Abstract
Immune complexes (IC) can induce cytokine production in vitro. While immune aggregates (IA) consisting of heat-aggregated gamma globulin (HAGG) as model IC increased interleukin (IL)-10 levels in cell cultures with native human serum, IL-12p40/p70 production was inhibited. Three series of experiments suggested that the effects of IA on IL-12 production depended on a functionally intact complement system: (1) heat-inactivation of serum inverted the inhibitory effect of IA on IL-12p40/p70 production; (2) IA-induced IL-12p40 production in a C4 deficient serum was lowered by addition of C4; and (3) addition of the peptide compstatin, which blocks C3 activation, mimicked the effects of heat inactivation on IL-12p40 levels. Neutralization of IL-12 resulted in modestly increased IL-10 levels, while neutralization of IL-10 had no effects on IL-12p40 production. IA-induced production of IL-10 was partially blocked by anti-FcγRII antibodies, whereas FcγR or CR blockade had no effect on IL-12p40 production. IC and local or systemic complement activation characterize rheumatoid arthritis, systemic lupus erythematosus and many malignancies. Different and complement-dependent effects on the production of IL-10 and IL-12 can be of importance in these diseases, where control of the complement system might be a way to direct IC-induced cytokine production in either a type 1 or type 2 direction.
Keywords: complement activation, immune complex, IL-10, IL-12
INTRODUCTION
Cytokines are of central importance in IC-associated disorders like systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and malignant diseases. The two monocyte-derived cytokines interleukin (IL)-10 and IL-12 have counteracting effects. While IL-10 is an anti-inflammatory cytokine with cytokine synthesis inhibitory effects [1], IL-12 is a major inducer of interferon (IFN)-γ, and thereby an important activator of T cell-mediated inflammation [2]. IL-10 also positively regulates proliferation and differentiation of B cells [3]. IL-10 and IL-12 have mutual inhibitory effects on the production of the other cytokine. Neutralization of IL-10 in mice increases IL-12 production [4] and when IL-12 is neutralized, increased expression of IL-10 message is observed [5]. This dichotomy reported for monocytes/macrophages is, however, not evident in all cell types, as IL-12 has been shown to increase IL-10 production from a human tumour T cell line, in parallel to enhancing IFN-γ production [6].
IC have been shown to increase the production of IL-6 and IL-10 from human PBMC in vitro [7–9], while at the same time decreasing the production of IL-12 [10]. We could repeat these latter findings in a native normal human serum (NHS) system using HAGG IA as model IC, but unexpectedly the IA-induced suppression of IL-12 production was exchanged by a stimulatory effect when heat-inactivated NHS devoid of a functionally intact complement system was used. The effect was seen only for IL-12, as production of IL-6 and IL-10 were stimulated by IA in the context of native NHS, whereas the effect was abolished in heat-inactivated NHS.
To test the hypothesis that the occurrence of either suppression or stimulation of IL-12 production by IC was dependent on the access to a functionally active complement system, IA stimulation was repeated in two other experimental systems. In one series of experiments a C4 deficient serum supplemented with C4 was used. In other experiments a system where the C3 activation blocking peptide compstatin was added to native NHS was utilized. We found that the reciprocal effects on IL-12p40 production could be repeated in these systems, with suppression or lower degree of IL-12p40 production occurring together with intact functional activity of the classical complement pathway.
MATERIALS AND METHODS
Blood donors and sera
Cells were obtained from healthy individuals after informed consent. The local ethical committee at the University Hospital in Uppsala had approved the investigation. The same NHS obtained as a whole blood donation was used for most of the experiments. In one experiment four sera from different blood donors were used. A C4 deficient serum was obtained from an SLE patient. All sera were centrifuged and frozen in −70°C within 4 h of sampling. Heat-inactivation, when appropriate, was performed with newly thawed serum in a water bath for 30 min at 56°C directly before use. Heat-inactivation reduced classical complement function to 0–2% of normal levels. Complement factor C4 was prepared in our laboratory essentially as described in [11]. Quantitative measurements of functional activity of the classical and alternative complement pathways were performed with a technique earlier described in our laboratory [12].
Preparation of peripheral blood mononuclear cells (PBMC)
Heparinized blood or buffy-coat preparations was diluted in phosphate buffered saline (PBS) at room temperature, and separated on Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden). Following two washings in PBS, the cells were suspended in RPMI-1640 (Flow Laboratories, Irvine, Scotland, UK) supplemented with glutamine, HEPES buffer, 1% Ultroser G®, Flow Laboratories), penicillin and streptomycin in adequate amounts. In individual experiments 10% NHS, either native, heat-inactivated, or mixtures between native and heat-inactivated was used. Cell concentrations were adjusted to 1000 000 PBMC/ml in supernatant (SN) cultures.
