Abstract
Common variable immunodeficiency (CVID) is a heterogeneous immunodeficiency that is accompanied by granulomatous lesions in 5–10% of cases. Why some patients develop granulomatous disease remains unclear. Here we describe a 12-year-old previously healthy girl who presented with pancytopenia and granulomatous lymphoproliferation subsequent to infection with Toxoplasma gondii. Loosely arranged non-fibrosing granulomas were observed in the liver, lymph nodes and lung, but no Toxoplasma tachyzoites could be demonstrated and polymerase chain reaction (PCR) and culture were negative for Toxoplasma and a wide range of other pathogens. While the patient had a normal peripheral B cell status at presentation, the development of CVID could be observed during the following months, leading to a loss of memory B cells. This was accompanied by an increasingly activated CD4+ T cell compartment and high serum levels of angiotensin-converting enzyme (ACE), tumour necrosis factor (TNF) and sCD25. Steroid therapy reduced pancytopenia, granulomatous lymphoproliferation and cytokine elevations, but did not improve the B cell status. This is the first report of an association of Toxoplasma infection with granulomatous CVID and provides one of the rare examples where the onset of CVID could be documented subsequent to an infectious disease.
Keywords: common variable immunodeficiency, granuloma, hypogammaglobulinaemia, sarcoidosis, Toxoplasma gondii
INTRODUCTION
Common variable immunodeficiency (CVID) is the phenotypic description of a clinically heterogeneous disorder. Several genetically defined primary immunodeficiency diseases can show a phenotype consistent with CVID, including hyper-IgM syndrome [1], X-linked lymphoproliferative disease [2] and inducible co-stimulator (ICOS) deficiency [3]. To date, however, the contribution of a genetic predisposition to CVID remains unclear in the majority of cases [4]. Infectious diseases have been implicated as a trigger for CVID [5] and a particularly well-characterized example is the development of CVID in some patients with X-linked lymphoproliferative disease who survive an acute Epstein–Barr virus (EBV) infection [2,6].
In approximately 5–10% of patients with CVID, sarcoid-like granulomatous lesions have been reported [7,8]. Non-caseating epitheloid granulomas have been found in the liver, spleen, lung, lymph nodes, bone marrow, skin and conjunctivae. It has been suggested that granulomatous CVID could be an atypical presentation of sarcoidosis on the genetic background of immunodeficiency [7]. Alternatively, the immunodeficiency could lead to a dysregulated immune response to an infectious agent resulting in widespread granulomatous reactions. However, no association of granulomatous CVID with particular infections has been reported. Overall, neither the pathogenesis of sarcoidosis nor that of CVID are well understood and the complex relationship between immunodeficiency, infection and formation of granulomas remains elusive.
Here we report a 12-year-old previously healthy patient who was diagnosed with pronounced granulomatous lymphoproliferation after infection with Toxoplasma gondii and subsequently developed CVID.
MATERIALS AND METHODS
Antibodies and flow cytometry
For surface stainings, 2 × 105 peripheral blood mononuclear cells (PBMCs) were stained for 30 min at 4°C with the following anti-human antibodies: CD4-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE), CD4-Cy-chrome (Cy) (clone RPA-T4), CD8-Cy (clone RPA-T8), CD3-antigen-presenting cell (APC) (clone HIT 3a), CD45RA-PE (clone HI 100), HLA-DR-FITC (clone G46-6), CD19-APC (clone HIB 19), CD27-FITC (clone M-A251), anti-IgD-PE (clone IA 6–2), anti-IgM-biotinylated (clone G20-127) and streptavidin-Cy (all antibodies from BD PharMingen, San Diego, CA, USA). T cell receptor (TCR) Vβ staining was performed using a panel of antibodies obtained from Immunotech, Hamburg, Germany). For intracellular stainings, 2 × 105 PBMCs in 200 µl Iscove's Modified Dulbecco's Medium (IMDM) 10% fetal calf serum (FCS) supplemented with 1 µl/ml Monensin (Golgistop, BD PharMingen) were stimulated for 3 h with phorbol myristate acetate (PMA) (final concentration: 2 × 10−6m) and ionomycin (final concentration: 10 µg/ml) in 96-well plates at 37°C. After 3 h, cells were harvested, washed and surface-stained. Intracellular cytokine staining was performed using the cytofix/cytoperm kit according to the instructions of the manufacturer (BD PharMingen). Cells were either stained with interferon (IFN)-gamma-PE (clone 4S.B3), tumour necrosis factor (TNF)-alpha-PE (clone MoAb11) or with isotype-PE (clone MOPC-21). Four-colour data acquisition was performed with a FACSCalibur (Becton Dickinson, Heidelberg, Germany). Data analysis was performed using CellQuest Software (Becton Dickinson Biosciences).
