Abstract
HIV-1 infected patients adherent to HAART and displaying stable increases in CD4 T-cell counts differ in their control of HIV replication and one might expect this to reflect depressed immune function. The importance of virological control in functional immune reconstitution was investigated in HIV-1 infected patients who maintained high or undetectable plasma HIV RNA levels over 2–4 years on HAART (discordant and complete responders, respectively). Immunocompetance and immune activation were assessed directly ex vivo and after a short period of culture, as HIV replication in cultures from viraemic patients may artificially depress responses. Expression of cytokine (interferon-γ, interleukin-5) and chemokine receptor (CCR5, CRTH2) mRNA were determined and soluble CD30 and NO2–/NO3– were measured in sera. Unstimulated cells from all patients had low levels of IFNγ mRNA relative to uninfected controls. Discordant responders had more IFNγ, IL-5 and CCR5 mRNA in mitogen-stimulated PBMC than complete responders, where the difference could be attributed to CD8-T-cells. Serum NO2–/NO3– levels were significantly higher in all patients than controls, with no difference between complete and discordant responders. Serum CD30 levels were significantly higher in discordant responders. These data indicate a persistent immune deficit in immune reconstituted patients irrespective of HIV viral load and associate persistent viral replication with lymphocyte activation, probably involving CD8 T-cells.
Keywords: cytokine, HAART, HIV, RT-PCR
INTRODUCTION
Highly active antiretroviral therapy (HAART) suppresses HIV replication, increases CD4 T-cell counts and increases survival of treated HIV-1 infected patients, including those who start HAART with advanced immunodeficiency [1–3]. However, CD4 T-cell counts never fully recover in previously immunodeficient patients and little is known about the functional integrity of the reconstituted cells. As HAART becomes more widely available, more immunodeficient patients will commence therapy. Their prognosis will depend on how well their immune systems function after several years on therapy.
Cytokine and lymphoproliferative responses to mitogens and antigens may recover following 1–2 years on HAART [2–5]. However, a history of persistently low CD4 T-cell counts prior to HAART has been associated with persistently impaired T-cell lymphoproliferative responses [6] and cytokine production [7]. The balance of type 1/type 2 (T1/T2) cytokines may influence immunocompetence in patients receiving HAART [8], as HIV disease progression is characterized by a shift from a predominant T1 to a T2 cytokine response [9] and HAART decreases expression and secretion of T2 cytokines in patients who achieve immunological and virological responses [10,11].
In some patients treated with HAART, CD4 T-cell counts increase despite persistent HIV viraemia. These ‘discordant’ or ‘discrepant’ responses [12–15] have been attributed to: impaired T-cell apoptosis by protease inhibitors (PI) [16], changes in T-cell turnover kinetics [17], or a reduced effect of PI-resistant HIV on thymic dysfunction [18]. The effect of discordance on the recovery of immune function is not known and has the potential to influence management of patients who are tolerating a given regimen without side-effects. Here we focus on a particular category of patients, the individuals who began treatment with advanced disease and have adhered to therapy and achieved reasonable recovery of CD4 T-cell counts.
All patients began treatment with severe immunodeficiency and retained a good CD4 T-cell response after at least 25 months of therapy. To obtain a snap-shot of the immune system in vivo (avoiding changes that may occur when cells are cultured), we quantified mRNA for markers of a T1 (interferon-γ (IFNγ), CCR5) or T2 (interleukin-5 (IL-5), CRTH2 [19]) cytokine environments in unstimulated peripheral blood mononuclear cells (PBMC) and in purified CD4 and CD8 T-cells. Stimulated cells were also assessed but the period of culture was minimized. Serum levels of NO2–/NO3–, a marker of T1 environment [20], and soluble (s)CD30, a T2 marker [21] were measured. This is the first study to utilize a sensitive RT-PCR technique to quantify cytokine mRNA levels directly ex vivo and address the role of persistent viral replication in a defined group of patients.
