Abstract
The expression of Mullerian inhibiting substance (MIS), a key molecule in sex differentiation and reproduction, is tightly regulated. It has been suggested that meiotic germ cells repress MIS expression in testicular Sertoli cells, although the substance responsible for this cell-cell communication remains unknown. Here, we present the cytokine tumor necrosis factor alpha (TNF-α) as a strong candidate for such a substance and its downstream molecular events. TNF-α inhibited MIS expression in testis organ cultures, and TNF-α−/− testes showed high and prolonged MIS expression. Furthermore, in transient-transfection assays TNF-α suppressed the MIS promoter that was activated by steroidogenic factor 1 (SF-1), one of the major transcription factors that regulate MIS expression. The modulation of SF-1 transactivation by TNF-α is through the activation of NF-κB, which subsequently interacts with SF-1 and represses its transactivation. The physical association of NF-κB with SF-1 was shown by yeast two-hybrid protein interaction, glutathione S-transferase pull-down, and coimmunoprecipitation (ChIP) analyses. ChIP assays also revealed that endogenous NF-κB, as well as SF-1, is recruited to the MIS promoter upon TNF-α signaling. SF-1-bound NF-κB subsequently recruits histone deacetylases to inhibit the SF-1-activated gene expression. These results may identify, for the first time, the responsible substance and its action mechanism underlying the repression of MIS expression by meiotic germ cells in the testis.
Mullerian inhibiting substance (MIS), also known as anti-Mullerian hormone (AMH), is essential in normal sex differentiation and reproductive function (for a review, see reference 48). Its expression is tightly regulated in tissue-specific and development-specific manners. MIS expression in the testis is restricted to Sertoli cells and is high in fetal-to-prepubertal mice, but it becomes low in pubertal-to-adult mice. Previous studies have suggested that MIS expression is regulated independently by meiotic germ cells (1, 38), androgens (39), and gonadotropins (24), although the factors that govern the complex expression of the MIS gene have not been fully understood. The MIS promoter contains a number of evolutionarily conserved elements, including those for steroidogenic factor 1 (SF-1), Sox9, and GATA-4, and these conserved elements and corresponding transcription factors have been shown to be required for the activation of MIS transcription (11, 43, 53).
SF-1, an orphan nuclear receptor, plays an important role in development and differentiation of the endocrine and reproductive systems (for reviews, see references 37 and 41). Molecular studies have revealed that many genes encoding steroidogenic enzymes and regulators of endocrine function are governed by SF-1 for their expression and contain SF-1 response elements within their proximal promoters. In testicular Sertoli cells, SF-1 is required for the expression of MIS (12, 43). Diverse coregulators have been reported to modulate the effect of SF-1 on gene transcription. WT-1 and GATA-4 activate SF-1-mediated MIS expression (34, 51), whereas DAX-1 represses MIS expression (20). Other proteins, such as steroid receptor coactivator 1 (SRC-1) (7), c-Jun (26), and DP103 (35), have also been shown to modulate the transactivation activity of SF-1, although the significance of these interactions remains elusive in terms of reproductive development and function.
NF-κB is a pivotal transcription factor governing the expression of early response genes involved in numerous cellular responses to a wide range of signals. The major form of NF-κB is a heterodimer of the p65 and p50 subunits. In the inactivated state, NF-κB is sequestered in the cytoplasm through its association with the inhibitor protein IκB. Activation of the NF-κB signaling cascade results in phosphorylation and subsequent degradation of IκB, allowing the translocation of NF-κB to the nucleus, where it induces transcription by binding to specific response elements (4). Previous studies have shown that NF-κB cross-talks with other proteins. The NF-κB transactivation function is modulated by proteins such as C/EBP, SRC-1, glucocorticoid receptor (GR), and RXR, resulting in the regulation of promoters with κB enhancer motifs (31-33, 45). Furthermore, NF-κB itself is able to repress the activity of steroid receptors, modulating a number of gene responses to hormonal stimuli (17, 31, 36).
There have been reports of an inverse relationship between germ cell meiosis and MIS expression (1, 38). Since transgenic mice overexpressing MIS have normal spermatogenesis (3), it has been suggested that meiotic germ cells repress MIS expression in Sertoli cells possibly by producing an inhibiting substance. The cytokine tumor necrosis factor alpha (TNF-α is produced in meiotic germ cells, spermatocytes, and spermatids (9, 44), whereas TNF-α receptor is detected in Sertoli cells (30). Furthermore, NF-κB, a downstream mediator of the TNF-α signaling cascade, is expressed at a high level in Sertoli cells and has been implicated in the regulation of mammalian spermatogenesis (10).
These previous studies allowed us to hypothesize that TNF-α may be the inhibiting substance that is secreted from meiotic germ cells and represses MIS expression in Sertoli cells, possibly through NF-κB activation. To test this hypothesis, we first examined whether MIS expression is repressed by TNF-α in organ-cultured testes and cultured mammalian cells, including primary Sertoli cells. Then, we investigated the involvement of NF-κB in the TNF-α repression of MIS expression and the possible downstream events. Our results suggest that TNF-α may act as a paracrine factor for the communication between meiotic germ cells and Sertoli cells to downregulate MIS expression in postnatal testis through the activation of NF-κB and its subsequent interaction with SF-1 on the MIS promoter. To our knowledge, this is the first molecular mechanism that has been suggested to explain the cellular regulation of MIS expression in the testis.
MATERIALS AND METHODS
Animals.
TNF-α knockout (B6;129S6-Tnftm1Gkl, stock no. 003008) and wild-type control (B6;129SF2, stock no. 101045) mice were purchased from The Jackon Laboratory (Bar Harbor, Maine). ICR mice and Sprague-Dawley rats were purchased from a commercial supplier (Daehan Laboratories, Daejeon, Korea). Thirty-two-day-old male TNF-α knockout mice were injected intraperitoneally with recombinant mouse TNF-α (Pierce Biotechnology, Inc.) at 50 μg/kg (body weight) for 6 h. Animals were kept and bred in a cage with water and chow available and were maintained under controlled conditions (12-h light and dark photoperiod, 50% humidity, 22°C). The ethical treatment of animals in the present study was carried out according to National Institutes of Health standards.
Plasmids.
Full-length SF-1 and its deletion mutants were subcloned in frame into pcDNA3flag and LexA202 vector to construct plasmids for in vitro translation and for LexA fusion proteins, respectively. Full-length SF-1 was obtained by EcoRI digestion of pCEP4-SF-1 (a gift from D. D. Moore, Baylor College of Medicine). SF-1DBD+PL was obtained by EcoRI-SacI digestion of the pcDNA3flag-SF-1 and SF-1LBD+AF2 region by SacI digestion of pcDNA3flag-SF-1. The proline-rich domain of SF-1 was amplified by PCR with forward (5′-GGAATTCAAGCTGGAGACCGGACCA-3′) and reverse (5′-CCCGCTCGAGCTCTGGTACATTGGGCCC-3′) primers. pcDNA3flag-SF-1DBD construct was generated by self-ligation of pcDNA3flag-SF-1 after digestion with ApaI. B42-p65 and B42-p50 constructs were as described previously (32).
MIS-Luc and Sox9 expression construct (pSGSox9) are kind gifts from P. K. Donahoe (Harvard Medical School, Boston, Mass.) and by P. Koopman (University of Queensland), respectively. Mammalian expression constructs of SF-1 mutant (S203A and AF2M4) were kindly provided by H. A. Ingraham (University of California at San Francisco). SFRE-Luc and GAL4-SF-1 constructs were kindly given by V. Laudet (Centre Nationale de la Recherche Scientifique UMR 49, Lyon, France) and D. D. Moore, respectively. Mammalian expression constructs of p65, p50, IκB, HDAC1, HDAC4, and HDAC5 were described previously (13, 32, 33). HDAC4 and HDAC5 were subcloned into NotI-XhoI site of pBluescript II KS under the T7 promoter to do in vitro translation. Glutathione S-transferase (GST)-p65 and GST-p50 constructs were as described previously (32, 33). Mouse MIS cDNA (pBAM5-mAMH) is a kind gift from R. Lovell-Badge (National Institute for Medical Research, London, United Kingdom). Super-IκBα retrovirus vector pLIκBαMSN was obtained from I. Verma (Salk Institute).
Organ culture.
Testes were collected from 7-day-old male ICR mice and put on a 0.45-μm-pore-size filter membrane in Dulbecco modified Eagle medium plus 0.5% fetal bovine serum supplemented or not supplemented with 20 ng of TNF-α/ml. Cultured testes at 32°C were collected at indicated time points and processed for Northern blot analysis.
Immunohistochemistry.