Preparation of heat-aggregated gamma globulin (HAGG)
HAGG IA was prepared as a source for artificial IC. Human IgG (Gammagard, Baxter, Belgium), 50 mg/ml, was heated to 63°C for 30 min, and diluted in PBS to the desired concentration immediately prior to cell culture. As the same mIgG preparation without heat aggregation served as control, IA and controls differed only in steric composition.
Cytokine enzyme-linked immunosorbent assays (ELISAs)
SN were harvested after 20 h of incubation. Previous investigations had shown that IC-induced cytokine levels in serum containing PBMC cultures to be maximal at this point. ELISAs were performed following a standard protocol [9], except that the alkaline phosphatase system was exchanged for a horseradish peroxidase system employing 3,3′-5,5′-tetramethylbenzidine (TMB; Dako A/S, Glostrup, Denmark) as substrate for the IL-10 and IL-12 ELISAs. Monoclonal antibodies 13A5 (IL-6), 9D7 (IL-10) and IL-12-I (IL-12) were used as primary antibodies at a concentration of 1 µg/ml, and biotinylated 39C3 (IL-6), 12G8 (IL-10), IL-12-II (IL-12p40) and IL-12-III (IL-12p35/p70) were used as secondary antibodies at concentrations of 2, 1 and 1 µg/ml, respectively. Monoclonal antibodies against IL-6 and IL-10 were obtained from Pharmingen (San Diego, CA, USA) and against IL-12 from Mabtech (Stockholm, Sweden). Standard curves were constructed with recombinant IL-6, IL-12p70 (R&D systems, Abingdon, UK) and IL-10 (a kind gift from Dr Satwant Narula, Schering Plough Research Institute, Kenilworth, NJ, USA). In additional attempts to measure the low IL-12p70 values, other ELISA reagents (IL-12p70 Duoset, R&D systems) were also used, without further success.
Reagents for blocking of cell surface receptors, complement activation and functional cytokine effects
For blocking studies of the interaction between IA and cell surface receptors, monoclonal antibodies IV.3 (Fab fragment) against FcγRII, preferentially recognizing FcγRIIa [13] and 3G8 (F(ab′)2 fragment) against FcγRIII were obtained from Medarex (Nutley, NY, USA). PBMC were incubated with FcγR blocking antibodies (1·5 µg/ml, optimal concentration defined in titration experiments) for 30 min at 4°C before the addition of HAGG or mIgG. The cell suspensions were then incubated 20 h before harvesting SN. Neutralization of IL-10 and IL-12 was performed with polyclonal goat antibodies with non-specific goat IgG as negative control (20 µg/ml, R&D systems). The circular peptide compstatin or a reduced linear control peptide (generous gifts from Professor John Lambris, Pennsylvania University, PA, USA) were added in varied concentrations to human native serum for 15 min at 37°C. Thereafter HAGG or mIgG (final concentration in cell cultures 100 µg/ml) were added for additional 20 min at 37°C before addition to cell cultures.
The following antibodies and reagents were used in attempts to block IC-induced responses through complement receptors: J3D3, 10 µg/ml (mouse IgG1, against human CR1 (CD35), Immunotech), 44, 10 µg/ml (mouse IgG1, against human CR3 (CD11b); Sigma), 2LPM19c 6·7 µg/ml (mouse IgG1, against human CR3 (CD11b), Dako), S5/1 10 µg/ml (mouse IgG2a against human CD88/C5aR, Immunotech); soluble CR1 (CD35), 50 µg/ml (a kind gift from Henry Marsh, Avant Pharmaceutical), peptide A7 (280 µg/ml synthesized by Åke Engström, Biomedical Center, Uppsala, Sweden) for the functional blocking of CR3(CD11b) [14]). As control antibodies H5 and 7-B4 (mouse IgG1 and IgG2a, respectively, kind gifts from Birgitta Heyman, Uppsala, Sweden) were used.