CFSE proliferation assay
PBMCs (5 × 106) were labelled with the fluorescent dye CFSE (Molecular Probes, Eugene, OR, USA) for 10 min and washed twice with ice-cold phosphate buffered saline (PBS); 2 × 105 CFSE-labelled PBMCs in a volume of 200 µl IMDM 10% FCS were stimulated with either ConA (10 µg/ml), ConA supplemented with interleukin (IL)-2 (20 U/ml) or phytohaemagglutinin (1·25 µg/ml) at 37°C. Control cells were cultivated in medium alone. On day 6, the cells were harvested, washed, surface-stained with anti-CD3 and anti-CD4 or anti-CD8 antibodies and analysed by flow cytometry.
Cytokine assays
Serum cytokines were determined using a solid-phase, two-site chemiluminescent enzyme immunometric assay on an automated analyser.
Histology and immunohistochemistry (IHC)
For histology and immunohistochemistry (IHC), formalin-fixed, paraffin-imbedded tissue blocks of lymph nodes, liver and lung were used. IHC of paraffin-embedded material was performed as described [9] using the Histostain™-Plus Staining kit (Zymed, S. San Francisco, CA, USA) in a Genesis PSP 200 robot (Tecan, Crailsheim, Germany). Antibodies used for IHC were provided by Novocastra (Dossenheim, Germany): anti-CD3 (1 : 20; clone PS1); anti-CD4 (1 : 40; clone 1F6); by Dako (Hamburg, Germany): anti-CD20 (1 : 2000; L26); anti-CD8 (1 : 10). Rabbit antisera were provided by Dako: anti-heavy chains of IgA, IgG, IgM (1 : 12000); anti-light chains κ/λ (1 : 8000 and 1 : 6000, respectively).
CASE REPORT
Lymphoproliferative granulomatous disease in a patient with acute toxoplasmosis
A 12-year-old girl presented with a 1-week history of fever, fatigue, malaise, weight loss and increasing abdominal circumference. The girl had been previously healthy with no particular susceptibility to infectious diseases. She had undergone uncomplicated vaccination with Bacille Calmette–Guérin (BCG) and the measles, mumps, rubella vaccine, had had an uneventful recovery from varicella infection and there was no history of significant airway infections. Two years before presentation she had received abdominal ultrasound for suspected appendicitis and was documented to have normal-sized spleen and liver with no structural abnormalities. Fifteen months before presentation she had had a prolonged facial fungal skin infection with Trichophyton rubrum, which resolved after 6 weeks of treatment with systemic itroconazole and local sertoconazole.
At presentation, she had pancytopenia (leucocytes 1560/µl, thrombocytes 73·000/µl, haemoglobin 11·1 g/dl) and slightly elevated C-reactive protein (15 mg/l). A chest X-ray was normal, but diffuse small nodular pulmonary lesions were found on computed tomography (CT) scan. Further imaging showed hepatomegaly and massive splenomegaly with tiny nodules dispersed throughout the splenic parenchyma and mediastinal as well as intra-abdominal lymphadenopathy. Extensive screening for infectious disease was performed, including typical and atypical mycobacteria, cryptococcus, aspergillus, histoplasma, pneumocystis, mycoplasma, bartonella, brucella, Toxoplasma, HIV, cytomelovirus (CMV) and Epstein–Barr virus (EBV). All results were negative except for elevated IgG [immunofluorescent test (IFT) 1 : 512] and IgA [immunosorbent agglutination assay (ISAGA) 1 : 64] antibody titres to T. gondii. Within the next 2 weeks there was a fourfold rise in Toxoplasma-specific IgG (IFT 1 : 8192) and a 12-fold rise in IgA (ISAGA > 1 : 4086). A recent infection with T. gondii was diagnosed, but this could neither explain the pulmonary findings nor the extensive lymphoproliferation. No Toxoplasma-specific antibiotic therapy was initiated.