Testes were dissected from male mice and fixed in 10% formalin. The tissues were embedded in paraffin blocks and cut into 3-μm sections. The sections were deparaffinized in xylene and rehydrated according to standard procedures. After being blocked with rabbit serum, the sections were incubated with goat anti-TNF-α antibody (1:150; Santa Cruz Biotechnology) for 60 min at room temperature, followed by the addition of biotinylated rabbit anti-goat immunoglobulin G (Zymed LAB-SA kit) for 30 min. The sections were then sequentially incubated with streptavidin peroxidase for 30 min and 3,3-diaminobenzidine chromogen for 5 min, counterstained with Meyer's hematoxylin, and mounted.
Transient-transfection assays.
HeLa, TM4, RAW264.7/super-IκBα, and CV-1 cells were maintained in Dulbecco modified Eagle medium (Life Technologies, Inc.) supplemented with 10% fetal bovine serum. Cells were plated in 24-well plates, and transfected with the indicated amount of expression plasmids, a reporter plasmid, and control lacZ expression plasmid pCMVβ (Clontech) by using Effectene reagent (Qiagen) according to the manufacturer's instructions. Total amounts of expression vectors were kept constant by adding appropriate amounts of pcDNA3. TNF-α and anti-TNF-α antibody (Endogen) were treated for 24 h after transfection. Trichostatin A (TSA) was added 20 h before the cells were harvested. The luciferase and β-galactosidase activities were assayed as described previously (32). The levels of luciferase activity were normalized to the lacZ expression.
Primary Sertoli cell culture.
Primary Sertoli cells were isolated from 20-day-old rat testes as previously described (27). Transfection of the primary cells was carried out by using Effectene reagent (Qiagen) as a described above for transient-transfection assays.
Northern blot analysis.
Total RNAs were prepared from testes by using Tri-Reagent (Molecular Research Center, Inc.). A total of 10 to 30 μg of total RNA was separated on a 1.2% denaturing agarose gel, transferred onto Zeta-Probe nylon membrane (Bio-Rad), and immobilized by UV cross-linking. The membrane was hybridized with a random-primed 32P-labeled mouse MIS or p450c17 cDNA probe as described previously (25). The membrane was reprobed for GAPDH as a loading control.
Yeast two-hybrid assay.
Plasmids encoding LexA fusions and B42 fusions were cotransformed into Saccharomyces cerevisiae EGY48 containing the lacZ reporter plasmid. The transformants were grown in the inducing medium and processed for liquid β-galactosidase assays as described previously (25).
GST pull-down assay.
GST, GST-p65, GST-p50, and GST-SF-1 fusion proteins were expressed in Escherichia coli BL21 cells and isolated with glutathione-Sepharose 4B beads (Pharmacia, Biotech AB, Sweden). Immobilized GST fusion proteins were then incubated with [35S]methionine-labeled proteins produced by in vitro translation by using the TNT-coupled transcription-translation system (Promega). The binding reactions were carried out in 250 μl of GST binding buffer (20 mM Tris-HCl [pH 7.9], 100 mM NaCl, 10% glycerol, 0.05% NP-40, 5 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol, 1.5% bovine serum albumin) for 4 h at 4°C. The beads were washed three times with 1 ml of GST binding buffer. Bound proteins were eluted by adding 20 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and then analyzed by SDS-PAGE and autoradiography.
Coimmunoprecipitation.
In vivo coimmunoprecipitation assays were performed with HeLa cells transfected with 500 μg of flag-SF-1, flag-HDAC4, or flag-HDAC5 expression plasmid with 250 μg of each of the p65 and p50 subunits of NF-κB. The cells were harvested with IPH cell lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 0.2 M sodium PPi, 0.1 M Na3VO4) 48 h after transfection. Whole-cell lysate (400 μg) was incubated with 8 μg of anti-flag antibody (Sigma) for 4 h at 4°C and further incubated for another 4 h after adding 20 μl of protein A-Sepharose CL-4B bead slurry (Amersham Pharmacia). Sepharose beads were washed three times with IPH buffer at 4°C. Bound proteins were separated by SDS-PAGE, transferred to nitrocellulose membrane (Sigma), subjected to Western blot analysis with anti-flag, anti-p65, and anti-p50 antibody (Santa Cruz Biotechnology), and then detected with an enhanced chemiluminescence kit (Amersham Pharmacia).
ChIP assay.
Seven-day-old testes were organ cultured at 32°C as described above and treated with 10 ng of TNF-α/ml for 6 h. Cultured testes were then cross-linked in 2% formaldehyde-0.2% glutaraldehyde for 40 min at room temperature. HeLa cells transfected with expression plasmids and the linearized reporter MIS-Luc were treated with 10 ng of TNF-α/ml for 6 h and cross-linked with 1% formaldehyde. After incubation with TSE I (100 mM Tris-HCl [pH 9.4], 10 mM dithiothreitol) for 20 min at 30°C, testes or cells were washed and processed for chromatin immunoprecipitation (ChIP) assay as previously described (42). Anti-SF-1 (a kind gift from K. Morohashi, National Institute for Basic Biology, Okazaki, Japan), anti-p65 (Santa Cruz Biotechnology), anti-hemagglutinin (anti-HA; Santa Cruz Biotechnology), or anti-Flag antibody (Santa Cruz Biotechnology) was used for immunoprecipitations. Immunoprecipitated DNA and input-sheared DNA were subjected to PCR with an MIS primer pair (sense, 5′-GTGTTTGGTAGTGGGGAGGG-3′; antisense, 5′-GGTGGTACAGCAAGGTCCGG-3′), which amplifies a 309-bp region spanning the endogenous MIS promoter (−293 to +16). As a negative control, PCRs were performed with either an actin primer pair (sense, 5′-GAGACCTTCAACACCCCAGCC-3′; antisense, 5′-CCGTCAGGCAGCTCATAGCTC-3′), which amplifies a 362-bp region spanning exon 4 of the β-actin gene, or a Luc primer pair (sense, 5′-GAAGGTTGTGGATCTGGATAC-3′; antisense, 5′-TTTCCGTCATCGTCTTTCCG-3′), which amplifies an ∼370-bp region spanning the C-terminal part of luciferase coding region of the reporter.
EMSA.
The preparation of nuclear extracts from HeLa cells and electrophoretic mobility shift assay (EMSA) were performed as described previously (16). As probes, complementary oligonucleotides containing the Igκ-chain binding site (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) or the SF-1 binding site from MIS promoter (SFRE, 5′-GGCCGGCACTGTCCCCCAAGGTCGC-3′) were labeled by a fill-in reaction in the presence of [α-32P]dCTP. For competition or supershift experiments, a 50-fold excess of unlabeled competitor oligonucleotide or antibody solution was incubated for 1 h at 4°C prior to the addition of the probe.
RESULTS
MIS expression is downregulated by TNF-α.
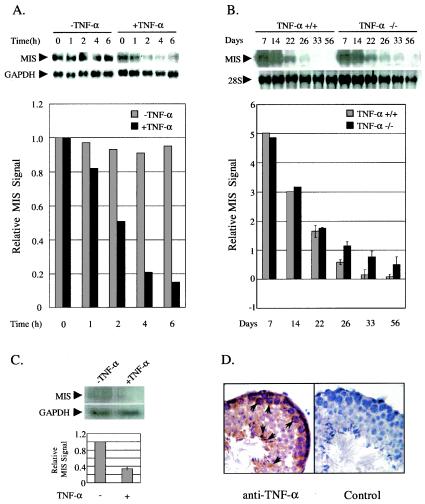
To test our hypothesis that TNF-α is the inhibiting substance for the meiotic repression of MIS expression in Sertoli cells, we performed organ culture of 7-day-old testes in the presence of TNF-α and examined the effect of TNF-α on MIS expression (Fig. 1A). MIS expression began to be reduced promptly and decreased approximately sixfold at 6 h after TNF-α treatment compared to the control testis without TNF-α treatment (Fig. 1A, top and bottom panels).
FIG. 1.