Statistics
Non-parametric statistics were used throughout the study. For comparisons between cytokine production under various cell culture conditions, and also for the comparison between HAGG- and mIgG-stimulated cultures, the Wilcoxon signed rank test was used. As IA stimulations always were performed with simultaneous stimulation of parallel cell cultures with HAGG and with mIgG, IA stimulation or suppression of cytokine responses were expressed as net HAGG cytokine induced response (pg/ml of cytokine in HAGG-stimulated cultures − pg/ml of cytokine in mIgG-stimulated cultures). Positive figures therefore denote stimulatory effects of IA, whereas negative figures denote inhibitory effects. P-values less than 0·05 were regarded as significant.
RESULTS
Opposite effects of heat-inactivation of NHS on the IA-induced production of IL-6/IL-10 and IL-12
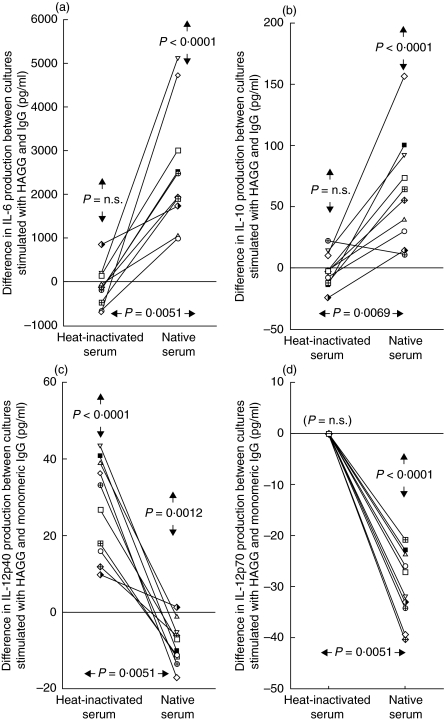
When PBMC from 10 healthy individuals were investigated in parallel concerning the effects of HAGG on cytokine production in medium containing native or heat-inactivated serum, uniform results appeared. A net stimulatory effect of HAGG on the production of IL-6 and IL-10 (P < 0·0001 for both IL-6 and IL-10) was found for all individuals in cultures with native serum. This effect was abolished when heat-inactivated serum was used (differences between sera P = 0·0051 and P = 0·0069, respectively, Fig. 1a,b). For the production of IL-12p40 the opposite situation was found, as all individuals but one showed an inhibitory effect on cytokine production of HAGG compared to monomeric IgG (mIgG) in native serum (P = 0·0012). On the contrary, all individuals showed a stimulatory effect of HAGG compared to mIgG on IL-12p40 production in heat-inactivated serum (difference between sera P = 0·0051, Fig. 1c). Parallel data were also obtained for IL-12p70 (Fig. 1d). IL-12p70 production was however, generally lower than for IL-12p40, and measurable quantities could be recorded only in all cultures with native serum and mIgG, whereas HAGG-stimulated cultures and cultures with heat-inactivated serum consistently yielded non-measurable amounts of IL-12p70. Addition of mIgG to cell cultures generally differed minimally from unstimulated cultures (data not shown).
Fig. 1.
Effects of native and heat-inactivated NHS on IA-induced cytokine production in 10 PBMC donors. PBMC (106/ml) were stimulated with HAGG or mIgG (100 µg/ml) in media containing 10% native or heat-inactivated NHS. After 20 h SN were collected and levels of (a) IL-6, (b) IL-10, (c) IL-12p40 and (d) IL-12p70 analysed by ELISA. Results are shown as differences in cytokine production between cultures stimulated with HAGG and mIgG, with a positive value signifying higher cytokine levels in SN in HAGG cultures compared to mIgG SN. P-values within vertical arrows in the upper part of the figures apply to the net differences between HAGG and mIgG-stimulated cultures. P-values within horizontal arrows in the lower part of the figures apply to differences in net HAGG-induced effects between cell cultures with heat-inactivated and native serum.
To exclude that the effects were dependent on a single serum, these experiments were repeated using two PBMC donors investigated with four different sera. The results obtained were compatible with the results in Fig. 1 (data not shown).
