INTRODUCTION
Dr Philip Ellman was an outstanding physician and master of both respiratory medicine and rheumatology, appreciating the frequent link between joint disease and diseases of the chest. Tuberculosis (TB) is a condition which occurs with increasing frequency in respiratory medicine, in rheumatology and in virtually every other speciality of medicine and surgery. This review covers some of the important issues surrounding TB today—current epidemiology, susceptibility to infection, some new diagnostic techniques and the particular difficulties in managing patients treated with new immuno-modulating drugs such as the tumour necrosis factor (TNF) alpha blockers. The treatment of TB is not included as this has changed very little since Dr Wallace Fox gave a comprehensive account to the Society in 1976.1
METHODS
Material for this review was collected by a variety of methods, including Medline searches using various key words depending on the section, personal experience and our own studies and publications.
TB—A BRIEF HISTORY
TB is one of the oldest diseases known to man. Modern molecular techniques have demonstrated specific amplicons of the Mycobacterium tuberculosis complex in skeletal remains of ancient peoples.2 More recently, TB became greatly feared in Europe and in the seventeenth century was famously described by John Bunyan as ‘the Captain of all these Men of Death’,3 and, a century later, as the ‘white plague’ by Oliver Wendell Holmes.4 Indeed, TB was responsible for 20% of all deaths in London in 1651 recorded in London's Bills of Mortality. Social change and improvements in housing in the early nineteenth century started the decline in TB that continued in the western world until the mid 1980s.
In 1840, George Bodington of Sutton Coldfield isolated victims of the disease in an airy house in the country,5 leading to development of sanatoria, initially in Germany. Robert Koch's discovery of the tubercle bacillus in 18826 proved that TB was an infectious disease. In 1944 the first effective medication for TB, streptomycin, was discovered, rapidly followed by para-aminosalicylic acid (PAS) and later isoniazid (1952), pyrazinamide (1954), ethambutol (1962) and the rifamycins in 1969.7 By the mid 1980s numbers of cases of TB had reached an all time low: the sanatoria were closed and physicians with a lifetime's experience of the disease had retired. Funding was withdrawn, the public health infrastructure dismantled and TB became a most unfashionable disease in which to be interested. TB was, however, still alive and well and from 1987, in the UK, numbers began to rise and have continued to do so.
EPIDEMIOLOGY
The rapid rise in incidence of TB globally led the World Health Organization to declare the disease a global emergency in 1998.8 It is estimated that one in three of the world's population is infected, resulting in millions of cases of active, infectious disease and some 3 million deaths per annum—a figure predicted to rise.9 The highest TB rates of >300 cases/100 000 of the population are generally found in the countries of sub-Saharan Africa. However, huge areas of the world, including the Indian Subcontinent, Far East, China and the former Soviet Union, have rates between 100 and 300 cases/100 000.
TB rates vary across the UK, the average being 13.4/100 000 (May 2005). The highest rates are found in London where the number of cases was 3163 in 2002, a doubling since 1998, and 3834 in May 2005—58% of the England and Wales total. Of the 33 London boroughs, 14 have rates greater than 40/100 000 with two reaching >70/100 000. Numbers in the indigenous population are, in fact, falling but numbers in new immigrants continue to rise: 72% of cases were born abroad and 45% of these entered the UK in the last 5 years. Although numbers of those co-infected with the human immunodeficiency virus (HIV) remain small, there has been a proportionately large increase since 1993 from 2.2% of TB patients to 3.3% in 1998. Most of this increase has been in London where 4.5% of TB cases were co-infected with HIV in 2000 (PHLS Enhanced TB Surveillance). There is concern that these numbers will continue to rise in parallel with the recent rise in all sexually transmitted diseases.
RISKS OF DEVELOPING ACTIVE TB DISEASE
Most TB seen in adults is reactivation of primary disease acquired in childhood. An immunologically naïve child is infected by a sputum smear positive adult, but frequently remains asymptomatic. After exposure, the next 12 months represents a critical time when an individual's cell-mediated immunity can contain the infection by forming granulomata in the lung and draining lymph nodes. Around 1.5% of those infected will progress to active disease within the first year following infection and 5-10% in the first 5 years.10 The remainder remain asymptomatic but develop latent infection with the potential to reactivate if the immune balance changes. If no co-morbid conditions intervene to lower immune defence, the lifetime risk of reactivation is 10%. Thus, the total lifetime cumulative risk of developing disease after being infected is around 15% under normal conditions. HIV co-infection, however, results in an annual risk of reactivation of 5-10%. Other co-morbid conditions that significantly alter the risk of reactivation are shown in Table 1.11
Table 1.