Downregulation of MIS expression by TNF-α in the testis. (A) MIS gene regulation by TNF-α in the organ-cultured testis. Testes from 7-day-old male mice were organ cultured in the presence or absence of 20 ng of TNF-α/ml and collected at the indicated time points for the preparation of total RNA. Testes from two to three mice were used for each time point. At the top of the panel, a Northern blot analysis of total RNAs from organ-cultured testes with 32P-labeled mouse MIS cDNA as a probe is shown. The expression of GAPDH was used as an internal control. At the bottom of the panel, MIS mRNA signal quantified by using a phosphorimager and normalized by determining the GAPDH mRNA level in each sample is shown. Data are representative of three similar experiments. (B) MIS expression during the development of TNF-α knockout testis. Total RNAs from TNF-α wild-type and mutant testes were prepared at different developmental days. Testes from two to three mice were combined to prepare total RNAs for day 7 to day 26 samples. A Northern blot analysis of the total RNAs from testes with 32P-labeled mouse MIS cDNA as a probe is shown in the top panel. 28S rRNA was used as an internal control. In the bottom panel, the MIS mRNA signal was quantified and normalized by using the 28S rRNA level in each sample. Two independent experiments were performed, and error bars represent the standard error of the mean (SEM). (C) Downregulation of MIS expression in TNF-α knockout testis by TNF-α treatment. Northern blot analysis was performed with total RNAs from testes of three 32-day-old TNF-α knockout mice, which were injected with or without 50 μg of TNF-α/kg. (D) Expression of TNF-α in meiotic germ cells. The immunohistology of mouse adult testis was evaluated with anti-TNF-α antibody. The negative control was processed with exclusion of the primary antibody. Strong signals in pachytene spermatocytes and elongated spermatids are indicated by arrows.
Since TNF-α suppressed MIS expression in the organ-cultured testis, we assumed that MIS expression might be altered in the testis of TNF-α knockout mice (28). In order to confirm this assumption, we measured MIS expression during testis development in TNF-α knockout mice and compared it to that of wild-type mice (Fig. 1B). In the postnatal testis of wild-type mice, MIS expression was gradually reduced and became hardly detectable at day 33. In the TNF-α knockout testis, however, MIS expression was reduced more slowly than in the wild-type testis and MIS transcripts were still detectable even at day 33 (Fig. 1B, top and bottom panels). The alteration of MIS expression was quite distinct starting around day 26, which is probably when the expression and secretion of TNF-α from germ cells in wild-type testis was well established.
The higher and prolonged MIS expression in TNF-α knockout mice was further confirmed by the reduced testicular testosterone level (approximately 2-fold lower than the wild-type at day 30) and lower expression level of p450c17 mRNA in postpubertal testes (data not shown). MIS has been reported to decrease testosterone production in vivo and in vitro by downregulating at least the steroidogenic enzyme p450c17 (49, 50).
To test whether TNF-α treatment would correct the misregulation of MIS expression in the TNF-α knockout testis, we provided TNF-α to TNF-α knockout mice at day 32 after birth. MIS expression was downregulated approximately threefold in the TNF-α-injected testis compared to the uninjected testis, confirming further the MIS gene regulation by TNF-α in vivo (Fig. 1C). In addition, immunohistology showed that TNF-α protein was expressed in meiotic germ cells, spermatocytes, in mouse testis (Fig. 1D), as previously proposed in mice (9) and recently reported in rats (44). Altogether, the results suggest that TNF-α downregulates MIS expression in the testis.
TNF-α represses MIS promoter through the modulation of SF-1 transactivation.
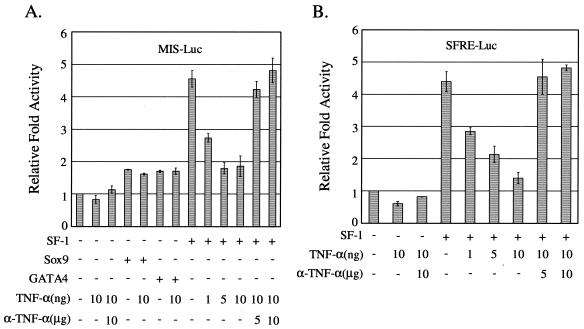
To investigate the molecular mechanism by which TNF-α inhibits MIS expression, we first examined whether the activity of the proximal MIS promoter was affected by TNF-α by using a reporter that contains the MIS promoter spanning −269 to +2, followed by the luciferase gene (Fig. 2A). Previous studies have established that the 180-bp proximal region of the MIS promoter containing binding sites for Sox9, GATA4, and SF-1 is sufficient to direct the initiation and maintenance of cell-specific expression of MIS gene (12). As shown in Fig. 2A, TNF-α treatment of HeLa cells transfected with a reporter MIS-Luc alone caused very weak, if any, reduction in the basal activity of MIS promoter, which was rescued by cotreatment of anti-TNF-α antibody. Since the meiotic repression of MIS expression in Sertoli cells in vivo occurs in the state of active MIS expression, we then examined the effect of TNF-α on the activated MIS promoter by Sox9, GATA4, and SF-1. Expression of Sox9 and GATA4 moderately activated the MIS promoter, and treatment with TNF-α did not affect the promoter activation. However, SF-1 expression activated the MIS promoter by approximately 4.5-fold and TNF-α treatment suppressed the SF-1-activated MIS promoter in a dose-dependent manner. TNF-α-suppression of SF-1 activity was blocked by cotreatment with anti-TNF-α antibody, indicating the specific effect of TNF-α.
FIG. 2.
Repression of MIS promoter by TNF-α through the negative regulation of SF-1 transactivation. HeLa cells were transiently transfected with a reporter alone or along with 10 ng of SF-1, GATA4, or SF-1 expression plasmid. After 24 h of transfection, they were treated with TNF-α alone (in nanograms/milliliter) or together with anti-TNF-α antibody (in micrograms/milliliter) for 24 h. (A) Cells were cotransfected with MIS-Luc reporter. (B) Cells were cotransfected with SFRE-Luc reporter. All values represent the mean ± the SEM of at least three separate transfection experiments.
To further confirm that TNF-α repression of the MIS promoter is accomplished through the modulation of SF-1 transactivation activity, we tested the effect of TNF-α signaling on SF-1 transactivation activity by using an SF-1 reporter plasmid SFRE-Luc that contains SF-1 response elements with a minimal promoter (52). SF-1 activated the reporter expression by approximately 4.5-fold, and TNF-α treatment suppressed the SF-1 transactivation activity in a dose-dependent manner, which was blocked by anti-TNF-α antibody (Fig. 2B). These results suggest that the TNF-α signaling cascade represses MIS expression through the inhibition of SF-1 transactivation.
Repression of SF-1 transactivation by NF-κB.
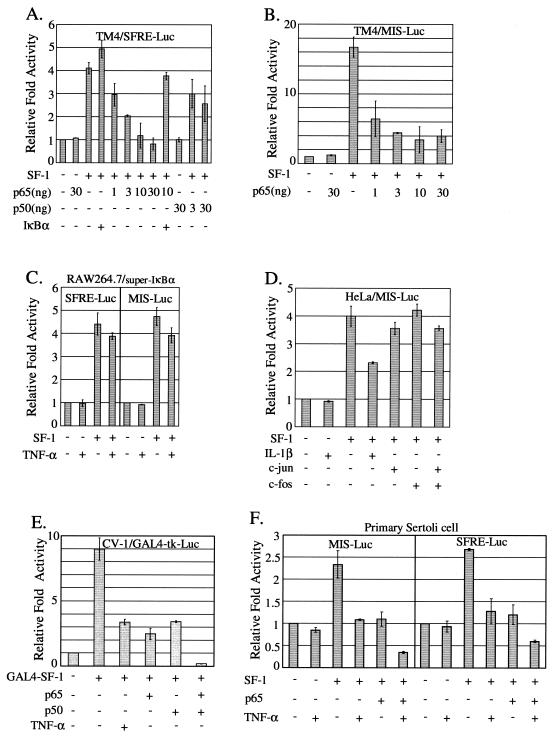
Since NF-κB, a well-known downstream mediator of TNF-α signaling cascade, is highly expressed in Sertoli cells (10), we then examined whether NF-κB is involved in the TNF-α repression of SF-1 transactivation for MIS gene regulation. TM4 Sertoli cells were cotransfected with SF-1 expression vector and an increasing amount of NF-κB subunit (p65 or p50) expression plasmid, along with a reporter SFRE-Luc (Fig. 3A). Coexpression of p65 inhibited SF-1 transactivation in a dose-dependent manner. This p65 inhibition of SF-1 transactivation was efficiently recovered by coexpression of IκB, the inhibitor of NF-κB, indicating that NF-κB influenced the inhibition of SF-1 transactivation. Coexpression of p50 also inhibited SF-1 transactivation in a dose-dependent manner, albeit to a lesser extent than p65. We also performed a parallel experiment with the proximal MIS promoter. Consistent with the above data (Fig. 3A), p65 inhibited the expression of MIS-Luc in a dose-dependent manner to an extent comparable to that of SFRE-Luc in TM4 cells (Fig. 3B). Similar results were observed with both SFRE-Luc and MIS-Luc reporters in HeLa cells (data not shown). Interestingly, p65 overexpression alone never completely blocked SF-1 transactivation on the MIS promoter in TM4 Sertoli cells, whereas 30 ng of p65 expression plasmid completely blocked the SF-1 transactivation to the basal level in HeLa cells (data not shown).
FIG. 3.