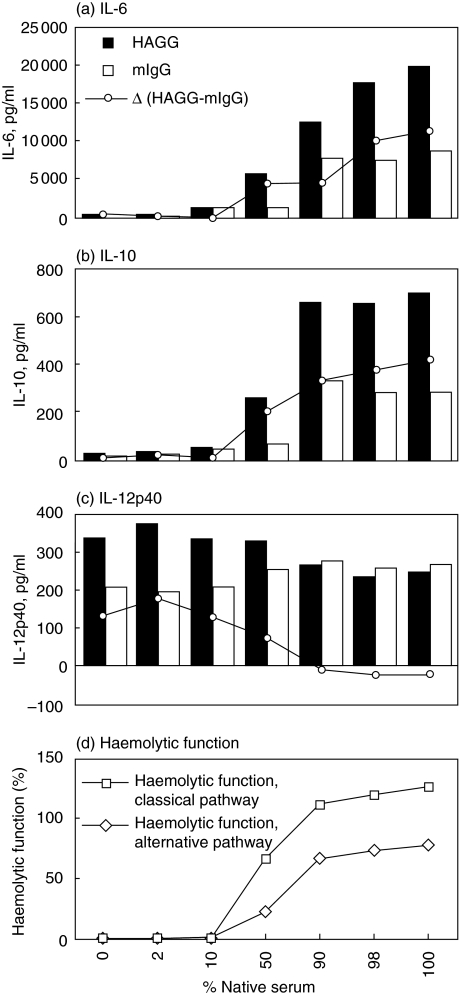
When normal serum was heat-inactivated and then mixed with the corresponding native serum in different ratios, the findings shown in Fig. 1 were found to be gradual. By increasing the percentage of native serum, the net HAGG-induced production of IL-6 and IL-10 both successively increased (Fig. 2a,b), whereas parallel measurement of IL-12p40 in the same cultures changed from net stimulation to net suppression of IL-12p40 production (Fig. 2c). As expected, addition of native serum to heat-inactivated serum dose-dependently restored complement function (Fig. 2d). Gradual substitution of the heat-inactivated serum with native serum increased the production of IL-6 and IL-10 both in cultures stimulated with HAGG and mIgG. The effect of native serum was most pronounced in HAGG-stimulated cultures, resulting in a net stimulatory effect of IA (Fig. 2a,b).
Fig. 2.
Effects of gradual heat-inactivation on IA-induced cytokine production. The same normal human serum as in Fig. 1 was heat-inactivated and mixed with native serum to obtain variation in percentage of native serum, and added (10% v/v of human serum altogether) to separate cell cultures. PBMC (106/ml) from a healthy donor were stimulated with HAGG or mIgG (100 µg/ml) and SN were collected after 20 h incubation and levels of (a) IL-6, (b) IL-10 and (c) IL-12p40 analysed by ELISA. In (d) functional activity of the classical and alternative complement pathways (12) of the corresponding serum mixtures in RPMI are shown. Data from one of four PBMC donors with similar results is shown.
Supplementation of a complement-deficient serum restores the IC-inhibitory effects on IL-12p40 production
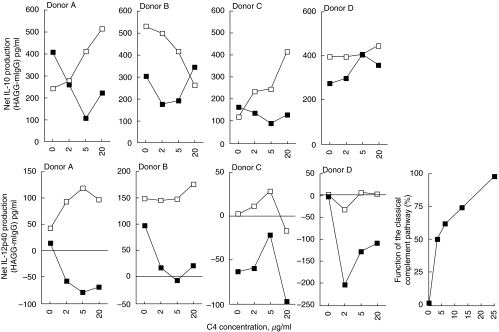
When normal native serum was replaced by a C4 deficient serum from an SLE patient and this serum gradually was reconstituted with C4, the effect differed between the cytokines. Addition of C4 either abolished a net positive effect of HAGG or changed the net HAGG-effect on IL-12p40-production from neutral to negative in cell cultures from four different PBMC donors. Addition of C4 to a complement sufficient normal serum did not induce any consistent changes in net HAGG-induced IL-12p40 production. Addition of C4 to the C4-deficient serum, on the other hand, did not induce any consistent effects on net HAGG-induced IL-10 production, (Fig. 3). Normal serum generally stimulated net HAGG-induced IL-6 production to a higher extent than the C4-deficient serum, but the data were inconclusive because of the pronounced IL-6-stimulatory effects of the addition of C4 both in C4 deficient serum and normal control serum (data not shown). IL-12p70 values were below the measurement range.
Fig. 3.
Effects of gradual reconstitution of C4-deficient serum on IA-induced cytokine production. Sera were incubated without or with increasing amounts of C4 together with HAGG or mIgG (100 µg/ml final concentration in SN) for 20 min in 37°C. The mixtures (final serum concentration 10%) were then added to PBMC cultures (106/ml) from four healthy donors. After 20 h SN were collected and levels of IL-10 and IL-12p40 determined by ELISA. Filled symbols show the results of C4 addition to the C4-deficient serum, whereas open symbols represent the results of C4 addition to a complement sufficient normal serum. Results are shown as differences in cytokine production between cultures stimulated with HAGG and mIgG, with a positive value signifying higher cytokine levels in HAGG culture SN compared to mIgG SN. A separate graph shows the effect on the function of the classical complement pathway after addition of increasing amounts of C4 to the C4 deficient serum.