Relative risk of developing active tuberculosis (TB) with different co-morbidities
| Clinical condition | Relative risk |
|---|---|
| Diabetes mellitus | 2-4 |
| Solid organ transplantation | 20-74 |
| Silicosis | 30 |
| Chronic renal failure / haemodialysis | 10-25.3 |
| Gastractomy | 2.5 |
| Contact smear +ve TB | 5-10 |
Some populations may be more susceptible to TB than others, and Asians and Africans present with more extrapulmonary TB than Caucasians. White populations with their origins in Europe are generally thought to have a lower incidence of TB than Asians and Africans.12,13 although few studies show this and others detect no difference.14
Parasitic infections
Parasitic infections can alter the host's ability to generate a protective immune response to mycobacteria, thus suggesting that co-infection with intestinal parasites may increase susceptibility to TB.15 It is estimated that some 2 billion people are infected with schistosomiasis and soiltransmitted helminths, particularly in countries where TB rates are also very high.16 These organisms engender chronic immune activation associated with a T helper (Th)-2 response. Immunoglobulin E (IgE) production is regulated by the Th2 cytokines interleukin (IL)-4 and IL-13 and is an important component of host protective immune responses against parasites but not against mycobacterial infections. A marked correlation has been found between total serum IgE concentrations and the incidence of TB in parts of Cape Town.17 Other studies have shown down regulation of Th1 cytokines and impaired Th1 responses in helminth infected subjects.18,19 The ability to generate a strong Th1 response is important in containing M. tuberculosis infection. During the first 3-4 weeks of primary M. tuberculosis infection, mice predominantly generate a Th1 response, but later also a Th2 response.20 In humans, increased Th2 cytokines in peripheral blood is associated with severe disease.21 Children with hereditary interferon-γ (IFN-γ, a Th1 cytokine) receptor 1 deficiency are prone to overwhelming infection with environmental mycobacteria.22 Together these data suggest that Th1 responses are protective, whereas Th2 responses are associated with chronic or more severe disease. This thus supports the hypothesis that coinfection resulting in a Th2 bias in the immune response could increase susceptibility to TB disease.
Vitamin D
New immigrants from countries of high prevalence are at increased risk of developing the disease. Stress and changes in diet may be involved and also probably the low vitamin D levels found in patients with TB.23,24 Vitamin D promotes activation of macrophages and restricts intracellular growth of M. tuberculosis by acting in synergy with IFN-γ released from activated CD4+ Th1 cells.25 The cytokine induces a 1-hydroxylase in human macrophages, converting inactive 25-hydroxy vitamin D3 to active 1,25-di-hydroxy vitamin D3 (calcitriol). This enables macrophages to inhibit intracellular replication of M. tuberculosis, and also sensitizes them to triggering by mycobacterial antigens to release tumour necrosis factor (TNF).
Vitamin D occurs in foods such as eggs and oily fish and is synthesized in the skin by ultraviolet radiation acting on the cutaneous precursor 7-dehydrocholesterol.26 Most people create sufficient vitamin D during the summer months to maintain normal levels throughout the year. Increased skin pigmentation, however, reduces the penetration of ultraviolet (UV) rays, hence reducing vitamin D formation. Furthermore, at 52 North (the latitude of London), the penetration of UV light is insufficient to initiate cutaneous production of vitamin D between October and March.27 The association between resistance to TB and vitamin D is nothing new. Before the development of antimicrobial agents for TB, both phototherapy (sunbathing or exposure to UV) and cod liver oil supplements (‘bottled sunlight’) were used to treat TB.