Inhibition of SF-1 transactivation by NF-κB. (A and B) The p65 and p50 subunits of NF-κB inhibited the transactivation of SF-1 with SFRE-Luc and MIS-Luc reporter. (A) TM4 cells were transiently cotransfected with 10 ng of SF-1 expression vector and increasing amounts of either p65 or p50 expression plasmid, along with SFRE-Luc. (B) TM4 cells were cotransfected with the indicated expression plasmids along with MIS-Luc. (C and D) NF-κB activation is involved in the TNF-α inhibition of SF-1 transactivation. (C) RAW264.7 cells expressing super-IκBα (stably transfected with pLIκBαMSN) were transfected with SF-1 expression vector, together with the indicated reporter, and treated or not treated with TNF-α. (D) HeLa cells were transfected with the indicated expression vectors (10 ng of SF-1 and 50 ng of c-jun and/or c-fos) and MIS-Luc and either treated or not treated with 10 ng of IL-1β/ml. (E) The p65 and p50 subunits are directly engaged in the repression of SF-1 transactivation. CV-1 cells were cotransfected with the indicated expression vectors (10 ng of GAL4-SF-1, 30 ng of p65 and/or p50), along with GAL4-tk-Luc reporter. (F) In primary Sertoli cells, TNF-α and the p65 subunit of NF-κB inhibited SF-1 transactivation, resulting in the repression of MIS promoter. Primary Sertoli cells isolated from 20-day-old rat testes were transiently transfected with the indicated expression vectors (20 ng of SF-1, 50 ng of p65), together with either MIS-Luc or SFRE-Luc. All values represent the mean ± the SEM of at least three separate experiments.
The involvement of NF-κB in TNF-α-mediated MIS gene repression was further confirmed by using super-IκBα-expressing cells and IL-β. In super-IκBα-expressing RAW264.7 cells that lack TNF-α-induced NF-κB activation, TNF-α signaling did not significantly repress SF-1-induced expression of both SFRE-Luc and MIS-Luc reporters (Fig. 3C). In addition, IL-β, another NF-κB-activating agent, was also able to inhibit the expression of MIS-Luc (Fig. 3D). On the other hand, overexpression of c-jun and/or c-fos did not significantly alter SF-1 transactivation with MIS-Luc, indicating no involvement of TNF-α-induced AP-1 activation in the TNF-α inhibition of SF-1 transactivation (Fig. 3D). Together, the results suggest that NF-κB is the essential factor mediating the TNF-α repression of MIS gene expression.
To further confirm the direct involvement of NF-κB in the repression of SF-1 transactivation, we simply tethered SF-1 to a promoter by using GAL4-SF-1 fusion protein and GAL4-tk-Luc reporter and investigated the effect of NF-κB coexpression on GAL4-SF-1 transactivation activity (Fig. 3E). As expected, TNF-α treatment or overexpression of either p65 or p50 repressed the SF-1 transactivation by three- to fourfold. Furthermore, coexpression of both p65 and p50 synergistically inhibited the SF-1 transactivation by approximately 40-fold.
Using primary Sertoli cells isolated from 20-day-old rat testes, we repeated the experiments with the proximal MIS promoter. Consistent with the above data, TNF-α treatment or p65 coexpression repressed the MIS promoter activated by SF-1. Furthermore, TNF-α treatment and p65 coexpression synergistically inhibited the MIS promoter (Fig. 3F). A parallel experiment with SFRE-Luc reporter gave results comparable to those with MIS-Luc (Fig. 3F). Taken together, these results strongly suggest that NF-κB, a mediator of TNF-α signaling cascade, is involved in the repression of MIS promoter through the suppression of SF-1 transactivation in Sertoli cells.
Interaction of SF-1 with NF-κB in vitro and in vivo.
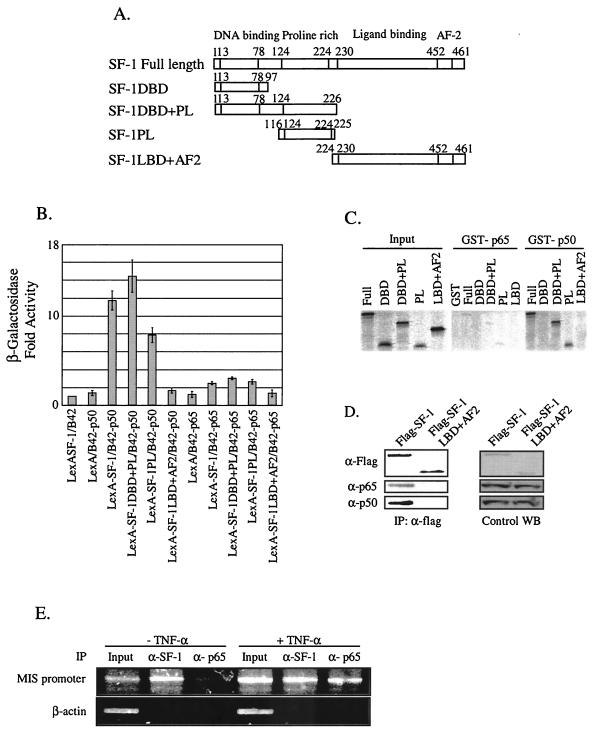
To verify that the functional interaction between NF-κB and SF-1 involved their physical association, we performed yeast two-hybrid analysis by using SF-1 fused to the LexA DNA-binding domain and either p65 or p50 fused to the B42 activation domain. As shown in Fig. 4B, LexA-SF1 fusion protein itself showed a basal level of β-galactosidase activity and so did either B42-p50 or B42-p65 alone. However, the presence of B42-p50 with LexA-SF-1 strongly induced β-galactosidase activity, whereas the presence of B42-p65 with LexA-SF-1 induced very weak activation of the reporter. The region of SF-1 protein responsible for its association with NF-κB was then determined by using LexA fusion proteins of serial SF-1 deletion mutants (Fig. 4A). The SF-1DBD+PL and SF-1PL interacted with p50 as strongly as did the full-length SF-1, but very weakly with p65 (Fig. 4B). LexA-SF-1LBD+AF2 fusion did not exhibit any significant interaction with either p50 or p65. The results indicate that SF-1 interacts with NF-κB mainly through the p50 subunit, and the SF-1 region, which is circumscribed by amino acids 116 to 225 and contains the proline-rich domain, is the major determinant for the interaction of SF-1 with NF-κB.
FIG. 4.
Association of NF-κB with SF-1 in vitro and in vivo. (A) Schematic diagrams of SF-1 full-length and deletion constructs. (B) The p50 subunit of NF-κB efficiently interacted with SF-1 via its proline-rich domain in yeast two-hybrid protein-binding assays. The interaction between SF-1 and either p65 or p50 was scored by the activation of β-galactosidase reporter. All values represent the mean ± the SEM of at least three independent colonies. (C) The p50 subunit directly interacted with SF-1 via its proline-rich domain in GST pull-down assays. [35S]methionine- labeled SF-1 and its deletion mutants were allowed to bind the GST fusion proteins of p65 and p50 subunits. Reactions were carried out with the equivalent amount of each protein, as determined by Coomassie blue staining (data not shown). Five percent of the labeled protein used in the binding reaction was loaded as input. (D) NF-κB was coimmunoprecipitated with SF-1. Flag-tagged full-length SF-1 expression plasmid or Flag-tagged SF-1LBD+AF2 was cotransfected with p65 and p50 expression plasmid into HeLa cells. Coimmunoprecipitations were conducted with anti-Flag antibody. Western blot analyses of immunoprecipitated materials were performed with anti-Flag, anti-p65, and anti-p50 antibody. Control Western blots (WB) are shown for the expression level of each protein. (E) NF-κB was recruited to the endogenous MIS promoter in the testis. ChIP assays were performed with organ-cultured 7-day-old testes treated or not treated with 10 ng of TNF-α/ml. Cross-linked DNA fragments were immunoprecipitated with anti-SF-1 or anti-p65 antibody, and the immunoprecipitates were analyzed by PCR with pairs of specific primers spanning the proximal promoter region of MIS. A control PCR for nonspecific immunoprecipitation was performed with primers specific to the β-actin coding region.
Direct physical interaction between SF-1 and NF-κB, and the region of SF-1 responsible for their interaction were also assessed by GST pull-down experiments. [35S]methionine-labeled SF-1 and its deletion mutants produced by in vitro translation were allowed to bind the GST fusion protein of either the p65 or p50 subunit of NF-κB (Fig. 4C). Although the p65 subunit did not interact with either SF-1 full-length or deletion mutants, the p50 subunit interacted with full-length SF-1, SF-1DBD+PL, and SF-1PL. SF-1DBD and SF-1LBD+AF2 did not interact with p50. These results are consistent with those from yeast two-hybrid analysis and suggest that SF-1 directly interacts with NF-κB through the proline-rich domain of the p50 subunit.