PATIENTS, MATERIALS AND METHODS
Patients
The study investigated 13 HIV-1 infected patients attending Outpatient Clinics at Royal Perth Hospital (RPH), selected from a comprehensive database on the basis of distinct immunological and virological responses to HAART (triple therapy including a PI). An immunological response was defined as an increase of the CD4 T-cell counts by >200 cells/µl above baseline value. A virological response was defined as a reduction in plasma HIV RNA to undetectable levels for >24 months prior to and including the time of evaluation. No patients had acute medical conditions at the time of sampling and all were judged to be adherent to therapy by their physicians. This is supported by the stable increases in their CD4 T-cell counts. PBMC and sera from healthy individuals with no evidence of exposure to HIV were obtained as controls. This work was approved by the Human Ethics Committee from Royal Perth Hospital and informed consent was obtained from all donors.
Plasma HIV RNA and CD4 and CD8 T-cell counts
Plasma HIV RNA levels were determined by a quantitative reverse transcriptase polymerase chain reaction technique (Amplicor™, Roche Diagnostics Systems, Branchburg, USA) every 3 months. The detection limit of the assay was 400 copies/ml from 1996 to 1998 and 50 copies/ml from 1998 onwards. CD4 and CD8 T-cell counts were determined from fresh blood specimens by flow cytometric analysis using a Coulter® EPICS® XL-MCL Flow Cytometer.
Isolation of peripheral blood mononuclear cells
Blood samples were collected in heparinized tubes and processed within 4 h. PBMC were isolated using Ficoll-Hypaque density gradient separation. Cells were resuspended in fetal calf serum with 10% dimethylsulphoxide and stored in liquid nitrogen. Sera collected at the same time were stored at −80°C. The viability of thawed cells was >90% by trypan blue exclusion. To determine the effects of in vitro stimulation, 0·5 × 106 thawed PBMC were cultured for 4 h in RPMI-1640 supplemented with 5% human type AB serum, 150 µg/ml penicillin and 16 µg/ml gentamycin in the presence of phorbol myristate acetate (PMA) and calcium-ionophore [5]. CD4 and CD8 T-cells were isolated from PBMC using magnetic beads (Dynal, Lake Success, NY, USA). The purity of each preparation was around 95% by flow cytometry.
Quantification of mRNA by real-time PCR
RNA was extracted using the RNeasy total RNA kit (Qiagen, USA). 14 µl of RNA was used for cDNA synthesis using Omniscript (Qiagen, Valencia, CA, USA). PCR was performed on a LightCycler™ (Roche Diagnostics, Castlehill, NSW, Australia) in 20 µl reaction volumes containing 1·25 mm dNTP, 20 pmol of each primer, 0·25 mg/ml bovine serum albumin and 1·5 unit Platinum Taq DNA polymerase. Forward and reverse primer sequences were 5′GATGACCCAGATCATGTTTGA3′ and 5′GACTC CATGCCCAGGAAGG AA3′ for β-actin, 5′CTCG GAAACGATGAAATATACA3′ and 5′CATATGGGTCCT GGCAGTAAC3′ for IFNγ and 5′AGGATGCTTCTGCATTT GAG3′ and 5′CTATTA TCCACTCGGTGTTC3′ for IL-5 [22] and 5′AATAATTGCAGTAGCTCTAACAG G3′ and 5′TTGAG TCCGTGTCACAAGCCC3′ for CCR5 [23] and 5′GAATGGAG TCATCCTCTTCGTG3′ and 5′AGCCGCTGGCGAACATGTT GAG3′ for CRTH2. Amplicons were 459 bp for β-actin, 90 bp for IFNγ, 394 bp for IL-5, 280 bp for CCR5 and 212 bp for CRTH2. The PCR protocol comprised an initial denaturation step at 95°C for 5mins, followed by 35 cycles of denaturation (95°C for 2 s), annealing (64°C for CCR5, CRTH2 and β-actin and 60°C for IL-5 and IFNγ for 10 s) and extension (72°C for 20 s). Amplification was monitored using SYBR Green fluorochrome (Sigma, St Louis, MO, USA) and generation of a single product was confirmed from the melting curve. Cytokine and chemokine receptor mRNA were quantified using standard curves generated from serial 10-fold dilutions of purified PCR products. The ratios of the amount of IFNγ, IL-5, CCR5 and CRTH2 PCR products to β-actin in each sample were calculated and expressed in arbitrary units. The lower limit of detection for the genes analysed was a ratio of 0·01. After in vitro stimulation, mRNA production was expressed as a fold-increase over mRNA in unstimulated PBMC. The intra- and inter-assay coefficients of variation for the real-time PCR assay were 10% and 15%, respectively.