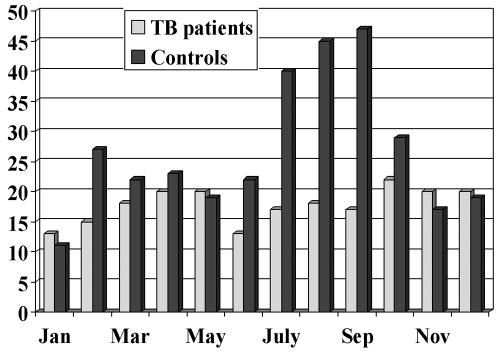
Vitamin D levels measured in patents with TB, and in age, sex and ethnically matched controls, showed both groups had mean levels below the normal range (40-120nmol/L). However, patients had significantly lower levels than controls (Table 2), and low levels were found in patients from all ethnic groups including Caucasians.28 Furthermore, only three TB patients (2.5%) had normal levels (all from the same family with a diet rich in fish) compared with 27 (22%) of the controls. Similarly, a much higher proportion of patients had severely deficient levels (<20nmol/L) than controls. Looking in detail at diet and sunlight exposure, there was no difference between the two groups,29 but the control group responded to increased sunlight in the summer months while the TB patients did not (Figure 1). This raises interesting questions over the handling of this vitamin in patients with TB. It is, however, unclear whether low vitamin D predisposes to TB or whether the disease itself somehow depresses the ability to absorb the vitamin. A recent study in Gujarati Asians suggests the former and has defined a role for polymorphisms in the vitamin D receptor gene.30
Table 2.
Serum vitamin D levels in tuberculous (TB) patients and age, sex and ethnically matched controls. TB patients have significantly lower levels than controls with significantly fewer patients with normal levels and more with severe deficiency
| Culture +ve TB (N=119) | Healthy contacts (N=123) | χ | |
|---|---|---|---|
| Mean ± SD | 18 [11.8] | 29 [15.9] | P<0.001 |
| Normal levels (>39nmol/L) | 3 (2.5%) | 27 (22%) | P<0.001 |
| Severe deficiency (<20nmol/L) | 81 (68%) | 46 (37%) | P<0.001 |
Figure 1.
Seasonal variation of serum vitamin D levels. Controls show a response to increased sunshine in the summer months while patients with tuberculosis (TB) show no response
Genetic susceptibility
It is likely that other genes are involved in susceptibility to human TB and additional association studies have implicated HLA-DR231 and variants of the NRAMP1 (natural resistance associated macrophage protein 1) gene,32 also known as solute carrier family 11A member 1, SLC11A1. NRAMP1 is located on chromosome 2q35 and is the most extensively studied of the non-major histocompatibility complex TB susceptibility genes. It is the human homologue of the mouse gene Nramp1, in which a single non-conservative amino acid substitution appears to control susceptibility to mycobacteria as well as leishmania and salmonella.33 NRAMP1 acts by activating microbicidal responses in the infected macrophage, and is therefore important in the early innate response to mycobacterial infection. In a recent study, two of five polymorphisms of SLC11A1 (NRAMP1) were significantly associated with TB in the South African coloured population in the Western Cape.34
In addition to host genetics and co-morbid disease, immune modifying drugs, which represent an important advance in the treatment of rheumatoid arthritis and other inflammatory conditions, significantly increase the risk of reactivation of latent disease (vide infra).
Table 3.
Clinical features of post primary tuberculosis (TB). Weight loss and night sweats significantly more likely to occur with TB than with non tuberculous disease. Presence of TB risk factors, positive skin test and upper lobe disease on chest radiograph (CXR) are also important features of TB (Ref. 36)
| Symptom | TB (N=47) | Non-TB (N=516) | Odds ratio |
|---|---|---|---|
| Cough | 81% | 77% | 1.27 |
| Fever | 70 | 59 | 1.64 |
| Weight loss | 64 | 27 | 4.74* |
| Night sweats | 55 | 27 | 3.29* |
| Dyspnoea | 47 | 50 | 0.88 |
| Chest pain | 27 | 26 | 1.08 |
Presence of TB risk factors or symptoms (odds ratio [OR] 7.9); positive skin test for TB (OR 13.2); upper lobe disease on chest X-ray (OR 14.6)
DIAGNOSIS
TB can occur in any organ of the body, but the most common sites are the lungs, peripheral lymph nodes, bones and joints. Making a diagnosis can be difficult, or may not even considered, when classic signs and symptoms are absent.
The tuberculin skin test (TST)
Primary TB usually occurs in children and is often asymptomatic. Development of a positive TST shows that infection has occurred. Until 2006, both Heaf and Mantoux tests were available. Unfortunately, the Heaf test, which is relatively easy to both administer and read, has now been withdrawn in the UK. Intradermal injection of purified protein derivative of tuberculin (PPD) results in a delayed hypersensitivity response, and the diameter of this response indicates the degree of positivity. While it can be a useful pointer in making a diagnosis, particularly in children and young adults, it should be interpreted with care in older adults. Previous tuberculous infection or disease will result in a persistent positive response. Corticosteroids, other immunosuppressants and renal disease will depress the response and co-infection with HIV will often give a negative reaction even in the presence of serious disease.