To determine the in vivo interaction of SF-1 with NF-κB, coimmunoprecipitation experiments were performed by using HeLa cells transfected with either Flag-tagged SF-1 or Flag-tagged SF-1LBD+AF2, along with expression plasmids of the p65 and p50 subunits of NF-κB. Whole-cell extracts were prepared and immunoprecipitation was carried out with anti-Flag antibody. Western blot analysis of the immunoprecipitated material revealed that SF-1 is associated with NF-κB in vivo (Fig. 4D). There was no detectable p65 or p50 in the Flag-immunoprecipitated material from the negative control cells transfected with SF-1LBD+AF2.
In order to determine whether endogenous SF-1 and NF-κB are recruited by the MIS promoter in vivo, we performed ChIP assays with organ-cultured 7-day-old testes that were treated with or without 10 ng of TNF-α (Fig. 4E)/ml. Without TNF-α treatment, only SF-1, not the p65 subunit (NF-κB), was associated with the MIS promoter. However, TNF-α treatment resulted in the recruitment of NF-κB by the MIS promoter. No signal was detected from the control PCR for nonspecific immunoprecipitation with primers specific to the β-actin coding region. These results suggest that, upon TNF-α signaling, NF-κB is recruited to the MIS promoter by SF-1 and downregulates MIS expression. This is consistent with the fact that NF-κB binding sites are not present within the MIS promoter that is sufficient to direct the initiation and maintenance of cell-specific MIS expression (12).
DNA-binding activity of SF-1 is not affected by NF-κB.
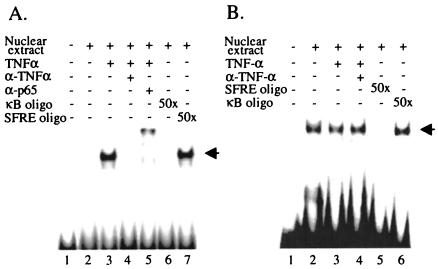
To explore how NF-κB activated by TNF-α signaling cascade affects SF-1 function, alteration of SF-1 DNA-binding activity was first accessed by EMSAs by using the SF-1 binding site from the MIS promoter as a probe (11). When HeLa cells were treated with TNF-α, NF-κB was activated and translocated into the nucleus, which was confirmed by the formation of NF-κB-DNA complex with κB enhancer oligonucleotide (16) (Fig. 5A). Nuclear extracts from unstimulated HeLa cells produced a readily detectable SF-1-DNA complex. The formation of the complex was eliminated by a 50-fold excess of the same unlabeled SF-1 oligonucleotide but not by a 50-fold excess of κB oligonucleotide, indicating that the complex formation was specific (Fig. 5B). This SF-1-DNA complex formation was not noticeably affected by either TNF-α treatment or the concomitant treatment of TNF-α with anti-TNF-α antibody (Fig. 5B). These results suggest that the repression of SF-1 transactivation by TNF-α signaling may not be accomplished by the alteration of SF-1 DNA-binding activity.
FIG. 5.
DNA-binding activity of SF-1 was not affected by NF-κB. HeLa cells were either not treated or treated with TNF-α or TNF-α together with anti-TNF-α antibody for 24 h. Nuclear extracts were prepared, and EMSAs were performed by using either the κB enhancer oligonucleotide (A) or the oligonucleotide of the SF-1 binding site from MIS promoter (B) as a probe. (A) TNF-α activated NF-κB, allowing it to form a NF-κB-DNA complex. (B) The formation of SF-1-DNA complex was not affected by TNF-α treatment. For competition or supershift experiments, a 50-fold excess of unlabeled competitor oligonucleotide or antibody solution was preincubated for 1 h at 4°C.
NF-κB represses SF-1 transactivation via recruitment of HDACs.
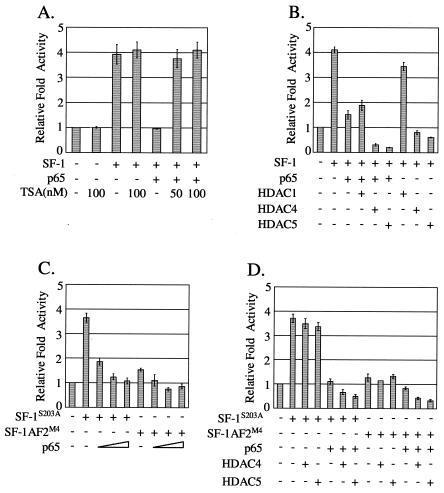
To search for a clue to the mechanism by which NF-κB represses SF-1 transactivation, we assessed the involvement of histone deacetylase (HDAC) by using the HDAC inhibitor TSA. In HeLa cells, cotransfection of SF-1 with p65 expression plasmid repressed SF-1 transactivation to the basal level, which was fully recovered with TSA treatment at a concentration of 50 to 100 ng/ml (Fig. 6A). SF-1 transactivation activity itself in the absence of p65 was not affected by TSA treatment. These results imply that HDACs are involved in the NF-κB repression of SF-1 transactivation.
FIG. 6.
HDACs were involved in the inhibition of SF-1 transactivation by NF-κB. (A) TSA rescued NF-κB inhibition of SF-1 transactivation. HeLa cells were cotransfected with 10 ng each of SF-1 and p65 expression plasmids, along with SFRE-Luc reporter, and treated with 50 or 100 nM TSA for 20 h before being harvested. As a control, cells transfected with SF-1 expression plasmid alone were treated with 100 nM TSA. (B) HDAC4 and HDAC5 were involved in the inhibition of SF-1 transactivation by NF-κB. HeLa cells were cotransfected with 10 ng of p65 expression plasmid, and 100 ng each of HDAC1, HDAC4, and HDAC5, along with 10 ng of SF-1 expression plasmid. p65 or each of the HDACs, was cotransfected with SF-1 expression plasmid as controls. (C) p65 inhibited the transactivation of the SF-1 mutants SF-1S203A and SF-1AF2M4. SF-1S203A and SF-1AF2M4 are phosphorylation-defective and transactivation activity-defective mutants, respectively. The 20 ng of SF-1 mutant and 3 to 30 ng of p65 expression plasmids were transfected. (D) Effects of both HDAC4 and HDAC5 coexpression on the transactivation of SF-1 mutants. Each expression plasmid of SF-1 mutants (20 ng) was cotransfected with p65 (10 ng), HDAC4 (100 ng), or HDAC5 (100 ng) into HeLa cells. All values represent the mean ± the SEM of at least three separate experiments.
NF-κB has been reported to recruit HDACs to negatively regulate its target gene expression (2), which let us test the possibility of recruitment of HDACs by NF-κB that is associated with SF-1. Each HDAC1, HDAC4, and HDAC5 expression plasmid, along with p65 and SF-1 expression plasmids, was cotransfected into HeLa cells, and their effects on NF-κB repression of SF-1 transactivation were assessed. As shown in Fig. 6B, HDAC4 and HDAC5 were able to further inhibit p65-repressed SF-1 transactivation by approximately 5- and 7.5-fold, respectively, whereas HDAC1 was unable to repress it. It is notable that expression of HDAC4 or HDAC5 alone significantly repressed SF-1 transactivation.
The inhibition effect of HDAC4 and HDAC5 on SF-1 transactivation in the absence of p65 coexpression made it difficult to confirm the NF-κB-dependent recruitment of HDACs to SF-1. To eliminate such interference, we searched for SF-1 mutants whose transactivation is still inhibited by p65 but not directly affected by HDAC4 and HDAC5 without NF-κB mediation. Two SF-1 mutants, the phosphorylation-defective (SF-1S203A) and AF-2 (SF-1-AF-2m4) mutants, were tested, since phosphorylation at Ser-203 and the AF-2 domain of SF-1 have been previously proposed to control its cofactor recruitments (15). As shown in Fig. 6C, the transactivation activities of both SF-1S203A and SF-1-AF-2m4 were repressed by p65 in a dose-dependent manner, although the repressed extents were different depending on the mutants. Meanwhile, the transactivation of both mutants was not significantly affected by coexpression of either HDAC4 or HDAC5 only (Fig. 6D), in contrast with the wild-type SF-1 (Fig. 6B). Consistent with the idea of NF-κB-mediated recruitment of HDACs to SF-1, coexpression of HDAC4 or HDAC5 further inhibited the NF-κB-repressed transactivation activity of both SF-1 mutants (Fig. 6D).
Considered together, these results suggest that NF-κB represses SF-1 transactivation by recruiting HDACs.
NF-κB directly recruits HDACs to the MIS promoter.