Immunohistological characterization of the granulomatous lesions
Ten days after admission, a diagnostic laparotomy was performed with histopathological examination of abdominal lymph node and liver biopsy specimens.
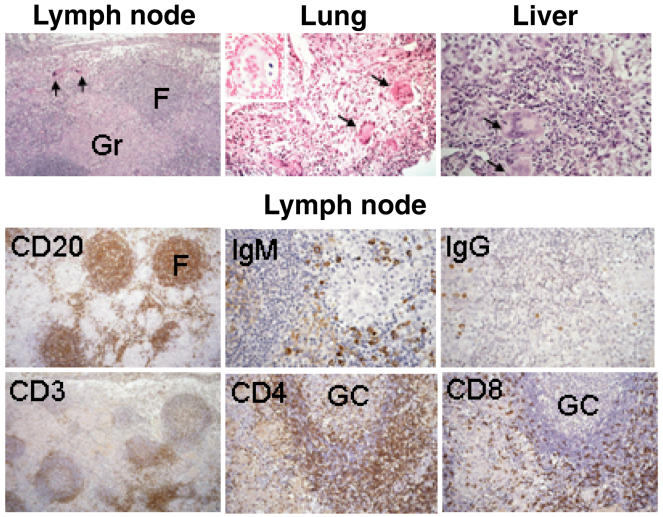
A transbronchial lung biopsy was performed 2 years later. In none of these specimens could Toxoplasma tachyzoites be demonstrated. Polymerase chain reaction (PCR) and culture were negative for Toxoplasma as well as for a large range of other pathogens, including mycobacteria, various viruses and fungi. The lymph node showed extensive non-caseating granulomas (Fig. 1). They were non-fibrosing, did not displace the structure of B cell follicles and were located in the T cell zone. The paracortical T cell zone was strongly reduced, there was no immature sinus histiocytosis that is characteristic of toxoplasmosis. There was a predominance of CD4+ T cells, which were also present in the mantle zone of lymphoid follicles with germinal centres. Stainings for immunoglobulins revealed few IgG+ plasma cells. There were numerous IgM-producing cells, while no IgA-producing cells could be demonstrated (Fig. 1). The lung biopsy revealed a fibrosing interstitial pneumonia with numerous scattered multi-nucleated giant cells (Fig. 1). Some giant cells had inclusion bodies staining positive for iron (Fig. 1, insert). Similar cellular aggregates consisting of mononuclear histiocytes and multi-nucleated histiocytic giant cells were found in the liver. However, neither in the lung nor in the liver were well-developed granulomas characteristic of sarcoidosis evident.
Fig. 1.
Histological analysis of perihepatic lymph node and liver biopsies obtained at diagnosis and a lung biopsy obtained 2 years later. H&E stains (upper panel) and immunohistological stains with the indicated antibodies (lower panels) are shown. The insert in the lung section shows a multinucleated giant cell with inclusion bodies positive for iron. Arrows indicate multinucleated giant cells. F: lymph node follicles, Gr: granulomas, GC: germinal centre.
