Abstract
Cutaneous infiltration of activated CD4+ T cells and eosinophils is an early event in blister formation during bullous pemphigoid (BP), suggesting that the trafficking of circulating leucocytes through the sites of inflammation, their activation and cytokine release is crucial in the pathogenesis of the disease. IL-16 is a major chemotactic factor able to recruit CD4+ cells in the skin during inflammation and to induce the expression of functional high-affinity interleukin (IL)-2 receptors, thus contributing to cellular activation and proliferation. We performed a study in order to evaluate the presence of IL-16 in skin samples and sera and blister fluids of patients affected with BP in active phase of the disease (n = 39), compared with healthy donors studied as control group. Ten patients were also evaluated before and after steroid therapy. Our results demonstrated that IL-16 was expressed strongly by keratinocytes and by dermal infiltrating CD4+ T lymphocytes in lesional skin of BP patients. High levels of IL-16 were detected in sera and blisters of BP, significantly higher in respect to healthy donors. When patients were investigated for the presence of eosinophil cationic protein (ECP) and soluble CD30 (sCD30) to reveal signs of eosinophils and Th2-cells activation, we found a positive correlation between IL-16 serum levels and both ECP and sCD30, suggesting that IL-16 is involved in Th2 lymphocytes and eosinophils recruitment during BP.
Keywords: bullous pemphigoid, IL-16
INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune skin disease clinically showing large, tense blisters on normal or erythematosus skin. The immunopathological hallmark of BP is the presence of tissue-bound and circulating IgG autoantibodies directed against two hemidesmosomal proteins designated as BP180 and BP230 [1]. Although a pathogenic relevance of anti-BP180 antibodies in blister formation has been demonstrated, it is well known that inflammatory cells and mediators other than autoantibodies are responsible for the immunological response leading to tissue damage during BP [2–4]. Early cutaneous infiltration of activated CD4+ T cells and eosinophils seems to be a crucial event in the development of bullous lesions and a few studies revealed that autoreactive T cell lines and clones recognizing the BP180 NC16A domain show the functional features of Th2 lymphocytes [5,6]. Indeed, several Th2-type cytokines and soluble mediators released by these cells, such as interleukin (IL)-4, IL-5, IL-6, soluble IL-2 receptor (sIL-2R) and soluble CD30 (sCD30), have been detected extensively in blood and blister fluids of patients with BP [7–10]; more recently, skin-homing T cells expressing the cutaneous lymphocyte-associated antigen (CD4+ CLA+) have been identified as the main source of IL-4 and IL-13 in the blood and in the skin of patients with active BP, thus accounting for an expansion of Th2-type immune reactions during the disease [11]. Taken together, these data make clear that the influx of circulating leucocytes through the sites of inflammation, their activation and cytokine release is a crucial immunological event in the pathogenesis of BP. In this light, an increased expression and release of eotaxin and thymus and activation-regulated chemokine (TARC), CC-chemokines involved in the chemotaxis of eosinophils and Th2 effector cells, has been documented in patients with BP, thus confirming the role of chemotactic factors in activating and recruiting inflammatory cells during tissue damage [12–15].
IL-16 has been described recently as a major chemotactic cytokine for a variety of CD4+ immune cells [16,17]; several studies have characterized IL-16 as an immunomodulatory cytokine contributing to the regulatory process of CD4+ cells recruitment and activation at sites of inflammation during Th2-mediated immune disorders such as asthma, autoimmune diseases, atopic dermatitis and HIV infection [18–27]. Because of the central role of CD4+ cells in tissue damage of BP, we decided to investigate IL-16 levels in sera and blister fluid of patients with BP by enzyme-linked immunosorbent assay (ELISA) and the expression of IL-16 in lesional skin by immunohistochemistry. We also evaluate serum levels of eosinophil cationic protein (ECP) and soluble CD30 (sCD30) as signs of eosinophils and Th2-cells activation, respectively. In a subset of BP patients treated with immunosuppressive therapy, serum levels of IL-16 were measured before and after treatment.