Treatment of native NHS with a peptide blocking complement activation increases net HAGG-induced IL-12p40 production
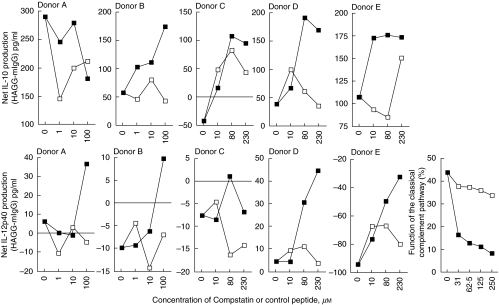
When NHS was treated with the C3 activation blocking peptide compstatin [15] in PBMC cultures from five healthy donors, the net HAGG-induced IL-12p40 production was dose-dependently increased in all cultures, compared to the effect of the same molar concentration of a reduced linear control peptide. Compstatin also increased net HAGG-induced IL-10 production compared to cultures with the linear control peptide in PBMC cultures from four of five donors at the highest compstatin concentration used, but the picture was not as uniform as for IL-12p40 (Fig. 4). IL-6 production was not investigated in this part of the study, and values for IL-12p70 were mostly below the standard curve in the ELISA.
Fig. 4.
Effects of gradual suppression of the classical complement pathway by compstatin. Compstatin (filled symbols) or a reduced control peptide (open symbols) were added to 100% normal human serum for 15 min at 37°C, whereupon HAGG or mIgG (100 µg/ml final concentration in the PBMC cultures) were added for an additional 20 min at 37°C. Sera were thereafter added to cultures (10% v/v) of PBMC from five healthy donors and incubated for 20 h. SN were then collected and analysed for IL-10 and IL-12p40 by ELISA. Results are shown as differences in cytokine production between cultures stimulated with HAGG and mIgG, with a positive value signifying higher cytokine levels in HAGG culture SN compared to mIgG SN. Compstatin concentrations in the graphs indicate final concentration in cell cultures. A separate graph show the effect on the classical complement function after addition of increasing amounts of compstatin (filled symbols) and control peptide (open symbols). The cytokine data are assembled from two experiments using different concentration ranges for compstatin.
Weak interdependency between IA-induced production of IL-10 and IL-12
Neutralization of IL-12 in HAGG cultures with heat-inactivated sera marginally increased the production of IL-10, whereas no effect was shown in native serum where the corresponding IL-12-levels were low. On the contrary, neutralization of IL-10 showed no obvious effects on IL-12p40 production in any system investigated (data not shown).
IC-induced IL-10 production is partially dependent on FcγRII, whereas IC-induced IL-12 production is not
In a recent paper we have shown that IC-induced production of IL-10 is partially dependent on FcγRII [9]. Similar findings were also obtained in the present study. Net IA-induced IL-10 production was, as expected, evident only in native serum, and in this medium anti FcγRII decreased HAGG-induced IL-10 production by 36%, whereas no suppression was noted with FcγRIII antibody fragments. No effect of blocking FcγRII or FcγRIII on IA-induced IL-12 production was noted with the antibody concentrations found to suppress the IA-induced production of IL-10 (data not shown).
No effect of complement receptor blockade on IA-induced production of IL-12p40
A panel of antibodies, peptides and soluble complement receptors was used in an attempt to functionally block the interaction between IC and CR1 (CD35), CR3 (CD11b) and CD88 (C5a receptor). No effect was noted on the production of IL-12p40 either in native or in heat-inactivated sera using concentrations earlier reported to functionally block the receptors. Also for IL-10, no consistent findings were obtained from complement receptor blocking experiments (data not shown).