Clinical features
A history of recent TB contact is the most important factor in making the diagnosis. Features of primary and post primary TB have been extensively described,35,36 but it is worth remembering that particularly primary and non-respiratory disease may be relatively asymptomatic, associated with non-specific symptoms or symptoms confined to a particular site, but not always with the classic triad of symptoms of fever, weight loss and night sweats. The presence of TB risk factors, a positive TST and upper lobe disease on the chest X-ray (CXR) have all been shown to be positively associated with culture proven TB.36 A multiplicity and long duration of symptoms should also alert the clinician to the diagnosis.
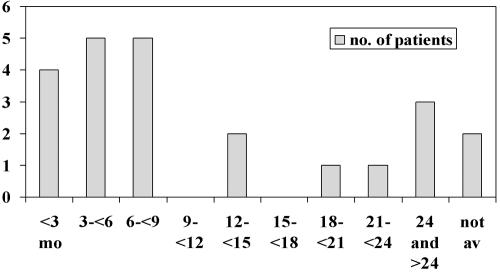
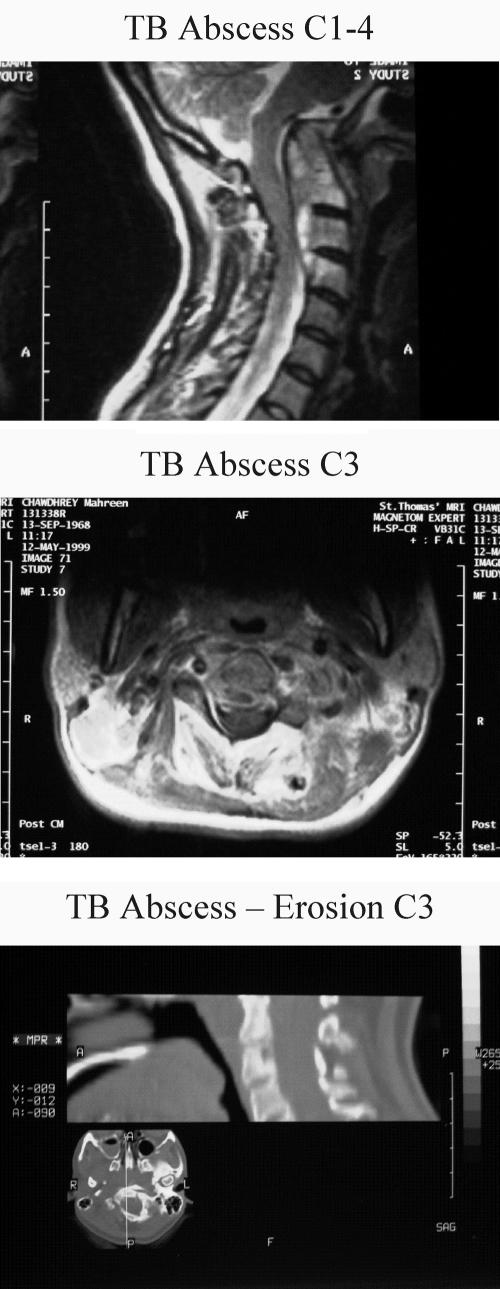
Lymph nodes, usually cervical, are the most common extra-pulmonary sites. Enlargement is gradual and usually painless, and the lack of warmth makes the classical ‘cold abscess’. Bone and joint TB accounts for some 10-15% of non-respiratory disease, with approximately 50% in the spine and 50% in a wide range of other bones and joints. Pain is the most common symptom and TB or other infective process should be considered in patients from ethnic minority groups complaining of back pain without history of injury and with or without neurological signs. Our own experience of patients with spinal TB suggests that this diagnosis is not entertained in a significant number of cases. The majority of our 23 patients had symptoms for several months before a diagnosis was made (Figure 2);37 one unfortunate patient with cervical spine TB was seen by various practitioners including psychiatrists over 2½ years before finally getting the right investigations and emergency surgery to prevent paraplegia (Figure 3).
Figure 2.
Time taken to diagnosis for 23 patients with spinal tuberculosis (TB). Vertical axis, number of patients; horizontal axis, months
Figure 3.