MATERIALS AND METHODS
Patients
We enrolled 39 patients affected with active BP (15 males and 24 females, aged from 52 to 71 years) attending our department of dermatology and never treated before they entered our study. The diagnosis of BP was established on the basis of both clinical and immunopathological evaluation and all patients showed a clinical picture of generalized BP, without mucosal involvement (median disease duration: 2·3 months, range 0–4); disease severity in terms of cutaneous extention of lesions (bullae and/or erythema) ranged from 5% to 30% of total body area. Direct immunofluorescence (DIF) performed on perilesional skin biopsies evidenced linear deposition of IgG and/or C3 along the basement membrane zone (BMZ) in all patients and circulating anti-BMZ autoantibodies, when present, were detected by indirect immunofluorescence (IIF) on monkey oesophagus with titres ranging from 40 to 320. IIF performed on 1 m NaCl human split-skin demonstrated epidermal side autoantibody binding in all patients. Concomitant pathologies were excluded on the basis of clinical and instrumental examinations. A subset of patients (n = 10) was studied after 2 weeks of systemic immunosuppressive therapy (oral administration of corticosteroids from 25 to 100 mg/die prednisolone) resulting in an improvement of clinical picture in terms of clearance of old blisters and absence of new skin lesions in all treated patients.
Evaluation of IL-16, ECP and sCD30 levels in sera and blister fluids
Sera for quantitative evaluation of circulating IL-16, ECP, sCD30 were obtained from all subjects with BP (n = 39) and from healthy donors (n = 10) studied as controls. In the group of BP patients treated with corticosteroids we collected serum samples before and after therapy to detect a possible modification induced by steroid treatment on circulating levels of IL-16 and of other Th2-related antibody-inducing cytokines such as IL-4 and IL-13. Blister fluids were taken from bullae of early onset (≤24 h) in five of them before treatment; as controls, suction blisters raised on the forearm of three healthy volunteers selected from the serum sample healthy donor group were also collected. Immunoenzymatic assays were performed using commercially available ELISA and fluoroimmunoenzymatic (FEIA) kits according to the manufacturer's instructions. The detection limits of each assay were as follows: 8 pg/ml for IL-16 (Bender MedSystems, Vienna, Austria), 1 U/ml for sCD30 (Dako, Glostrup, Denmark), 0·13 pg/ml for IL-4 (R&D Systems, Minneapolis MN, USA), 32 pg/ml for IL-13 (R&D Systems) and 0·5 µg/l for ECP (CAP System FEIA, Pharmacia, Uppsala, Sweden). All sera and blister fluids, stored at−80°C until use, were evaluated in duplicate.
Immunohistochemical evaluation of cellular infiltrate and IL-16 in lesional skin of BP patients
Lesional skin specimens were obtained from lesions of early appearance (≤24 h) in 10 patients with BP and control skin biopsies were taken from normal volunteers (n = 2). Serial cryostat sections (5 µm) were cut frozen, coated on polylysine pretreated slides, air-dried overnight at room temperature and processed for immunohistochemistry using an HRP two-steps amplified method (EnVision + TM, peroxidase, Dako Corp., Carpinteria, CA, USA) as described previously [14]. Mouse antihuman CD4 (Becton Dickinson, San José, CA, USA), mouse antihuman EG2 (Pharmacia, Uppsala, Sweden) and rabbit antihuman IL-16 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used as primary antibodies. Diaminobenzidine and 3-amino-9-ethylcarbazolo (liquid DAB + and AEC + Substrate-Chromogen System, Dako Corp.) were used as chromogen substrates to reveal CD4, EG2 and IL-16 staining, respectively.
Statistical analysis
Data were evaluated by means of the SigmaStat 2·0 statistical software. Non-parametric Mann–Whitney U-test was used to compare BP patients and control groups and Wilcoxon signed-rank test for paired data revealed differences between BP blister fluids and corresponding sera and in response to treatment; a P-value of less than 0·05 was considered to be statistically significant. Correlation coefficient (r) was determined by Spearman's rank correlation coefficient matrix.
