antibody responses to the therapeutic enzyme may be desirable if not required in some patients to maximize efficacy and minimize adverse effects [27,28]. Thus, our experiments provide a framework for potential use of MTX in the clinic for the reduction of antibody responses to protein therapies such as ERT.
Acknowledgments
We thank Jen Tedstone, Lea Ritterova, Megan O’Brien and Shannon Macauley for excellent technical assistance and Dr John McPherson for critical review of the manuscript. This work was supported entirely by Genzyme Corporation.
References
- 1.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–35. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Richards SM. Immunologic considerations for enzyme replacement therapy in the treatment of lysosomal storage disorders. Clin Appl Immunol Rev. 2002;2:241–53. [Google Scholar]
- 3.Kingma W, Rosenberg M, Richards SM. Antibody formation in patients receiving Imiglucerase and impact on safety and clinical response. Gaucher Clin Perspect. 1998;6:8–11. [Google Scholar]
- 4.Richards SM, Olson TA, McPherson JM. Antibody response in patients with Gaucher disease after repeated infusion with macrophage-targeted glucocerebrosidase. Blood. 1993;82:1402–9. [PubMed] [Google Scholar]
- 5.Rosenberg M, Kingma W, Fitzpatrick MA, Richards SM. Immunosurveillance of alglucerase enzyme therapy for Guacher patients: induction of humoral tolerance in seroconverted patients after repeat administration. Blood. 1999;93:2081–8. [PubMed] [Google Scholar]
- 6.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 7.Desnick RJ, Ioannou YA, Eng CM. Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis for inherited disease. New York: McGraw-Hill; 2001. pp. 3733–74. [Google Scholar]
- 8.Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystem disorder: expert recommendations for diagnosis, management and enzyme replacement therapy. Ann Intern Med. 2003;138:338–46. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Ioannou Y, Zeidner K, et al. Generation of a mouse model with alpha-galactosidase A deficiency. Am J Human Genet. 1996;59:A208. [Google Scholar]
- 10.Ioannou Y, Zeidner K, Gordon RE, Desnick R. Fabry disease preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme deficient mice. Am J Human Genet. 2001;68:14–25. doi: 10.1086/316953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Lin XY, Zhang K, et al. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003;13:305–13. doi: 10.1093/glycob/cwg034. [DOI] [PubMed] [Google Scholar]
- 12.Gotlib RW, Bishop DF, Wang AM, et al. The entire genomic sequence and cDNA expression of mouse α-galactosidase A. Biochem Molec Med. 1996;57:139–48. doi: 10.1006/bmme.1996.0020. [DOI] [PubMed] [Google Scholar]
- 13.Schad VC, Garman RD, Greenstein JL. The potential use of T cell epitopes to alter the immune response. Semin Immunol. 1991;3:217–24. [PubMed] [Google Scholar]
- 14.Wauben MH. Immunological mechanisms involved in experimental peptide immunotherapy of T-cell-mediated diseases. Crit Rev Immunol. 2000;20:451–69. [PubMed] [Google Scholar]
- 15.Liblau RS, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:3131–6. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Switzer S, Wallner BP, Briner TJ, Sunshine GH, Bourque CR, Luqman M. Bolus injection of aqueous antigen leads to a high density of T-cell-receptor ligand in the spleen, transient T-cell activation and anergy induction. Immunology. 1998;94:513–22. doi: 10.1046/j.1365-2567.1998.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–600. [PubMed] [Google Scholar]
- 18.Brooks DA, Kavanos R, Hopwood JJ. Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trends Molec Med. 2003;9:450–3. doi: 10.1016/j.molmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Wight J, Paisley S, Knight C. Immune tolerance induction in patients with haemophilia A with inhibitors: a systematic review. Haemophilia. 2003;9:436–63. doi: 10.1046/j.1365-2516.2003.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Hyman LR, Kovacs K, Steinberg AD. Drug-induced tolerance: selective induction with immunosuppressive drugs and their synergistic interaction. Int Arch Allergy Appl Immunol. 1975;48:248–60. doi: 10.1159/000231311. [DOI] [PubMed] [Google Scholar]
- 21.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard J-P. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paillot R, Genestier L, Fournel S, Ferraro C, Miossec P, Revillard J-P. Activation-dependent lymphocyte apoptosis induced by methotrexate. Transplant Proc. 1998;30:2348–50. doi: 10.1016/s0041-1345(98)00648-4. [DOI] [PubMed] [Google Scholar]
- 23.Jobanputra P, Wilson J, Douglas K, Burls A. A survey of British rheumatologists’ DMARD preferences for rheumatoid arthritis. Rheumatology. 2003;42:1230–6. doi: 10.1093/rheumatology/keh003. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PC. Anti-TNF-alpha therapy for rheumatoid arthritis: an update. Int Med. 2003;42:15–20. [PubMed] [Google Scholar]
- 25.Antoni C, Kalden JR. Combination therapy of chimeric monoclonal anti-tumor necrosis factor alpha antibody (infliximab) with methotrexate in patients with rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:S73–7. [PubMed] [Google Scholar]
- 26.Wagner CL, Schantz A, Barnathan E, et al. Consequences of immunogenicity to the therapeutic monoclonal antibodies ReoPro and Remicade. Dev Biol. 2003;112:37–53. [PubMed] [Google Scholar]
- 27.Kishnani P, Voit T, Nicolino M, et al. Enzyme replacement therapy with recombinant human acid alpha glucosidase (rhGAA) in infantile Pompe disease (IPD): results from a phase 2 study. Pediatr Res. 2003;53:259A. [Google Scholar]
- 28.Hunley TE, Corzo D, Dudek M, et al. Nephrotic syndrome (NS) complicating recombinant human acid alpha-glucosidase (rhGAA) replacement therapy for Pompe disease. Pediatr Res. 2003;53:532A. doi: 10.1542/peds.2003-0988-L. [DOI] [PubMed] [Google Scholar]