Magnetic resonance imaging scans of the neck in a patient with cervical spine tuberculosis. (a) A large paraspinal abscess from C1 to C4; (b) abscess at C3; and (c) erosion of the body of C3
Investigations
A number of non-specific markers can aid diagnosis—e.g. anaemia, low lymphocyte count, raised monocytes, erythrocyte sedimentation rate and C-reactive protein and abnormal liver function tests. Elevated levels of adenosine deaminase, an enzyme produced by lymphocytes, have been found in cerebrospinal and pleural fluids associated with TB. A CXR should be performed in anyone with a suspicion of TB at whatever site, as non-pulmonary disease often occurs in association with pulmonary disease. Every effort should be made to obtain material for microbiological culture by sending a portion of any biopsy in either normal saline or a plain pot. This both confirms the diagnosis and obtains material for drug susceptibility testing. This is crucial in the modern world where levels of drug resistance are rising. Isoniazid resistance is currently found in 6-7% of all isolates in England and Wales, and multi-drug resistance (i.e. resistance to both isoniazid and rifampicin) in around 1%. Acid fast bacilli can be seen by either Ziehl-Neelsen or fluorescent auramine staining in sputum smears, fine needle aspirates and biopsy specimens, and have been traditionally grown on Lowenstein-Jensen medium. However, this method is slow, taking from 2-4 weeks for microscopy positive and 4-8 weeks for microscopy negative material. Newer, automated liquid culture systems using either radiometric, fluorimetric or colorimetric detection of changes in O2 consumption/CO2 production are generally faster and have higher recovery rates than solid media.38 They are, however, expensive and it is not practical to have a system in every district general hospital. The economic value of the system is best when processing 10-20 000 samples per annum. It is likely that there will be 1-2 units in each strategic health authority, with more in urban conurbations.11 Optimal recovery is, however, achieved through a combination of rapid automated liquid culture and solid Lowenstein-Jensen slopes, and three consecutive sputum specimens are required.39
New immunological tests
T cell-based rapid blood tests which measure IFN-γ production by stimulated peripheral blood T cells may overcome some difficulties with the TST. These tests use the antigens early secretion antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) which are more specific for M. tuberculosis than PPD. They are encoded by genes located within the region of difference 1 of the M. tuberculosis genome but are absent from BCG strains and most non-tuberculous mycobacteria. Two commercial tests are available: the QuantiFERON-TB Gold (Cellestis, Australia) uses enzyme-linked immunosorbent assay to measure release of IFN-γ in whole blood in response to stimulation by ESAT-6 and CFP-10, and the T SPOT-TB assay (Oxford Immunotec, UK) which measures the number of T cells producing IFN-? by enzyme linked immunospot assay (ELISPOT). Studies using these tests have been reviewed by Pai et al., 40 but evidence is still inadequate on their value in different clinical situations. Furthermore, they are more expensive than the TST and require laboratory facilities and trained personnel. Their increased specificity may, however, prove useful in certain patient groups, e.g. those starting anti-TNF-α treatment for inflammatory disease.
TNF-α BLOCKADE
Three TNF-α blocking drugs are available for the treatment of inflammatory diseases, particularly rheumatoid arthritis, Crohn's disease, ankylosing spondylitis, psoriatic arthropathy and juvenile polyarticular idiopathic arthritis. Infliximab is a murine human chimeral monoclonal antibody against TNF-α and causes lysis of cells expressing membrane TNF. Etanercept is a fusion protein that binds free TNF-α using the soluble portion of TNF-receptor 2 coupled with an Fc moiety. Adalimumab, licensed in 2003, is a recombinant fully humanized monoclonal antibody against TNF.
TNF-α is a potent pro-inflammatory cytokine produced mainly by monocytic cells but also expressed by T, B and natural killer cells. It exists in soluble and transmembrane forms and two receptors have been identified. While it has anti-tumour and anti-viral activity and stimulates apoptosis of infected cells, it is also implicated in shock, cachexia, rheumatoid arthritis and Crohn's disease. The role of TNF-α in the immune response to mycobacteria in humans is incompletely understood, but in animal models it plays a central role in the formation of granulomata and containment of disease.41 Figure 4 shows the likely role of TNF-α in the immune response to mycobacterial infection, and it is clear that blocking it will inhibit this response.