DISCUSSION
In this paper we show that IA-induced production of IL-6/IL-10 and IL-12 are regulated separately. The effects on IL-10 differ from the effects on IL-12; a cytokine with opposite biological effects to IL-6/IL-10. In PBMC cultures with native NHS, IA stimulated the production of IL-6 and IL-10 while the production of IL-12 was inhibited, compared to control cell cultures. This inhibiting effect on IL-12 production was reversed when serum was heat-inactivated, while the same treatment abolished the stimulatory effect of IA on IL-6 and IL-10 production. When NHS was exchanged with a C4-deficient serum devoid of functional classical complement pathway, IA either showed weak stimulatory effects or no effects on IL-12p40 production. Restoration of complement function in this serum with C4 neutralized or inverted the enhancing effects on IA-induced IL-12p40 production, while at the same time no consistent effects were seen on the production of IL-10. In a third experimental setting we added compstatin, a C3-binding peptide that inhibits complement activation, to native NHS. In all five experiments performed, this treatment induced a higher IA-induced production of IL-12p40 compared to a reduced linear control peptide. IL-10 production, however, also showed an increase in four of five PBMC cultures. The effects on IL-10 production were mediated partially through FcγRII in cultures with native NHS, while the IA-induced effects on IL-12p40 production are mediated most probably through other means than FcγRII or FcγRIII. However, no reagents used for the blocking of complement receptors CR1 (CD35), CR3 (CD11b) or CD88/C5a receptor have hitherto shown any consistent effect on the IA-stimulated production of IL-10 or IL-12p40. We will proceed in investigating these and other receptors utilizing additional blocking agents.
The three systems used for the studies of IC-induced cytokine production differ considerably, and all three have limitations. Heat-inactivation arrests both the classical and the alternative pathway of complement activation, and act primarily on the heat-labile complement components C1q and factor B. Additional serum proteins might, however, also be denatured or damaged by heating. The C4 deficient serum was obtained from an SLE patient, and increased levels of many cytokines, including IL-6 and IL-10, as well as IC and autoantibodies, are found regularly in SLE sera. Compstatin is known to inhibit the classical pathway of complement activation at low doses, but will also block the alternative pathway when higher concentrations are used [15]. Notwithstanding these methodological differences, inhibition of complement activity uniformly resulted in a relative HAGG-induced increase in the production of IL-12p40.
Theoretically, two independent systems might influence IL-12p40 production in cell cultures stimulated with IC. One system can be enhancing, shown by the stimulatory effects of IC in heat-inactivated NHS, in C4 deficient serum or in compstatin-treated native NHS. In the presence of a functionally active complement system, a suppressive system is added and the net effect instead becomes inhibitory, or at least neutralizes the stimulatory effects of IC. This latter system varies with the degree of complement function of the classical complement pathway. IC induce the classical complement pathway, thereby exposing an array of complement products on the IC surfaces, together with soluble products of complement activation. The effects of IA on IL-12 production found in native serum or in reconstituted C4 deficient serum could possibly be an effect exerted through one or several of these components. Recently two papers have shown strong inhibitory effects on monocyte IL-12 production exerted by the anaphylatoxin C5a in human in vitro cultures [16,17].
One limitation of our study is the low levels of IL-12p70 measured in the SN. Despite the use of two different ELISA systems, the levels were very low and were only possible to interpret for the experiments using heat-inactivation, where the results for IL-12p70 were in agreement with those for IL-12p40. As the p40 homodimer has been shown to function as an IL-12p70 antagonist [18], we are also interested in evaluating the IC-induced effects on IL-12p70 production in other cell culture systems.
The different effects of IA on the production of IL-10 and IL-12 in systems with different functional activities of the classical complement cascade can have importance for the natural course in rheumatic diseases with local or systemic activation of the complement system, as well as in malignant and lymphoproliferative diseases. In SLE, an IC-mediated systemic autoimmune disease with hyperactive B-lymphocytes and increased spontaneous antibody production, the production of IL-10 [19] and IL-6 [20] from monocytes is increased. The expression and levels of IL-12 are, on the other hand, decreased and correlated negatively with disease activity and IL-10 levels [21,22].
The counteracting effects between IL-10 and IL-12 are pivotal also in RA pathogenesis: levels of IL-12 are increased in RA joints compared to peripheral blood [23,24] and the production of IFN-γ is increased accordingly in arthritis joints [25]. Exogenously delivered IL-12 has been shown to induce RA exacerbation [26]. Stimulation with type 2 cytokines such as IL-10 have been found beneficial in experimental arthritis models [27] and also in RA in vitro [28], even if large-scale clinical studies hitherto have been disappointing.