RESULTS
IL-16, sCD30 and ECP levels in sera and blister fluids
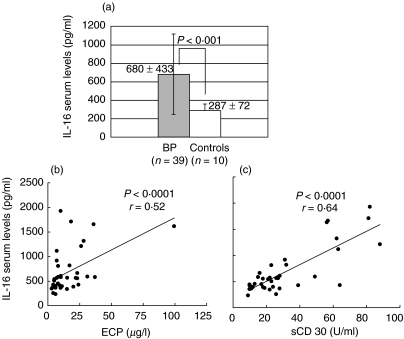
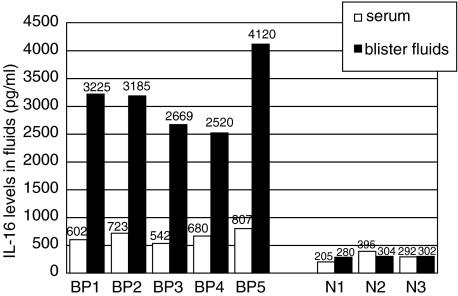
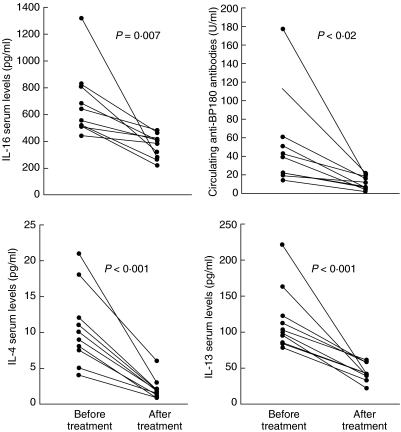
Large amounts of IL-16 were detected both in sera and blister fluids of BP patients. As shown in Fig. 1a, mean serum levels of IL-16 in BP resulted increased significantly when compared to healthy donors (680 ± 433 versus 287 ± 72 pg/ml, P < 0·001). Mean levels of ECP and sCD30 were also higher in BP serum samples when compared to controls (17 ± 21 versus 5 ± 7 ng/ml, P = 0·002; 31 ± 18 versus 10 ± 3 U/ml, P < 0·001, respectively) and, more interestingly, we could detect in BP subjects a positive correlation between circulating IL-16 and both ECP (P < 0·0001, r = 0·52) and sCD30 (P < 0·0001, r = 0·64) (Fig. 1b). A commercially available ELISA kit (MBL, Nagoya, Japan) was also used to determine the presence of circulating anti-BP-180 antibodies, which were found elevated in all patients with levels ranging from 13 to 196 U/ml (n.v. = 9 U/ml). In BP blister fluids IL-16 levels were significantly higher than those detected in corresponding serum samples (3144 ± 627 versus 670 ± 103 pg/ml, P < 0·001) and in suction blister fluid from normal donors (P = 0·03) while no difference was detected in IL-16 concentration between serum and blister fluid of normals (300 ± 95 versus 295 ± 13 pg/ml) (Fig. 2). In 10 patients evaluated before and after oral corticosteroid administration a significant reduction of circulating IL-16 (682 ± 258 versus 366 ± 89 pg/ml, P = 0·007) and anti-BP180 antibodies (55 ± 52 versus 11 ± 7 U/ml, P < 0·02) was observed during the treatment, in parallel with the clinical improvement of skin lesions (Fig. 3). To better reveal the immunosuppressive effect of corticosteroids we also measured serum levels of IL-4 and IL-13, thus documenting that both cytokines, whose mean levels in BP patients were higher in respect to controls at the beginning of treatment (10·5 ± 5·3 versus 1·5 ± 0·3 pg/ml, P < 0·0001 for IL-4; 116 ± 44 versus 56 ± 7 pg/ml, P < 0·0001 for IL-13), were reduced significantly after therapy (2·1 ± 1·5 pg/ml, P < 0·001; 43·6 ± 12·2 pg/ml, P < 0·001) (Fig. 3).
Fig. 1.
Serum levels of IL-16 in patients with active BP (n = 39) are significantly higher than those detected in healthy control subjects (n = 10) (a) and show a significant correlation between both ECP (r = 0·52, P < 0·0001) and sCD30 (r = 0·64, P < 0·0001) (b).
Fig. 2.
IL-16 concentration in blister fluids and corresponding sera of 5 bullous pemphigoid patients (BP) and three normal donors (N) evaluated as control group. Each single bar corresponds to IL-16 value detected in serum (white bar) or blister fluid (black bar) of a single subject. Mean values of IL-16 in blister fluids of BP (MV ± S.D. = 3144 ± 627 pg/ml) are about fivefold higher than corresponding IL-16 serum levels (MV ± S.D. = 670 ± 103 pg/ml).