Rapid decline in B cell function subsequent to Toxoplasma infection
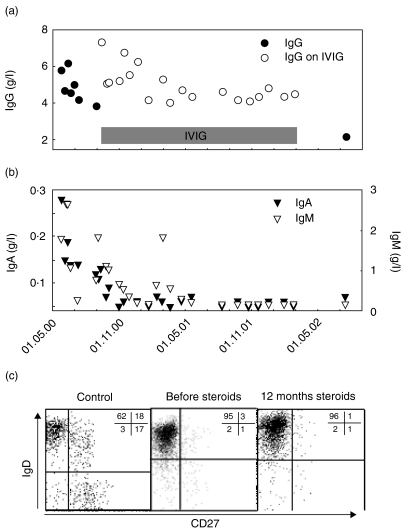
At the time of presentation, the patient was able to mount normal antibody responses, as evidenced by the strong serological response to Toxoplasma in two antibody classes. Retrospectively determined specific IgG to diphteria and tetanus antigens were in the normal range, but there were no antibodies to polio virus, despite regular childhood vaccination. Total IgG was slightly diminished, IgA was normal and IgM slightly elevated (Fig. 2a,b). Flow cytometry 5 weeks after admission showed a normal distribution of lymphocyte subsets with 15% B cells, 8% natural killer (NK) cells and 74% T cells with a CD4/CD8 ratio of 2·5 and an absolute CD4 count of 716/µl. Within the next 4 months, IgG dropped to 3·8 g/l and IgA and IgM dropped below detection limit (Fig. 2a,b). The diagnosis of granulomatous CVID was made and the patient was treated with IVIG (400 mg/kg every 4 weeks) and prednisone (maintenance dose of 10 mg/d). The pancytopenia improved, the hepatosplenomegaly regressed and there were no infectious complications. One-and-a-half years later the patient stopped all treatment. Five months after that she was admitted with severe sepsis caused by Group C streptococci. At that time, IgG had dropped to 2·0 g/l (Fig. 2a); IgA and IgM were undetectable (Fig. 2b). Antibodies to Toxoplasma could no longer be detected. The percentage of B cells had decreased to 5%, the absolute number was 77/µl (age-related normal range: 200–600/µl). Analysis of the B cell phenotype revealed an almost complete absence of CD27+ memory B cells including IgD− switched memory B cells (Fig. 2c). IVIG treatment was re-initiated, but the patient refused steroids.
Fig. 2.
Course of serum levels for IgG (a), IgA and IgM (b). The horizontal bar indicates the period of IVIG treatment. Expression of CD27 and IgD on CD19+ B cells in peripheral blood obtained from a healthy donor (c; left) and the patient before (c; middle) and after 1 year of steroid therapy (c; right).
Increasing activation status but impaired proliferation of CD4+ T cells
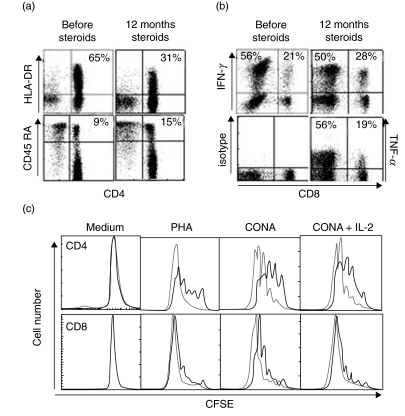
In the absence of steroid therapy the patient again developed pancytopenia and massive splenomegaly. Analysis of the T cell compartment at this time (free from infection and with no steroids for 10 months) revealed a significant increase of the CD4/CD8 ratio to 6·3 with an absolute CD4 count of 985/µl. The CD4+ T cells showed an activated phenotype with 65% expressing HLA-DR (normal range 2–12%) and only 9% being CD45RA+ (normal range 38–72%) (Fig. 3a). Vβ usage among CD4+ T cells in peripheral blood as determined with 21 Vβ-specific antibodies did not differ significantly from five healthy controls. Upon stimulation with PMA and ionomycin, 56% of CD4+ T cells produced IFN-γ and 56% produced TNF (normal range 5–35%) (Fig. 3b). While proliferation of CD8+ T cells to ConA and PHA was similar to healthy control cells, the response of CD4+ T cells was reduced significantly (Fig. 3c). These findings were confirmed on three occasions using three different control donors.
Fig. 3.
T cell status of the patient. The analysis was performed when the patient was free from infection and without steroid therapy for 10 months. Expression of HLA-DR and CD45RO on CD3+ CD4+ T cells in peripheral blood obtained from the patient before (a; left) and after 1 year of steroid therapy (a; right). Intracellular staining for IFN-γ and TNF in short-term stimulated CD3+ T cells (b). Proliferative response of patient (bold line) and healthy donor (slim line) cells to stimulation with the indicated mitogens or medium alone, analysed separately for CD4+ (upper panel) and CD8+CD3+ T cells (lower panel) (c).
Clinical and immunological response to steroid treatment
The patient was encouraged to re-initiate steroid therapy at a maintenance dose of 7·5 mg/d. After 1 year of therapy, the pancytopenia had largely resolved and the spleen size had significantly decreased. A pulmonary CT scan indicated progressive scarring fibrosis of previous lesions rather than actively spreading granulomatous disease. Pulmonary function tests showed mild peripheral obstruction. There were no signs of recovery of the B-cell compartment. However, the CD4/CD8 ratio returned to 3·6 and the signs of T cell activation decreased slightly. Elevated serum levels of TNF, soluble CD25 and ACE also responded well to steroid treatment (Table 1). Of note, in the 3·5 years since diagnosis there have been no episodes of sinopulmonary infections associated typically with CVID, nor have there been any opportunistic infections.