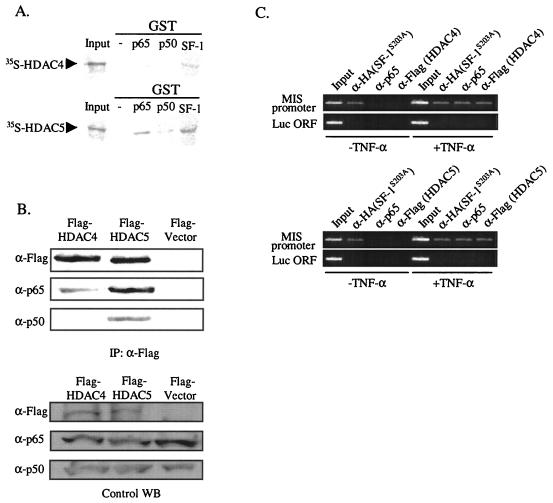
To determine whether NF-κB directly associates with HDAC4 or HDAC5, we performed GST pull-down assays. Both [35S]methionine-labeled HDAC4 and HDAC5 produced by in vitro translation were allowed to bind the GST fusion protein of the p65 or p50 subunit of NF-κB and the GST fusion protein of SF-1 (Fig. 7A). The p65 subunit interacted efficiently with HDAC5 and weakly with HDAC4, if at all. It is notable that SF-1 itself interacted with HDAC4 and HDAC5.
FIG. 7.
Association of NF-κB with HDAC4 and HDAC5 in vitro and in vivo. (A) p65 and SF-1 directly interact with HDAC5. 35S-labeled HDAC4 and HDAC5 were allowed to bind GST fusion proteins of p65, p50, or SF-1. Reactions were carried out with the equivalent amount of each protein, as determined by Coomassie blue staining (data not shown). Ten percent of the labeled protein used in the binding reaction was loaded as input. (B) NF-κB associates with HDAC4 and HDAC5 in vivo. p65 and p50 expression plasmids were cotransfected with either Flag-tagged HDAC4 or HDAC5 expression plasmid into HeLa cells. Coimmunoprecipitations were carried out with anti-Flag antibody, and Western blot analyses of the immunoprecipitated materials were performed with anti-Flag (HDACs), anti-p65, and anti-p50 antibody. As a negative control, p65 and p50 expression plasmid were cotransfected with Flag empty vector. Control Western blots (WB) are shown for the expression level of each protein. (C) Endogenous NF-κB recruits HDACs to the MIS promoter. ChIP assays were performed with HeLa cells, transfected HA-tagged SF-1S203A, and either Flag-tagged HDAC4 or Flag-tagged HDAC5 and treated or not treated with 10 ng of TNF-α/ml. Cross-linked DNA fragments were immunoprecipitated with anti-HA, anti-p65, or anti-Flag antibody, and the immunoprecipitates were analyzed by PCR with pairs of specific primers spanning the promoter region of MIS. A control PCR for nonspecific immunoprecipitation was performed with primers specific for the luciferase coding region.
We also performed coimmunoprecipitation experiments to confirm the association of NF-κB with HDACs in vivo. HeLa cells were cotransfected with each of Flag-tagged HDAC4, flag-tagged HDAC5, and flag vector, along with both p65 and p50 subunits of NF-κB. Whole-cell extracts were prepared, and immunoprecipitations were carried out with anti-Flag antibody. Western blot analysis of the immunoprecipitated material showed tighter association of HDAC5 than HDAC4 with NF-κB in vivo (Fig. 7B).
To show whether endogenous NF-κB recruits HDACs to the MIS promoter, we performed ChIP assays with HeLa cells cotransfected with HA-tagged SF-1S203A and either Flag-tagged HDAC4 or HDAC5 and then treated with or not treated with 10 ng of TNF-α/ml (Fig. 7C). Without TNF-α treatment, neither SF-1S203A, nor the endogenous p65 subunit (NF-κB) nor HDACs were associated with the MIS promoter. However, with TNF-α treatment, the endogenous p65 subunit (NF-κB) and HDACs, as well as SF-1S203A, were recruited to the MIS promoter. No signal was detected from the control PCR for nonspecific immunoprecipitation with primers specific to the luciferase coding region, which is ca. 3.3 kb upstream of the MIS promoter in the MIS-Luc reporter that was linearized at the SphI site located between the MIS promoter and luciferase coding region. These results suggest that NF-κB, which is recruited to the MIS promoter by SF-1 upon TNF-α signaling, recruits HDACs to inhibit MIS expression.
DISCUSSION
The present study suggests the molecular mechanism for the meiotic repression of MIS expression in the postnatal testis by demonstrating TNF-α inhibition of MIS promoter and its subsequent downstream molecular events. Furthermore, the regulatory mechanism of SF-1 transactivation by NF-κB, which is defined in the present study, adds yet another level to the already complex regulation of gene expression controlled by SF-1. To our knowledge, this is the first report to address the role of TNF-α signaling in the regulation of Sertoli cell function and to describe its action mechanism at the molecular level.
TNF-α represses MIS expression in the testis and is a strong candidate for the germ cell inhibitor.
Previous studies have suggested that meiotic germ cells repress MIS expression in Sertoli cells, possibly by secreting an inhibiting substance. However, no candidate substance for such a germ cell inhibitor has been addressed. In the present work, we provide several pieces of evidence that TNF-α is able to repress MIS expression, using organ-cultured testis, TNF-α−/− testis, and cultured cells. These results, considered together with the expression of TNF-α in meiotic germ cells (Fig. 1D) (9, 44), suggest that TNF-α is a strong candidate for the germ cell inhibitor that downregulates MIS expression in the postnatal testis. However, we cannot rule out the possibility that factors other than TNF-α are also involved in the meiotic repression of MIS expression in the testis.
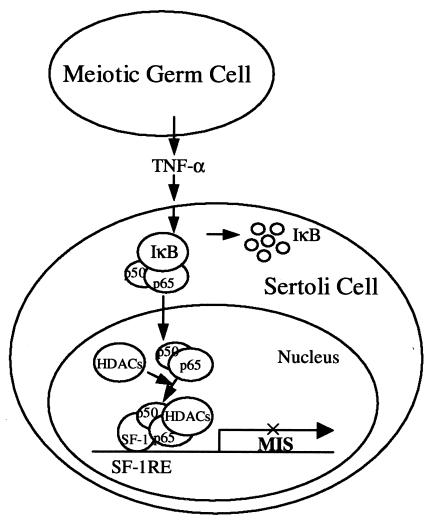
The present study demonstrates that TNF-α inhibition of MIS expression involves NF-κB activation, NF-κB recruitment by SF-1 to the MIS promoter, and subsequent HDAC recruitment by NF-κB. SF-1 directly interacts with the p50 subunit of NF-κB, whereas p65 (RelA) subunit is the major component to recruit HDACs. On the basis of the findings in the present study, we propose a working model wherein TNF-α signal from meiotic germ cells activates NF-κB in Sertoli cells. The activated NF-κB consequently moves to the nucleus and interacts with SF-1 on the MIS promoter, recruiting HDACs and/or HDAC-containing complex(es) to repress gene expression (Fig. 8).
FIG. 8.
Model for the downregulation of MIS expression by TNF-α signaling. TNF-α signaling from meiotic germ cells activates NF-κB in Sertoli cells. The activated NF-κB moves to the nucleus, interacts with SF-1 that is bound to the SF-1 binding site (SF-1RE) within the MIS promoter, and recruits HDACs and/or HDAC-containing complex(es) to repress the gene expression.
Pubertal repression of MIS expression in Sertoli cells.
The MIS level in serum is inversely correlated to the testosterone level in serum in males after the neonatal period and has been suggested to be a useful marker for predicting the onset of testicular pubertal maturation. Previous studies have suggested that MIS expression might be regulated via multiple routes, including meiotic germ cells and androgens. The drastic drop of the postnatal MIS expression in both TNF-α+/+ and TNF-α−/− testes in the present study (Fig. 1B) also suggests that there must be other mechanisms for the repression of MIS in the absence of TNF-α. The postnatal downregulation of SF-1 in Sertoli cells (18, 19, 47), as well as the increased production of androgen in the pubertal testis, might be responsible for such downregulation of MIS since SF-1 is a major transcription factor for MIS expression and androgen represses the expression of MIS (39). A synergy between the meiotic entry of germ cells and androgen downregulation of MIS expression has also been observed in mice (1).
Molecular mechanisms explaining the cellular and hormonal regulation of MIS expression in the postnatal testis have remained elusive. In the present study, we propose that TNF-α is the substance responsible for germ cell inhibition of MIS expression in Sertoli cells and that its action mechanism includes NF-κB inhibition of SF-1 transactivation. This inhibition mechanism through protein-protein interaction is consistent with the fact that NF-κB binding sites are not present within the proximal MIS promoter that is sufficient to direct the initiation and maintenance of cell-specific MIS expression (12).