IL-12 is important for the T cell defence against tumours [29]. IL-10 can, on the other hand, stimulate tumour growth through a general inhibition of cytokine production in tumour-infiltrating macrophages [30]. Many solid tumours and leukaemias are associated with circulating IC [31] that might be associated with immune suppression, both in experimental tumours in mice [32] as well as in human tumours [33]. Such immune suppression is dependent on intact Fc fragments, as IC containing only F(ab′)2 fragments are ineffective. The suppressive effect is dependent on serum, and a synergy with non-defined serum factor(s) has been shown [34]. These data are compatible with the hypothesis that IC-mediated and FcγR-dependent production of IL-10 can be responsible for the immune suppression, and that serum with an intact complement system is needed for IC-augmented IL-10 production in parallel to suppressed production of IL-12.
In RA and SLE as well as in malignant diseases the findings described in this paper might have either beneficial or detrimental consequences, dependent on the current access to a functionally active complement system. Further studies concerning the mechanisms behind our findings are warranted.
Acknowledgments
This investigation was supported by grants from the Swedish Research Council, the Swedish Society of Medicine, King Gustav V's 80-years Fund, the Swedish League Against Rheumatism, the Ugglas Foundation, the Lions foundation, the Golge Foundation, the Jeppsson Foundation, the Hierta Foundation, the Crafoord Foundation, the Groschinsky Foundation, the Grönberg Foundation, the Bergvall foundation, the Dahlin foundation, the Carlsson foundation, the Viberg foundation, the Nanna Svartz Foundation and the Swedish fund for research without animal experiments. We thank Dr R. A. Harris for linguistic advice.
References
- 1.Isomäki P, Luukkainen R, Saario R, Toivanen P, Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996;39:386–95. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- 2.Adorini L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell Mol Life Sci. 1999;55:1610–25. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 4.Wilcoxen SC, Kirkman E, Dowdell KC, Stohlman SA. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J Immunol. 2000;164:6237–43. doi: 10.4049/jimmunol.164.12.6237. [DOI] [PubMed] [Google Scholar]
- 5.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–9. [PubMed] [Google Scholar]
- 6.Heike M, Schlaak J, Heyl S, Schulze-Bergkamen H, Schmitt U, Meyer zum Buschenfelde KH. Contrary roles of IL-4 and IL-12 on IL-10 production and proliferation of human tumour reactive T cells. Scand J Immunol. 1997;45:221–6. doi: 10.1046/j.1365-3083.1997.d01-386.x. [DOI] [PubMed] [Google Scholar]
- 7.Berger S, Balló H, Stutte HJ. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes. a network of pro- and antiinflammatory cytokines dependent on the antigen-antibody ratio. Eur J Immunol. 1996;26:1297–301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 8.Berger S, Ballo H, Stutte HJ. Distinct antigen-induced cytokine pattern upon stimulation with antibody-complexed antigen consistent with a Th1 − >Th2-shift. Res Virol. 1996;147:103–8. doi: 10.1016/0923-2516(96)80223-8. [DOI] [PubMed] [Google Scholar]
- 9.Rönnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann Rheum Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger S, Chandra R, Balló H, Hildenbrand R, Stutte HJ. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. Eur J Immunol. 1997;27:2994–3000. doi: 10.1002/eji.1830271136. [DOI] [PubMed] [Google Scholar]
- 11.Lundwall A, Malmheden I, Stålenheim G, Sjöquist J. Isolation of component C4 of human complement and its polypeptide chains. Eur J Biochem. 1981;117:141–6. doi: 10.1111/j.1432-1033.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson UR, Nilsson B. Simplified assays of hemolytic activity of the classical and alternative pathways. J Immunol Meth. 1984;72:49–59. doi: 10.1016/0022-1759(84)90432-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Herik-Oudijk IE, Westerdaal NA, Henriquez NV, Capel PJ, Van De Winkel JG. Functional analysis of human Fc gamma RII (CD32) isoforms expressed in B lymphocytes. J Immunol. 1994;152:574–85. [PubMed] [Google Scholar]
- 14.Mesri M, Plescia J, Altieri DC. Dual regulation of ligand binding by CD11b I domain. Inhibition of intercellular adhesion and monocyte procoagulant activity by a factor X-derived peptide. J Biol Chem. 1998;273:744–8. doi: 10.1074/jbc.273.2.744. [DOI] [PubMed] [Google Scholar]