Table 1.
Serum cytokines before and after 1 year of steroid therapy
| Normal values | Diagnosis | Before steroids | 1 year on steroids | |
|---|---|---|---|---|
| TNF (pg/ml) | <3 | 122 | 48 | 16 |
| IL-1β (pg/ml) | <5 | <5 | <5 | <5 |
| sCD25 (U/ml) | <1000 | 8806 | 5323 | 3052 |
| ACE (U/l) | 15–45 | 88 | 186 | 54 |
DISCUSSION
This case report describes the development of granulomatous CVID subsequent to infection with T. gondii. Only a few cases of CVID evolving after an infectious disease have been reported so far. None of these have been related to toxoplasmosis.
The patient had a largely normal immune status at the time of clinical presentation, as indicated by the uneventful vaccination and infection history, normal antibody titres to diphtheria and tetanus, normal B and T cell numbers as well as a brisk response to Toxoplasma in several antibody classes. However, the histological lymph node analysis obtained 10 days after admission revealed relevant changes. Although germinal centres were still intact, only few IgG positive cells and no IgA positive cells could be detected and plasma cells were rare. Subsequently, a decline in total and specific antibody levels could also be observed in peripheral blood. Later analysis revealed absence of memory B cells among both IgM+ and IgM- subgroups corresponding to CVID type Ib according to the classification proposed by Warnatz et al. [10].
The exact role of the Toxoplasma infection in this case is difficult to define. Although there was clear serological evidence for a recent infection, the clinical and histopathological findings were not typical for toxoplasmosis. Also, microbiological studies of lymph node, liver and lung specimen could not detect the parasite in situ. The widespread granulomatous reaction is therefore unlikely to be a consequence of an uncontrolled Toxoplasma infection. It is possible that the toxoplasmosis induced macrophage and T cell activation including production of TNF and other cytokines involved in granuloma formation. However, whether these events triggered or enhanced the granulomatosis must remain speculative. The relationship between the Toxoplasma infection and CVID is also unclear. An existing immunodeficiency may have predisposed to the symptomatic Toxoplasma infection. Chronic toxoplasmosis has been reported in a single CVID patient, but no granulomatous disease was noted [11]. On the other hand, Toxoplasma infection may have enhanced the development of CVID which we could follow closely in the months after the infection.
More than 40 patients with granulomatous CVID have been reported [7,8,11–16] and some common immunological features have been observed. In particular, high levels of TNF and the presence of the TNF allele +488A have been associated previously with the development of granulomatous disease in CVID patients [14]. Most CVID patients with splenomegaly or granulomatous disease also showed a decreased CD4/CD8 ratio, with a predominance of activated CD8+ T cells [17,18]. The drastic polyclonal activation of CD4+ T cells and the significantly increased CD4/CD8 ratio observed in our patient are unusual and add to the heterogeneity of the disease. The histological features are also variable. A few illustrations in previous papers have shown the compact and organized structure of sarcoid granulomas with numerous epitheloid and multi-nucleated giant cells, a dense layer of lymphocytes and concentric fibrosis [8]. In most reports, however, the granulomatous lesions appeared loosely arranged, less well organized and were similar to those in our patient [12,15,16,19–21]. At present, it remains unclear whether this just reflects the ‘immaturity’ of the granuloma or indicates differences in the pathogenesis of granulomatous disease in sarcoidosis versus CVID.
In summary, we report a patient who developed granulomatous CVID subsequent to infection with T. gondii. A better understanding of the relationship between immunodeficiency, infection and formation of granulomas will be needed to establish the pathogenesis of this complex disorder.
Acknowledgments
We thank Professor Marion Schneider (Experimentelle Anästhesie, University of Ulm) for measuring serum cytokines and Professor Matthias Brandis for continuous support. This work was supported by the SFB 620: project A4.