The molecular mechanism for androgen repression of MIS expression during the pubertal period has also been unknown. However, a recent report that ligand-bound androgen receptor (AR) directly interacts with SF-1 and represses the SF-1-activated transcription of the LHβ subunit (23) may provide insight into the mechanism of androgen repression of the MIS gene. That is, in a similar way, androgen-bound AR interacts with SF-1 on the MIS promoter, repressing SF-1 transactivation, which consequently results in the suppression of MIS expression. This protein interaction model of the mechanism is attractive because androgen-responsive elements are not found on the MIS gene from 3,750 bp upstream of the transcription site to the polyadenylation site (40), a fact which suggests intermediate or alternative pathways of AR function. Further studies are necessary to determine whether this working model fits the androgen repression of the MIS gene and, if so, to explain how the meiotic entry and androgen synergistically affect MIS expression through the action of both of their mediators on SF-1.
NF-κB signaling cascades in the testicular function.
Recent studies suggest that NF-κB may have a role in regulating mammalian spermatogenesis, its activation being regulated during spermatogenesis. The p65 and p50 subunits of NF-κB are stage-specifically found in the nuclei of spermatocytes and spermatids and constitutively expressed at high levels in the nuclei of Sertoli cells (10). However, the mechanisms responsible for such NF-κB activation in germ cells and Sertoli cells are not understood. In addition, Sertoli cells also contain an inducible pool of NF-κB, since the cytokine TNF-α has been shown to increase DNA-binding and transcription activities of NF-κB in Sertoli cells. A number of genes expressed in the testis, including TNF-α, androgen receptor, and Fas ligand, have been shown to be regulated by NF-κB in other cells (6, 29, 46). In addition, NF-κB is able to act as a corepressor of steroid receptors, altering gene responses to hormonal stimuli (17, 31, 36). However, the precise physiological role of NF-κB in the testis has not been unraveled.
In the present study we postulate, as a mechanism for meiotic inhibition of MIS expression, that NF-κB activated by TNF-α functions as a corepressor of the orphan nuclear receptor SF-1. It will be worth investigating whether the same signal pathway and mechanism are generally applicable to the regulation of genes whose expression is governed by SF-1 and downregulated during puberty, such as inhibin alpha subunit (21). Previous studies have revealed that NF-κB stimuli, such as interleukin-1 (IL-1) and TNF-α, inhibit steroidogenesis by downregulating the transcription of steroidogenic enzymes such as p450c17 and 3β-HSD in Leydig cells (14, 54). Based on our present study, the transcriptional inhibition of steroidogenic enzymes in Leydig cells by TNF-α and IL-1 may be accomplished by a similar mechanism through NF-κB activation and its repression of SF-1 transactivation. In light of the facts that the expression of many genes is regulated by SF-1 and that NF-κB is a ubiquitous transcription factor, the mechanism identified here might be one of the common and significant means of gene responses to NF-κB signaling cascades.
NF-κB as a new modulator of SF-1 activity and a recruiter of class II HDACs.
The transactivation activity of SF-1 is regulated by protein interaction with diverse coregulatory proteins. WT-1, GATA-4, and DAX-1, which are coexpressed with SF-1 in several tissues, including testis and ovary, either enhance or repress SF-1 transactivation. The combination of interactions might result in temporal and spatial fine-tuning of the expression of SF-1 target genes such as MIS. Other transcription factors and coregulators have been also shown to modulate SF-1 transactivation. However, the precise mechanisms responsible for their modulation of SF-1 activity are not well understood. Some SF-1 coactivators, such as CBP and SRC-1, themselves have histone acetyltransferase activity, and DAX-1, at least, recruits the corepressor N-CoR for its repression of SF-1 (7, 8). In the present study, in particular, we identify NF-κB as another important modulator of SF-1 transactivation.
Besides functioning as a transcription factor, NF-κB has been reported to act as a corepressor of steroid hormone receptors such as AR, GR, and estrogen receptor (17, 31, 36), although the molecular mechanism for its action remains unknown. In the present study, we demonstrate that NF-κB also acts as a corepressor of the orphan nuclear receptor SF-1. Furthermore, we show that SF-1-bound NF-κB recruits HDAC(s). NF-κB interacts with multiple HDAC isoforms, including HDAC1 (2), HDAC2 (22), and HDAC3 (5). In addition, we revealed here that NF-κB is able to associate with class II HDACs, HDAC4, and HDAC5 (Fig. 6 and 7), showing the capacity of NF-κB to interact with diverse HDACs.
Acknowledgments
We thank R. Lovell-Badge, P. K. Donahoe, P. Koopman, H. A. Ingraham, V. Laudet, I. Verma, and D. D. Moore for providing the pBAM5-mAMH, MIS-Luc, pSGSox9, SF-1 mutant (S203A and AF2M4), SFRE-Luc, pLIκBαMSN, and SF-1 constructs, respectively. We also thank Y. S. Ahn for technical assistance.
This work was supported by a Korean Research Foundation grant (KRF-2002-070-C0007) and a Hormone Research Center grant (2001G0101).
REFERENCES
- 1.Al-Attar, L., K. Noel, M. Dutertre, C. Belville, M. G. Forest, P. S. Burgoyne, N. Josso, and R. Rey. 1997. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J. Clin. Investig. 100:1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner, B. P., S. D. Westerheide, and A. S. Baldwin, Jr. 2001. The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol. Cell. Biol. 21:7065-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behringer, R. R., R. L. Cate, G. J. Froelick, R. D. Palmiter, and R. L. Brinster. 1990. Abnormal sexual development in transgenic mice chronically expressing Müllerian inhibiting substance. Nature 345:167-170. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets IκB to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 6.Collart, M. A., P. Baeuerle, and P. Vassalli. 1990. Regulation of tumor necrosis alpha transcription in macrophages: involvement of four B-like motifs and of constitutive and inducible forms of NF-κB. Mol. Cell. Biol. 10:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford, P. A., J. A. Polish, G. Ganpule, and Y. Sadovsky. 1997. The activation function-2 hexamer of steroidogenic factor-1 is required, but not sufficient for potentiation by SRC-1. Mol. Endocrinol. 11:1626-1635. [DOI] [PubMed] [Google Scholar]
- 8.Crawford, P. A., C. Dorn, Y. Sadovsky, and J. Milbrandt. 1998. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol. Cell. Biol. 18:2949-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De, S. K., H.-L. Chen, J. L. Pace, J. S. Hunt, P. F. Terranova, and G. C. Enders. 1993. Expression of tumor necrosis factor in mouse spermatogenic cells. Endocrinology 133:389-396. [DOI] [PubMed] [Google Scholar]
- 10.Delfino, F. J., and W. H. Walker. 1998. Stage-specific nuclear expression of NF-κB in mammalian testis. Mol. Endocrinol. 12:1696-1707. [DOI] [PubMed] [Google Scholar]
- 11.De Santa Barbara, P., N. Bonneaud, B. Boizet, M. Desclozeaux, B. Moniot, P. Sudbeck, G. Scherer, F. Poulat, and P. Berta. 1998. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol. Cell. Biol. 18:6653-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuili, G., W. H. Shen, and H. A. Ingraham. 1997. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian inhibiting substance, in vivo. Development 124:1799-1807. [DOI] [PubMed] [Google Scholar]
- 13.Grozinger, C. M., C. A. Hassig, and S. L. Schreiber. 1999. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 96:4868-4873.10220385 [Google Scholar]
- 14.Hales, D. B. 1992. Interleukin-1 inhibits Leydig cell steroidogenesis primarily by decreasing 17 α-hydroxylase/C17-20 lyase cytochrome p450 expression. Endocrinology 131:2165-2172. [DOI] [PubMed] [Google Scholar]
- 15.Hammer, G. D., I. Krylova, Y. Zhang, B. D. Darimont, K. Simpson, N. L. Weigel, and H. A. Ingraham. 1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol. Cell 3:521-526. [DOI] [PubMed] [Google Scholar]