Serological assays
A commercial antibody pair ELISA kit (Bender MedSystem, Burlinghame, CA, USA) was used to assay levels of sCD30. Levels of NO2–/NO3– were measured colourimetrically according to the manufacturer's instructions (Cayman Chemical Company, Ann Arbor, MI, USA). Limits of detection and the interassay coefficients of variance for each assay [24] were: sCD30, 3·1–100 IU/ml, 8%, and NO2–/NO3–, 5–35 µm, 12%.
Statistical analysis
All results are given as median (range). Differences were analysed with nonparametric Wilcoxon Rank Sum Tests unless otherwise stated. Differences assessed at P ≤ 0·05 were considered statistically significant.
RESULTS
Characteristics of HIV-1 infected patients
All HIV-1 infected patients started HAART with <50 CD4 T-cells/µl. Seven patients achieved immunological and virological responses at all times on HAART (complete responders). Six patients failed to control HIV replication on HAART, despite increases in their CD4 T-cell counts (discordant responders). CD4 T-cell counts and plasma HIV RNA levels prior to HAART and the duration of HAART before sampling were similar for complete and discordant responders (Table 1). A similar proportion of patients from each group received NNRTI based regimes (3/7 complete responders versus 2/6 discordant responders). More discordant responders received PI based regimes than complete responders (6/6 versus 4/7). Four discordant responders but no complete responders carried HIV-1 with resistance mutations affecting protease inhibitors (data not shown). At the time of the study, patients with discordant responses had less memory (CD4+ CD45RO +) T-cells than complete responders (P = 0·01) (Table 1), whilst naïve (CD4+ CD45RA +) T-cell counts were similar in the two groups.
Table 1.
Characteristics of HIV-1 infected patients who maintained undetectable HIV viral load (complete responders) and patients with persistent viral replication (discordant responders) following HAART
| Complete responders (n = 7) | Discordant responders (n = 6) | |
|---|---|---|
| Age (years) | 41 (31–66) | 35 (31–41) |
| Time on HAART (months) | 42 (35–52) | 49 (25–54) |
| Nadir CD4 T-cell count (cells/µl) | 18 (0–48) | 18 (4–26) |
| CD4 T-cell count at time of assay (cells/µl) | 483 (375–609)* | 298 (230–450) |
| Nadir CD8 T-cell count (cells/µl) | 333 (220–702) | 284 (164–949) |
| CD8 T-cell count at time of assay (cells/µl) | 1275 (819–1740) | 1369 (840–1917) |
| CD4+ CD45RA + count at time of assay (cells/µl) | 123 (56–211) | 144 (64–391) |
| CD4+ CD45RO + count at time of assay (cells/µl) | 300 (218–384)‡ | 185 (153–266) |
| Baseline HIV RNA (log10 copies/ml) | 5·9 (5·2–6·7) | 5·8 (5·1–6·1) |
| HIV RNA at time of assay (log10 copies/ml) | 1·7 (1·7–2·6) | 4·2 (3·3–5·8)§ |
Results are presented as median (range) and P–values are determined by Wilcoxon Rank Sum Test.
Complete responders significantly higher than discordant responders (P = 0·005);
Complete responders significantly higher than discordant responders (P = 0·01);
Discordant responders significantly higher than complete responders (P = 0·001)
IFNγ, IL-5, CCR5 and CRTH2 mRNA in unstimulated CD4 and CD8 T-cells
All HIV-1 infected patients had significantly less IFNγ mRNA in unstimulated unfractionated PBMC and purified CD4 and CD8 T-cells than control subjects (Table 2). Cytokine and chemokine receptor mRNA in unstimulated CD4 and CD8 T-cells were similar in complete and discordant responders. However, discordant responders had significantly higher IFNγ mRNA in CD8 T-cells than CD4 T-cells (P = 0·02), whereas levels of IFNγ mRNA in the two T-cell subsets were similar in complete responders and healthy controls. CCR5 mRNA was also marginally higher in CD8 T-cells than CD4 T-cells from discordant responders (P = 0·08). IL-5 mRNA was below the limit of detection in unstimulated PBMC or purified CD4 or CD8 T-cells from healthy controls and HIV-1 infected patients (data not shown).