REFERENCES
- 1.Durandy A, Honjo T. Human genetic defects in class-switch recombination (hyper-IgM syndromes) Curr Opin Immunol. 2001;13:543–8. doi: 10.1016/s0952-7915(00)00256-9. [DOI] [PubMed] [Google Scholar]
- 2.Morra M, Silander O, Calpe S, et al. Alterations of the X-linked lymphoproliferative disease gene SH2D1A in common variable immunodeficiency syndrome. Blood. 2001;98:1321–5. doi: 10.1182/blood.v98.5.1321. [DOI] [PubMed] [Google Scholar]
- 3.Grimbacher B, Hutloff A, Schlesier M, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–8. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 4.Vorechovsky I, Cullen M, Carrington M, Hammarstrom L, Webster AD. Fine mapping of IGAD1 in IgA deficiency and common variable immunodeficiency: identification and characterization of haplotypes shared by affected members of 101 multiple-case families. J Immunol. 2000;164:4408–16. doi: 10.4049/jimmunol.164.8.4408. [DOI] [PubMed] [Google Scholar]
- 5.Spickett GP, Farrant J, North ME, Zhang JG, Morgan L, Webster AD. Common variable immunodeficiency: how many diseases? Immunol Today. 1997;18:325–8. doi: 10.1016/s0167-5699(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 6.Soresina A, Lougaris V, Giliani S, et al. Mutations of the X-linked lymphoproliferative disease gene SH2D1A mimicking common variable immunodeficiency. Eur J Pediatr. 2002;161:656–9. doi: 10.1007/s00431-002-1083-9. [DOI] [PubMed] [Google Scholar]
- 7.Fasano MB, Sullivan KE, Sarpong SB, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Baltimore) 1996;75:251–61. doi: 10.1097/00005792-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127:613–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ströbel P, Nanan R, Gattenlöhner S, et al. Reversible monoclonal lymphadenopathy in autoimmune lymphoproliferative syndrome with functional Fas (CD95/APo-1) defiency. Am J Surg Pathol. 1999;23:829–37. doi: 10.1097/00000478-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+) IgM(-) IgD(-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 11.Shachor J, Shneyour A, Radnay J, Steiner ZP, Bruderman I. Toxoplasmosis in a patient with common variable immunodeficiency. Am J Medical Sci. 1984;287:36–8. doi: 10.1097/00000441-198405000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Ameratunga R, Becroft DM, Hunter W. The simultaneous presentation of sarcoidosis and common variable immune deficiency. Pathology. 2000;32:280–2. [PubMed] [Google Scholar]
- 13.Kanathur N, Byrd RP, Jr, Fields CL, Roy TM. Noncaseating granulomatous disease in common variable immunodeficiency. South Med. 2000;93:631–3. [PubMed] [Google Scholar]
- 14.Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–41. [PubMed] [Google Scholar]
- 15.Spickett GP, Zhang JG, Green T, Shrimankar J. Granulomatous disease in common variable immunodeficiency. effect on immunoglobulin replacement therapy and response to steroids and splenectomy. J Clin Pathol. 1996;49:431–4. doi: 10.1136/jcp.49.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutor G, Fabel H. Sarcoidosis and common variable immunodeficiency. A case of a malignant course of sarcoidosis in conjunction with severe impairment of the cellular and humoral immune system. Respiration. 2000;67:204–8. doi: 10.1159/000029488. [DOI] [PubMed] [Google Scholar]
- 17.Aukrust P, Lien E, Kristoffersen AK, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency − possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 18.Wright JJ, Wagner DK, Blaese RM, Hagengruber C, Waldmann TA, Fleisher TA. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990;76:2046–51. [PubMed] [Google Scholar]
- 19.Crofts MJ, Joyner MV, Sharp JC, Costello J, Vergani D. Sarcoidosis associated with combined immunodeficiency. Postgrad Med. 1980;56:263–5. doi: 10.1136/pgmj.56.654.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelstein AD, Miller A, Zimelman AP, Rocklin RE, Neiman RS. Adult severe combined immunodeficiency and sarcoid-like granulomas with hypersplenism. Am J Hematol. 1978;5:55–62. doi: 10.1002/ajh.2830050108. [DOI] [PubMed] [Google Scholar]
- 21.Perks WH, Petheram IS. Familial combined cellular and humoral immune defect with multisystem granulomata. Thorax. 1978;33:101–5. doi: 10.1136/thx.33.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]