Post marketing surveillance of infliximab to the end of 2001 showed 70 cases of TB among 147 000 patients treated. Those developing TB came from areas of low endemicity, 40 had extrapulmonary TB and 17 disseminated disease—a rate of 25% compared with 15% in other groups of immunocompromised patients. Furthermore, they showed poor granuloma formation and minimal macrophage apoptosis.42 The majority of cases occurred within three cycles of treatment, with a median of 12 weeks after starting treatment. In the USA, the TB rate in rheumatoid arthritis patients treated with infliximab was estimated to be 24.2/100 000 as against 6.2/100 000 in rheumatoid patients not treated with infliximab—a fourfold increase. By March 2003, 234 cases had been reported and the rate had risen to 48/100 000.
By 2002, 121 000 patients had received etanercept and the case rate was then 21/100 000.43 Unlike infliximab, there seemed to be no consistent temporal relationship in the development of TB associated with etanercept, with disease occurring between 1 and 20 months (median of 11.5 months). This difference is perhaps explained by the different modes of action between the two drugs, dosage administration and length of treatment.
Adalimumab is relatively new but 13 cases of TB have been reported in 2468 patients treated with this drug, most occurring within 8 months of starting therapy.44
These figures have led to a requirement to screen all patients for active and latent TB before commencing anti-TNF-α therapy. The manufacturers recommend a skin test in all patients but, with 79% already taking other immunosuppressive therapy, this would be unhelpful in the majority. The ELISPOT and QuantiFERON-TB Gold tests have not been evaluated in this group of patients but may well prove useful in the future. All patients should have a CXR to detect both active disease and signs of previous disease. Active disease must be treated or excluded before starting anti-TNF therapy, and patients at particular risk of latent infection (previous TB inadequately treated, abnormal CXR, new immigrant from an area of high prevalence) may require TB chemoprophylaxis.45 Chemoprophylaxis itself carries some risk, particularly of drug induced hepatitis. Rifampicin, isoniazid and pyrazinamide can all cause hepatitis—the risk increasing with age and occasionally fatal. Other troublesome drug side effects are also more common in the elderly. Frequently, a single drug (isoniazid) will be used for chemoprophylaxis and, if given in the presence of active disease, there is a significant risk of developing drug resistance.
Two chemoprophylactic regimens are currently used in the UK. In the USA, 2 months of pyrazinamide plus rifampicin has been used, but the hepatitis risk is extremely high (6648/100 000 in 569 patients in three trials) and associated with several fatalities. This regimen is not used in the UK where we use either 6 months of isoniazid alone or 3 months of isoniazid plus rifampicin. Isoniazid alone has a weighted average risk of hepatitis of 278/100 000 in 18 815 patients treated in three large trials.45 The advantages of rifampicin plus isoniazid for 3 months are the reduced length of treatment and the use of two drugs rather than one. However, our limited experience with this regimen gives a calculated hepatitis risk of 1766/100 000 in 170 patients.45
The annual risk of developing TB depends on who you are and where you are from. In the white, UK-born population the risk increases with age from 0.8 to 10.9/100 000, whereas the risk in someone of Indian subcontinent origin increases with age from 28.7 to 405.7/100 000. The highest risk in Black Africans is 314/100 000 in the 15-34 age group. Anyone born abroad of any ethnic group has an increased risk of 10-20 times, but this reduces after 5 years in the UK.
Using the above figures, we have developed a risk benefit analysis of the risk of developing TB (as above multiplied by a factor of five for the increased risk of treatment with anti-TNF agents) against the risk of developing hepatitis with chemoprophylaxis. Further details can be found in the British Thoracic Society Guidelines.45 The recommendations made in these guidelines can be summarised as follows:
All patients should have a CXR and active disease excluded if this is abnormal
Those with active disease should receive standard chemotherapy for at least 2 months, and ideally a full course, before starting anti-TNF therapy
Those with past TB or abnormal CXR with inadequate or no previous treatment are at high risk of reactivation and should have chemoprophylaxis, ideally completed before starting anti-TNF treatment
In those with an abnormal CXR and a history of TB adequately treated, use the risk benefit calculation.
Most patients will have a normal CXR and here the TST may be useful in those not on immunosuppressive therapy.
TST positive (Mantoux 6-14mm with no BCG or 15+mm with BCG), use the risk benefit calculation
TST negative (Mantoux <6mm with or without BCG), start anti-TNF therapy and observe
All TB treatment or TB prophylaxis should be supervised by a chest physician.
We should remember that TB presenting in these patients is frequently extra-pulmonary, particularly in non Caucasians.