- 16.Han, S. J., J. H. Choi, H. M. Ko, H. W. Yang, I. W. Choi, H. K. Lee, O. H. Lee, and S. Y. Im. 1999. Glucocorticoids prevent NF-κB activation by inhibiting the early release of platelet-activating factor in response to lipopolysaccharide. Eur. J. Immunol. 29:1334-1341. [DOI] [PubMed] [Google Scholar]
- 17.Heck, S., K. Bender, M. Kullmann, M. Gottlicher, P. Herrlich, and A. C. B. Cato. 1997. IκB-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J. 16:4698-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda, Y., D. S. Lala, X. Luo, E. Kim, M. P. Moisan, and K. L. Parker. 1993. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol. Endocrinol. 7:852-860. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda, Y., W. H. Shen, H. A. Ingraham, and K. L. Parker. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 8:654-662. [DOI] [PubMed] [Google Scholar]
- 20.Ito, M., R. Yu, and J. L. Jameson. 1997. DAX-1 inhibits SF-1 mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol. Cell. Biol. 17:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, M., Y. Park, J. Weck, K. E. Mayo, and J. L. Jameson. 2000. Synergistic activation of the inhibin alpha-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol. Endocrinol. 14:66-81. [DOI] [PubMed] [Google Scholar]
- 22.Ito, K., P. J. Barnes, and I. M. Adcock. 2000. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol. Cell. Biol. 20:6891-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen, J. S., and J. H. Nilson. 2001. AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol. Endocrinol. 15:1505-1516. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda, T., M. M. Lee, C. M. Haqq, D. M. Powell, T. F. Manganaro, and P. K. Donahoe. 1990. Müllerian inhibiting substance ontogeny and its modulation by follicle-stimulating hormone in the rat testes. Endocrinology 127:1825-1832. [DOI] [PubMed] [Google Scholar]
- 25.Lee, Y. S., H.-J. Kim, H. J. Lee, J. W. Lee, S.-Y. Chun, S.-K. Ko, and K. Lee. 2002. Activating signal cointegrator 1 is highly expressed in murine testicular Leydig cells and enhances the ligand-dependent transactivation of androgen receptor. Biol. Reprod. 67:1580-1587. [DOI] [PubMed] [Google Scholar]
- 26.Li, L. A., E. F. Chiang, J. C. Chen, N. C. Hsu, Y. J. Chen, and B. C. Chung. 1999. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol. Endocrinol. 13:1588-1598. [DOI] [PubMed] [Google Scholar]
- 27.Lim, K., J.-H. Yoo, K.-Y. Kim, G.-R. Kweon, S.-T. Kwak, and B.-D. Hwang. 1994. Testosterone regulation of proto-oncogene c-myc expression in primary sertoli cell cultures from prepubertal rats. J. Androl. 15:543-550. [PubMed] [Google Scholar]
- 28.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui, K., A. Fine, B. Zhu, A. Marshak-Rothstein, and S. Ju. 1998. Identification of two NF-κB sites in mouse CD95 ligand (Fas ligand) promoter: functional analysis in T-cell hybridoma. J. Immunol. 161:3469-3473. [PubMed] [Google Scholar]
- 30.Mauduit, C., V. Besset, V. Caussanel, and M. Benahmed. 1996. Tumor necrosis factor receptor p55 is under hormonal (follicle stimulating hormone) control in testicular Sertoli cells. Biochem. Biophys. Res. Commun. 224:631-637. [DOI] [PubMed] [Google Scholar]
- 31.McKay, L. I., and J. A. Cidlowski. 1998. Cross-talk between nuclear factor-κB and the steroid hormone receptors: mechanism of mutual antagonism. Mol. Endocrinol. 12:45-56. [DOI] [PubMed] [Google Scholar]
- 32.Na, S. Y., S. K. Lee, S. J. Han, H. S. Choi, S. Y. Im, and J. W. Lee. 1998. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor κB-mediated transactivations. J. Biol. Chem. 273:10831-10834. [DOI] [PubMed] [Google Scholar]
- 33.Na, S. Y., B. Y. Kang, S. W. Chung, S. J. Han, X. Ma, G. Trinchieri, S. Y. Im, J. W. Lee, and T. S. Kim. 1999. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NF-κB. J. Biol. Chem. 274:7674-7680. [DOI] [PubMed] [Google Scholar]
- 34.Nachtigal, M. W., Y. Hirokawa, D. L. Enyeart-Van Houten, J. N. Flanagan, G. D. Hammer, and H. A. Ingraham. 1998. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445-454. [DOI] [PubMed] [Google Scholar]
- 35.Ou, Q., J. F. Mouillet, X. Yan, C. Dorn, P. A. Crawford, and Y. Sadovsky. 2001. The DEAD box protein DP103 is a regulator of steroidogenic factor-1. Mol. Endocrinol. 15:69-79. [DOI] [PubMed] [Google Scholar]
- 36.Palvimo, J. J., P. Reinikainen, T. Ikonen, P. J. Kallio, A. Moilanen, and O. A. Janne. 1996. Mutual transcriptional interference between RelA and androgen receptor. J. Biol. Chem. 271:24151-24156. [DOI] [PubMed] [Google Scholar]
- 37.Parker, K. L., and B. P. Schimmer. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr. Rev. 18:361-377. [DOI] [PubMed] [Google Scholar]
- 38.Rey, R., L. al-Attar, F. Louis, F. Jaubert, P. Barbet, C. Nihoul-Fekete, J. L. Chaussain, and N. Josso. 1996. Testicular dysgenesis does not affect expression of anti-Mullerian hormone by Sertoli cells in premeiotic seminiferous tubules. Am. J. Pathol. 148:1689-1698. [PMC free article] [PubMed] [Google Scholar]
- 39.Rey, R., F. Mebarki, M. G. Forest, I. Mowszowicz, R. L. Cate, Y. Morel, J. L. Chaussain, and N. Josso. 1994. Anti-Müllerian hormone in children with androgen insensitivity. J. Clin. Endocrinol. Metab. 79:960-964. [DOI] [PubMed] [Google Scholar]
- 40.Rey, R., and N. Josso. 1996. Regulation of testicular anti-Müllerian hormone secretion. Eur. J. Endocrinol. 135:144-152. [DOI] [PubMed] [Google Scholar]
- 41.Sadovsky, Y., and C. Dorn. 2000. Function of steroidogenic factor 1 during development and differentiation of the reproductive system. Rev. Reprod. 5:136-142. [DOI] [PubMed] [Google Scholar]
- 42.Shang, Y., X. Hu, J. Direnzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852. [DOI] [PubMed] [Google Scholar]
- 43.Shen, W. H., C. C. Moore, Y. Ikeda, K. L. Parker, and H. A. Ingraham. 1994. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651-661. [DOI] [PubMed] [Google Scholar]
- 44.Siu, M. K. Y., W. M. Lee, and C. Y. Cheng. 2003. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology 144:371-387. [DOI] [PubMed] [Google Scholar]
- 45.Stein, B., P. C. Cogswell, and A. S. Baldwin, Jr. 1993. Functional and physical associations between NF-κB and C/EBP family members: a Rel domain-bZIP interaction. Mol. Cell. Biol. 13:3964-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supakar, P. C., M. H. Jung, C. S. Song, B. Chatterjee, and A. K. Roy. 1995. Nuclear factor κB functions as a negative regulator for the rat androgen receptor gene and NF-κB activity increases during the age-dependent desensitization of the liver. J. Biol. Chem. 270:837-842. [DOI] [PubMed] [Google Scholar]
- 47.Tamura, M., Y. Kanno, S. Chuma, T. Saito, and N. Nakatsuji. 2001. Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech. Dev. 102:135-144. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira, J., S. Maheswaran, and P. K. Donahoe. 2001. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr. Rev. 22:657-674. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira, J., E. Fynn-Thompson, A. H. Payne, and P. K. Donahoe. 1999. Mullerian-inhibiting substance regulates androgen synthesis at the transcriptional level. Endocrinology 140:4732-4738. [DOI] [PubMed] [Google Scholar]
- 50.Trbovich, A. M., P. M. Sluss, V. M. Laurich, F. H. O'Neill, D. T. MacLaughlin, P. K. Donahoe, and J. Teixeira. 2001. Mullerian inhibiting substance lowers testosterone in luteinizing hormone-stimulated rodents. Proc. Natl. Acad. Sci. USA 98:3393-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tremblay, J. J., and R. S. Viger. 1999. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol. Endocrinol. 13:1388-1401. [DOI] [PubMed] [Google Scholar]
- 52.Vanacker, J. M., E. Bonnelye, S. Chopin-Delannoy, C. Delmarre, V. Cavailles, and V. Laudet. 1999. Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-alpha). Mol. Endocrinol. 13:764-773. [DOI] [PubMed] [Google Scholar]
- 53.Viger, R. S., C. Mertineit, J. M. Trasler, and M. Nemer. 1998. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125:2665-2675. [DOI] [PubMed] [Google Scholar]
- 54.Xiong, Y., and D. B. Hales. 1997. Differential effects of tumor necrosis factor-alpha and interleukin-1 on 3 β-hydroxysteroid dehydrogenase/delta 5→delta 4 isomerase expression in mouse Leydig cells. Endocrine 7:295-301. [DOI] [PubMed] [Google Scholar]