Fig. 3.
Comparison of serum IL-16, circulating anti-BP180 antibodies, IL-4 and IL-13 in BP patients (n = 10) between before and after treatment with oral corticosteroid, showing that all parameters are significantly decreased after therapy.
Cutaneous expression of IL-16 in lesional skin of BP patients
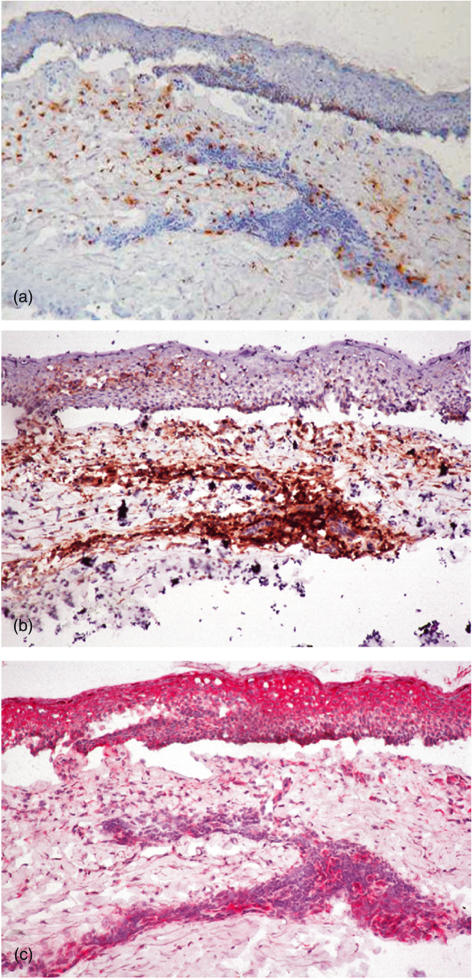
All samples processed for immunohistochemistry were characterized histologically by the presence of an inflammatory infiltrate consisting mainly of lymphocytes and eosinophils. Qualitative analysis of immunohistochemical staining showed high expression IL-16 in BP lesions. As shown in Fig. 4 in a representative sample, scattered EG2+ eosinophils were present in upper and middle dermis (Fig. 4a) while inflammatory infiltrate beneath the bullae was composed mainly of CD4+ T lymphocytes (Fig. 4b). A strong immunoreactivity for IL-16 was detected in the upper and middle dermis of lesional skin, documenting the same pattern of distribution when compared to CD4+ cells in consecutive sections, especially in perivascular infiltrate of middle dermis. IL-16 expression was also detected in epidermal keratinocytes, especially around the BP blister but also throughout the epidermis (Fig. 4c). No immunoreactivity for IL-16 was detected in normal skin samples.
Fig. 4.
Immunohistochemical staining of EG2+ eosinophils (a), CD4+ lymphocytes (b) and IL-16 (c) in serial cryostat sections from a representative sample of bullous pemphigoid (BP) lesional skin, showing immunoreactive IL-16 in both dermal CD4+ infiltrate and epidermal keratinocytes (HRP two-steps amplified method, DAB and AEC chromogen substrates, haematoxylin counterstaining, ×250).