CONCLUSIONS
This review has looked at our increasing understanding of the risks inherent in developing active tuberculous disease, methods of diagnosis and the particular problems in patients treated with anti-TNF-α immunomodulating drugs. Treatment has been omitted because little has changed over the past 30 years. New drugs have not been developed, although some of the newer fluoroquinolones such as moxifloxacin, sparfloxacin, levofloxacin and ofloxacin show promising activity against M. tuberculosis. There is little doubt that, with today's shrinking world, TB will continue to thrive unless we commit more resources to fund research into understanding host and environmental risk factors and the development of new and shorter treatment regimens.
Acknowledgments This review is based on the Philip Ellman Lecture presented to the Divisions of Respiratory Medicine and Rheumatology, The Royal Society of Medicine, 2 February 2005. This review was not sponsored or funded. Ethical approval was obtained from the Guy's and St Thomas' ethics committee for the vitamin D study described.
I am grateful to Professor Peter Ormerod for his assistance in presenting the anti-TNFα data.
Competing interests None declared.
References
- 1.Fox W. The Modern Management and Therapy of Pulmonary Tuberculosis. Proc Roy Soc Med 1977;70: 4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zink AR, Grabner W, Nerlich AG. Molecular identification of human tuberculosis in recent and historic bone tissue samples: The role of molecular techniques for the study of historic tuberculosis. Am J Phy Anthropol 2005;126: 32-47 [DOI] [PubMed] [Google Scholar]
- 3.Bunyan J. In: Brown, J, ed. The Life and Death of Mr Badman. Cambridge: Cambridge University Press, 1905
- 4.Myers JA. Captain of All These Men of Death. Tuberculosis Historical Highlights. St Louis: Warren H Green, 1977
- 5.Bodington G. An Essay on the Treatment and Cure of Pulmonary Consumption. London: Sinopkin, Marshall, Hamilton and Kent, 1840
- 6.Koch R. “Die Aetiologie und die Bekampfung der Tuberkulose”, lecture delivered to the Physiological Society of Berlin, 24 March 1882 (trans. W. de Rouville). Med Classics 1938;2: 853-80 [Google Scholar]
- 7.Ryan F. Tuberculosis: The Greatest Story Never Told. Yorkshire: Swift, 1992
- 8.World Health Organization. WHO Global Tuberculosis Programme: Global TB Control. Geneva: WHO, 1998: 237
- 9.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle 1991;72: 1-6 [DOI] [PubMed] [Google Scholar]
- 10.Chiba Y, Kurihara T. Development of pulmonary tuberculosis with special reference to the time interval after tuberculin conversion. Bull Int Union Tuberc 1979;54: 263-4 [Google Scholar]
- 11.NICE Guidelines. Tuberculosis. Clinical Diagnosis and Management of Tuberculosis, and Measures For Its Prevention and Control. London: Royal College of Physicians of London, 2006 [PubMed]
- 12.Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med 1990;322: 422-7 [DOI] [PubMed] [Google Scholar]
- 13.Talbot EA, Moore M, McCray E, Binkin NJ. Tuberculosis among foreign-born persons in the United States, 1993-1998. JAMA 2000;284: 2894-900 [DOI] [PubMed] [Google Scholar]
- 14.Hoge CW, Fisher L, Donnell HD Jr, et al. Risk factors for transmission of Mycobacterium tuberculosis in a primary school outbreak: lack of racial difference in susceptibility to infection. Am J Epidemiol 1994;139: 520-30 [DOI] [PubMed] [Google Scholar]
- 15.Bentwich Z, Kalinkovitch A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of helminth infections change the face of AIDS and tuberculosis? Immunol Today 1999;20: 485-7 [DOI] [PubMed] [Google Scholar]
- 16.Savioli L, Stansfield S, Bundy DA, et al. Schistosomiasis and soiltransmitted helminth infections: forging control efforts. Trans R Soc Trop Med Hyg 2002;96: 577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyers AD, vanRie A, Adams J, Fenhalls G, Gie RP, Beyers N. Signals that regulate the host response to M. tuberculosis. Novartis Foundation Symposium, 1998;217: 145-57 [PubMed] [Google Scholar]
- 18.Stadecker MJ, Hernandez HJ. The immune response and immunopathology in infection with Schistosoma mansonii: a key role of major egg antigen Sm-p40. Parasite Immunol 1998:20: 217-21 [DOI] [PubMed] [Google Scholar]
- 19.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansonii. J Expl Med 1991;173: 159-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme IM, Roberts AD, Griffin JP, et al. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol 1993;151: 518-25 [PubMed] [Google Scholar]