- 15.Sahu A, Soulika AM, Morikis D, Spruce L, Moore WT, Lambris JD. Binding kinetics, structure–activity relationship, and biotransformation of the complement inhibitor compstatin. J Immunol. 2000;165:2491–9. doi: 10.4049/jimmunol.165.5.2491. [DOI] [PubMed] [Google Scholar]
- 16.Wittmann M, Zwirner J, Larsson VA, et al. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–9. [PubMed] [Google Scholar]
- 17.Braun MC, Lahey E, Kelsall BL. Selective suppression of IL-12 production by chemoattractants. J Immunol. 2000;164:3009–17. doi: 10.4049/jimmunol.164.6.3009. [DOI] [PubMed] [Google Scholar]
- 18.Ling P, Gately MK, Gubler U, et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–27. [PubMed] [Google Scholar]
- 19.Llorente L, Richaud-Patin Y, Wijdenes J, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw. 1993;4:421–7. [PubMed] [Google Scholar]
- 20.Lacki JK, Samborski W, Mackiewicz SH. Interleukin-10 and interleukin-6 in lupus erythematosus and rheumatoid arthritis, correlations with acute phase proteins. Clin Rheumatol. 1997;16:275–8. doi: 10.1007/BF02238963. [DOI] [PubMed] [Google Scholar]
- 21.Liu TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 22.Liu TF, Jones BM. Impaired production of IL-12 in system lupus erythematosus. II. IL-12 production in vitro is correlated negatively with serum IL-10, positively with serum IFN-gamma and negatively with disease activity in SLE. Cytokine. 1998;10:148–53. doi: 10.1006/cyto.1997.0269. [DOI] [PubMed] [Google Scholar]
- 23.Bucht A, Larsson P, Weisbrot L, et al. Expression of interferon-gamma (IFN-gamma), IL-10, IL-12 and transforming growth factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA) Clin Exp Immunol. 1996;103:357–67. doi: 10.1111/j.1365-2249.1996.tb08288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim W, Min S, Cho M, et al. The role of IL-12 in inflammatory activity of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 2000;119:175–81. doi: 10.1046/j.1365-2249.2000.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rönnelid J, Berg L, Rogberg S, Nilsson A, Albertsson K, Klareskog L. Production of T-cell cytokines at the single-cell level in patients with inflammatory arthritides: enhanced activity in synovial fluid compared to blood. Br J Rheumatol. 1998;37:7–14. doi: 10.1093/rheumatology/37.1.7. [DOI] [PubMed] [Google Scholar]
- 26.Peeva E, Fishman AD, Goddard G, Wadler S, Barland P. Rheumatoid arthritis exacerbation caused by exogenous interleukin-12. Arthritis Rheum. 2000;43:461–3. doi: 10.1002/1529-0131(200002)43:2<461::AID-ANR29>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim KN, Watanabe S, Ma Y, Thornton S, Giannini EH, Hirsch R. Viral IL-10 and soluble TNF receptor act synergistically to inhibit collagen-induced arthritis following adenovirus-mediated gene transfer. J Immunol. 2000;164:1576–81. doi: 10.4049/jimmunol.164.3.1576. [DOI] [PubMed] [Google Scholar]
- 28.Chomarat P, Vannier E, Dechanet J, et al. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432–9. [PubMed] [Google Scholar]
- 29.Golab J, Zagozdzon R. Antitumor effects of interleukin-12 in pre-clinical and early clinical studies. Int J Mol Med. 1999;3:537–44. doi: 10.3892/ijmm.3.5.537. [DOI] [PubMed] [Google Scholar]
- 30.Salazar-Onfray F. Interleukin-10: a cytokine used by tumors to escape immunosurveillance. Med Oncol. 1999;16:86–94. doi: 10.1007/BF02785841. [DOI] [PubMed] [Google Scholar]
- 31.Segal-Eiras A, Croce MV. Immune complexes in human malignant tumours. A review. Allergol Immunopathol. 1984;12:225–32. [PubMed] [Google Scholar]
- 32.Gorczynski RM, Kilburn DG, Knight RA, Norbury C, Parker DC, Smith JB. Nonspecific and specific immunosuppression in tumour-bearing mice by soluble immune complexes. Nature. 1975;254:141–3. doi: 10.1038/254141a0. [DOI] [PubMed] [Google Scholar]
- 33.Jerry LM, Lewis MG, Cano P. Anergy, anti-antibodies and immune complex disease: a syndrome of disordered immune regulation in human cancer. In: Martin M, Dionne L, editors. Immunocancerology in solid tumors. New York: Stratton; 1976. pp. 63–79. [Google Scholar]
- 34.Kilburn DG, Fairhurst M, Levy JG, Whitney RB. Synergism between immune complexes and serum from tumor-bearing mice in the suppression of mitogen responses. J Immunol. 1976;117:1612–7. [PubMed] [Google Scholar]