DISCUSSION
In this study we showed that: (i) IL-16 serum levels in BP patients were significantly higher in respect to healthy control subjects and were related to both ECP and sCD30; (ii) blister fluid IL-16 levels were significantly higher in BP patients than those detected in normal donors and were increased about fivefold in respect to corresponding serum samples; (iii) serum IL-16 levels decreased significantly in patients with BP treated with oral corticosteroid; and (iv) IL-16 was expressed by epidermal keratinocytes and infiltrating CD4+ lymphocytes in BP lesional skin. BP is a blistering autoimmune disease associated to autoantibodies that recognize hemidesmosomal proteins of basement membrane zone. It is now well known that, in addition to autoantibodies deposition and complement activation, cell-mediated immune reactions are key events in the pathogenesis of BP [1–4]. Bullous lesions are infiltrated by inflammatory cells, consisting mainly of activated T lymphocytes and eosinophils since the early stage of blister formation. Large production and release of cytokines and soluble mediators from these cells has been documented extensively both in bullae and sera of patients affected with BP, often related to disease activity and skin involvement [7–11]. Several studies have shown tissue deposition and high concentration of eosinophil cationic protein (ECP) and major basic protein (MBP) in BP sera and blister fluid, and the presence of a strong eosinophil-colony stimulating activity seems to be derived from lesional infiltrating T lymphocytes [28,29]. Furthermore, our previous studies performed on BP patients demonstrated high levels of circulating IL-4 and sCD30, a Th2 activation marker, in relation to the disease activity [10]. On the whole, these data suggest that activation of Th2 lymphocytes able to recruit and activate eosinophils in inflammed skin is a major step in the pathomechanism of tissue damage during BP. IL-16 has been characterized as a chemoattractant for a variety of CD4-bearing immune cells (lymphocytes, monocytes, activated eosinophils, dendritic cells, neuronal cells); it is produced by several immune (CD8 and CD4 T cells, mast cells, eosinophils, dendritic cells) and non-immune (epithelial cells, fibroblasts) cell types both constitutively and under stimuli such as histamine, antigen, mitogen, cytokines and complement fragments [16]. In addition to cell migration, IL-16 induces functional high-affinity IL-2 receptors on CD4+ cells, thus contributing to cellular activation and proliferation; moreover, it is able to activate expression and production of proinflammatory cytokines such as IL-1β, IL-6, IL-15 and tumour necrosis factor (TNF)-α in human monocytes [17]. The role of IL-16 in human diseases has been largely investigated, although the specific biological function is still a matter of debate. In rheumatoid arthritis, for instance, contradictory studies demonstrated an anti-inflammatory and regulatory activity of IL-16 [18,19] as well as a capability in promoting inflammatory responses [20,21]. In other autoimmune diseases such as SLE and in HIV-1 infection, IL-16 serum levels correlate with disease progression [22,23]; its presence in bronchoalveolar lavage of asthmatic patients and in the skin or sera of patients with atopic dermatitis is related strictly to the earliest infiltration of CD4+ T cells and eosinophils and to other markers of inflammation [24–27]. Our study provides the first evidence for IL-16 production during BP, showing that high amounts of IL-16 can be detected in BP sera and blister fluid and that systemic corticosteroids may control inflammatory response by significantly reducing circulating levels of IL-16, IL-4 and IL-13. Furthermore, the decrease of IL-16 levels after treatment was observed in parallel to both clinical improvement of skin lesions and to the reduction of anti-BP180 circulating antibodies, whose detection is well established as a useful laboratory tool in monitoring BP. From these findings, considered as a whole, we suggest that IL-16 serum levels could reflect disease activity in BP patients. We also showed that IL-16 is expressed strongly in both keratinocytes and infiltrating CD4+ cells of lesional skin; although eosinophils may express CD4 molecule on their surface under activation [30] and are able to produce IL-16 [31], no staining for IL-16 was osberved in EG2+ eosinophils in our data. We performed immunohistochemical examination on skin lesions of early onset (<24 h), characterized histologically by a prevalent infiltration of T lymphocytes, so that a possible contribution of eosinophils to IL-16 production cannot be excluded when considering later stages of BP tissue inflammation. Recent literature data have demonstrated that lesional keratinocytes of BP patients may be a source of chemokines, such as eotaxin [12,13] and TARC [15], supporting the involvement of chemotactic factors in the pathogenesis of the disease. The significant release of IL-16 observed in the bloodstream of BP patients could be regarded as a systemic sign of IL-16 production by keratinocytes and CD4+ T cells at sites of skin inflammation. We further confirm that serum levels of sCD30, a soluble marker released from activated Th2 lymphocytes, are elevated during active BP and the correlation observed between circulating IL-16 and both sCD30 and ECP may account for the involvement of this cytokine in the specific recruitment of Th2 lymphocytes and eosinophils in the active phase of BP. According to our results, we may suggest a further mechanism in the pathogenesis of BP by which, since the early stage of the disease, IL-16 derived from lesional keratinocyte and inflammatory lymphocytes and released in the bloodstream is responsible for chemotaxis of autoreactive CD4+ Th2 cells that, through local production and release of other cytokines and IL-16 itself, are able to maintain and amplify immunological process underlying blister formation.
Acknowledgments
The authors gratefully thank Miss Monica Biondi and Miss Gilda Stillo for their skilful technical assistance while performing ELISA assays and immunohistochemical procedures and Dr Enrico Scala for his valuable suggestions in revising the manuscript.