- 21.Baliko Z, Szereday L, Szekeres-Bartho J. Th2 biased immune responses in cases with active Mycobacterium tuberculosis infection and tuberculin allergy. FEMS Immunol Med Microbiol 1998;22: 199-204 [DOI] [PubMed] [Google Scholar]
- 22.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996;335: 1941-9 [DOI] [PubMed] [Google Scholar]
- 23.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax 1985;40: 187-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect 2005;50: 432-7 [DOI] [PubMed] [Google Scholar]
- 25.Rook GAW. The role of vitamin D in tuberculosis. Am Rev Respir Dis 1988;138: 768-70 [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D and the skin photobiology, physiology and therapeutic efficacy for psoriasis. In: Heersche JNM, Kannais A, eds. Bone and Mineral Research, Vol 7. Amsterdam: Elsevier, 1990: 313-66
- 27.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D synthesis in human skin. J Clin Edocrinol Metab 1988;67: 373-8 [DOI] [PubMed] [Google Scholar]
- 28.Sita-Lumsden A, Swaminathan R, Milburn HJ. Sun related vitamin D deficiency and reactivation of tuberculosis. Thorax 2003;58: iii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sita-Lumsden A, Lapthorn G, Weekes E, Milburn HJ. Diet related vitamin D deficiency and reactivation of tuberculosis. Eur Respir J 2004; 24(suppl 48): 416S [Google Scholar]
- 30.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms among Gujerati Asians in west London: a case-control study. Lancet 2000;355: 618-21 [DOI] [PubMed] [Google Scholar]
- 31.Singh SP, Mehra NK, Dinley HB, Pande JN, Vaidya MC. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis 1983; 148: 676-81 [DOI] [PubMed] [Google Scholar]
- 32.Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NPAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med 1998;338: 640-4 [DOI] [PubMed] [Google Scholar]
- 33.Vidal SM, Malo D, Vogan K, Skamene E, Gross P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 1993;73: 469-85 [DOI] [PubMed] [Google Scholar]
- 34.Hoal EG, Lewis L-A, Jamieson SE, et al. SLC11A1 (NRAMP1) but not SLC11A2 (NRAMP2) polymorphisms are associated with susceptibility to tuberculosis in a high-incidence community in South Africa. Int J Tuberc Lung Dis 2004;12: 1464-71 [PubMed] [Google Scholar]
- 35.Milburn HJ. Primary tuberculosis. Curr Opin Pulm Med 2001;7: 133-41 [DOI] [PubMed] [Google Scholar]
- 36.Brandli O. The clinical presentation of tuberculosis. Respiration 1998; 65: 97-105 [DOI] [PubMed] [Google Scholar]
- 37.Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J 2006;82: 46-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piersimoni C, Scarparo C, Callegaro A, et al. Comparison of MB/Bact alert 3D system with radiometric BACTEC system and Lowenstein-Jensen medium for recovery and identification of mycobacteria from clinical specimens: a multicentre study. J Clin Microbiol 2001;39: 651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvell JD, Hadley WK, Ng VL. Increased sensitivity of the BACTEC 460 mycobacterial radiometric broth culture system does not decrease the number of respiratory specimens required for a definitive diagnosis of pulmonary tuberculosis. J Clin Microbiol 2000;38: 3608-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pai M, Riley LW, Colford JM Jr. Interferon-γ assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Inf Dis 2004;4: 761-76 [DOI] [PubMed] [Google Scholar]
- 41.Roach RR, Bean AGD, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and cell clearance of mycobacterial infection. J Immunol 2002;168: 4620-7 [DOI] [PubMed] [Google Scholar]
- 42.Keane J, Gershon SK, Braun MM. Tuberculosis and treatment with infliximab. N Engl J Med 2002;346: 625-6 [Google Scholar]
- 43.Manadan AM, Mohan AK, Cote TR, Siegal JN, Seqieira W, Block JA. Tuberculosis and etanercept. Proceedings of American College of Rheumatology Conference Atlanta, GA: ACR, 2002: abstract 356
- 44.Abbott Laboratories. Package literature for HUMIRA, July 2004. httpwww.humira.com (accessed June 2005)
- 45.Ormerod LP, Milburn HJ, Gillespie SH, Ledingham J, Rampton DS. For the British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in adult patients due to start anti-TNFalpha treatment. Compiled on behalf of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax 2005;60: 800-516055611 [Google Scholar]