References
- 1.Schmidt E, Brocker EB, Zillikens D. New aspects on the pathogenesis of bullous pemphigoid. Hautarzt. 2000;51:637–45. doi: 10.1007/s001050051188. [DOI] [PubMed] [Google Scholar]
- 2.Hertl M. Humoral and cellular autoimmunity in autoimmune bullous skin disorders. Int Arch Allergy Immunol. 2000;122:91–100. doi: 10.1159/000024364. [DOI] [PubMed] [Google Scholar]
- 3.Nestor MS, Cochran AJ, Ahmed AR. Mononuclear cell infiltrates in bullous disease. J Invest Dermatol. 1987;88:172–5. doi: 10.1111/1523-1747.ep12525315. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko F, Minagawa T, Takiguchi Y, Suzuki M, Itoh N. Role of cell-mediated immune reaction in blister formation of bullous pemphigoid. Dermatology. 1992;184:34–9. doi: 10.1159/000247496. [DOI] [PubMed] [Google Scholar]
- 5.Budinger L, Borradori L, Yee C, et al. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest. 1998;102:2082–9. doi: 10.1172/JCI3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eming R, Budinger L, Riechers R, et al. Frequency analysis of autoreactive T-helper 1 and 2 cells in bullous pemphigoid and pemphigus vulgaris by enzyme-linked immunospot assay. Br J Dermatol. 2000;143:1279–82. doi: 10.1046/j.1365-2133.2000.03901.x. [DOI] [PubMed] [Google Scholar]
- 7.Zillikens D, Ambach A, Schuessler M, Dummer R, Hartmann A, Burg G. The interleukin-2 receptor in lesions and serum of bullous pemphigoid. Arch Dermatol Res. 1992;284:141–5. doi: 10.1007/BF00372706. [DOI] [PubMed] [Google Scholar]
- 8.D’Auria L, Cordiali Fei P, Ameglio F. Cytokines and bullous pemphigoid. Eur Cytokine Netw. 1999;10:123–34. [PubMed] [Google Scholar]
- 9.Engineer L, Bhol K, Kumari S, Razzaque Ahmed A. Bullous pemphigoid: interaction of interleukin 5, anti-basement membrane zone antibodies and eosinophils. A preliminary observation. Cytokine. 2001;13:32–8. doi: 10.1006/cyto.2000.0791. [DOI] [PubMed] [Google Scholar]
- 10.De Pità O, Frezzolini A, Cianchini G, Ruffelli M, Teofoli P, Puddu P. T-helper 2 involvement in the pathogenesis of bullous pemphigoid: role of soluble CD30 (sCD30) Arch Dermatol Res. 1997;289:667–70. doi: 10.1007/s004030050259. [DOI] [PubMed] [Google Scholar]
- 11.Teraki Y, Hotta T, Shiohara T. Skin-homing interleukin-4 and -13-producing cells contribute to bullous pemphigoid. remission of disease is associated with increased frequency of interleukin-10-producing cells. J Invest Dermatol. 2001;117:1097–102. doi: 10.1046/j.0022-202x.2001.01505.x. [DOI] [PubMed] [Google Scholar]
- 12.Wakugawa M, Nakamura K, Hino H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. 2000;143:112–6. doi: 10.1046/j.1365-2133.2000.03599.x. [DOI] [PubMed] [Google Scholar]
- 13.Shrikhande M, Hunziker T, Braathen LR, Pichler WJ, Dahinden CA, Yawalkar N. Increased coexpression of eotaxin and interleukin 5 in bullous pemphigoid. Acta Derm Venereol. 2000;80:277–80. doi: 10.1080/000155500750012162. [DOI] [PubMed] [Google Scholar]
- 14.Frezzolini A, Teofoli P, Cianchini G, et al. Increased expression of eotaxin and its specific receptor CCR3 in bullous pemphigoid. Eur J Dermatol. 2002;12:27–31. [PubMed] [Google Scholar]
- 15.Kakinuma T, Wakugawa M, Nakamura K, Hino H, Matsushima K, Tamaki K. High level of thymus and activation-regulated chemokine in blister fluid and sera of patients with bullous pemphigoid. Br J Dermatol. 2003;148:203–10. doi: 10.1046/j.1365-2133.2003.05066.x. [DOI] [PubMed] [Google Scholar]
- 16.Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol. 2000;67:757–66. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 17.Mathy NI, Scheuer W, Lanzendorfer M, et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100:63–9. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. J Immunol. 1999;162:4293–9. [PubMed] [Google Scholar]
- 19.Blaschke S, Schulz H, Schwarz G, Blaschke V, Muller GA, Reuss-Borst M. Interleukin 16 expression in relation to disease activity in rheumatoid arthritis. J Rheumatol. 2001;28:12–21. [PubMed] [Google Scholar]
- 20.Kaufmann J, Franke S, Kientsch-Engel R, Oelzner P, Hein G, Stein G. Correlation of circulating interleukin 16 with proinflammatory cytokines in patients with rheumatoid arthritis. Rheumatology. 2001;40:474–5. doi: 10.1093/rheumatology/40.4.474. [DOI] [PubMed] [Google Scholar]
- 21.Lard LR, Roep BO, Toes RE, Huizinga TW. Enhanced concentrations of interleukin 16 are associated with joint destruction in patients with rheumatoid arthritis. J Rheumatol. 2004;31:35–9. [PubMed] [Google Scholar]
- 22.Lee S, Kaneko H, Sekigawa I, Tokano Y, Takasaki Y, Hashimoto H. Circulating interleukin-16 in systemic lupus erythematosus. Br J Rheumatol. 1998;37:1334–7. doi: 10.1093/rheumatology/37.12.1334. [DOI] [PubMed] [Google Scholar]
- 23.Center DM, Kornfeld H, Ryan TC, Cruikshank WW. Interleukin 16. implications for CD4 functions and HIV-1 progression. Immunol Today. 2000;21:273–80. doi: 10.1016/s0167-5699(00)01629-7. [DOI] [PubMed] [Google Scholar]
- 24.Laberge S, Pinsonneault S, Varga EM, et al. Increased expression of IL-16 immunoreactivity in bronchial mucosa after segmental allergen challenge in patients with asthma. J Allergy Clin Immunol. 2000;106:293–301. doi: 10.1067/mai.2000.108112. [DOI] [PubMed] [Google Scholar]
- 25.Taha RA, Laberge S, Hamid Q, Olivenstein R. Increased expression of the chemoattractant cytokines eotaxin, monocyte chemotactic protein-4, and interleukin-16 in induced sputum in asthmatic patients. Chest. 2001;120:595–601. doi: 10.1378/chest.120.2.595. [DOI] [PubMed] [Google Scholar]
- 26.Laberge S, Ghaffar O, Boguniewicz M, Center DM, Leung DY, Hamid Q. Association of increased CD4+ T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. J Allergy Clin Immunol. 1998;102:645–50. doi: 10.1016/s0091-6749(98)70282-9. [DOI] [PubMed] [Google Scholar]
- 27.Frezzolini A, Paradisi M, Zaffiro A, et al. Circulating interleukin 16 (IL-16) in children with atopic/eczema dermatitis syndrome (AEDS): a novel serological marker of disease activity. Allergy. 2002;57:815–20. doi: 10.1034/j.1398-9995.2002.23687.x. [DOI] [PubMed] [Google Scholar]
- 28.Borrego L, Maynard B, Peterson EA, et al. Deposition of eosinophil granule proteins precedes blister formation in bullous pemphigoid. Comparison with neutrophil and mast cell granule proteins. Am J Pathol. 1996;148:897–909. [PMC free article] [PubMed] [Google Scholar]
- 29.Kawachi Y, Otsuka F. Eosinophil-colony stimulating activity in blister fluid of bullous pemphigoid. J Dermatol Sci. 1997;15:51–4. doi: 10.1016/s0923-1811(97)00600-2. [DOI] [PubMed] [Google Scholar]
- 30.Lucey DR, Dorsky DI, Nicholson-Weller A, Weller PF. Human eosinophils express CD4 protein and bind human immunodeficiency virus 1 gp120. J Exp Med. 1989;169:327–32. doi: 10.1084/jem.169.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim KG, Wan HC, Bozza PT, et al. Human eosinophils elaborate the lymphocyte chemoattractants: IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996;156:2566–70. [PubMed] [Google Scholar]