Table 2.
Ex vivo levels of IFNγ, CCR5 and CRTH2 mRNA in PBMC and purified CD4 and CD8 T-cells in HIV-1 infected patients
| Controls (n = 7) | Complete responders (n = 7) | Discordant responders (n = 6) | |
|---|---|---|---|
| IFNγ | |||
| Unfractionated | 155 (0–214) | 32 (2·6–133)* | 53 (1·5–101)* |
| CD4 | 111 (0–154) | 9·9 (0–154) | 1·6 (0–5·5)† |
| CD8 | 125 (33–191) | 12 (2–119)‡ | 37 (4·4–115)‡ |
| CCR5 | |||
| Unfractionated | 265 (0–425) | 47 (9·2–226) | 92 (2·0–165) |
| CD4 | 270 (0–436) | 34 (3·3–263) | 4·5 (0–67) |
| CD8 | 293 (0–501) | 23 (8·8–227) | 78 (5·9–218 |
| CRTH2 | |||
| Unfractionated | 4·7 (0–6·7) | 2·3 (0·2–4·5)†† | 3·9 (0–5·6) |
| CD4 | 1·9 (0–6·5) | 0·6 (0–6·5) | 0·1 (0–1·6)¶ |
| CD8 | 2·8 (2·1–4·1) | 0·6 (0·1–3·7)** | 0·1 (0–1·1)** |
IFNγ, CCR5 and CRTH2 mRNA were normalized against β-actin mRNA. Results are presented as median (range) and P–values are determined by Wilcoxon Rank Sum Test.
Complete responders and discordant responders significantly lower than controls (P = 0·05 and P = 0·03, respectively);
Discordant responders significantly lower than controls (P = 0·02, respectively);
Complete responders and discordant responders significantly lower than controls (P = 0·007 and P = 0·048, respectively);
Complete responders significantly lower than controls (P = 0·05);
Discordant responders significantly lower than controls (P = 0·04, respectively)
Complete responders and discordant responders significantly lower than controls (P = 0·02 and P = 0·003, respectively)
IFNγ, IL-5, CCR5 and CRTH2 mRNA in PBMC after stimulation with a mitogen
Control subjects and complete responders had similar increases in IFNγ, IL-5, CCR5 and CRTH2 mRNA production in PBMC following stimulation with PMA/calcium-ionophore (Table 3). In contrast, discordant responders had significantly more IFNγ mRNA in stimulated PBMC than complete responders or healthy controls (P = 0·04 and P = 0·005, respectively). CCR5 and IL-5 mRNA production were also marginally higher in discordant responders than complete responders (P = 0·07 and P = 0·06, respectively) and CCR5 mRNA was significantly higher in discordant responders than healthy controls (P = 0·05).
Table 3.
IFNγ, IL-5, CCR5 and CRTH2 mRNA production in unfractionated PBMC in HIV-1 infected patients after 4 h stimulation with PMA/calcium-ionophore
| Controls (n = 7) | Complete responders (n = 7) | Discordant responders (n = 6) | |
|---|---|---|---|
| IFNγ | 35 (1·7–63) | 22 (2·9–277) | 108 (50–176)* |
| IL-5 | 6·5 (0–67) | 3·3 (0·8–16) | 26 (0·1–89)† |
| CCR5 | 0·9 (0–2) | 1·0 (0·3–7·2) | 1·6 (1·1–8·6)‡ |
| CRTH2 | 0·8 (0–2·5) | 1·2 (0·2–13) | 1·1 (0–1·5) |
IFNγ, IL-5, CCR5 and CRTH2 mRNA were normalized against β-actin mRNA and values in the table represent fold-increase over unstimulated PBMC. Results are presented as median (range) and P–values are determined by Wilcoxon Rank Sum Test.
Discordant responders significantly higher than controls and complete responders (P = 0·005 and P = 0·04, respectively);
Discordant responders marginally higher than complete responders (P = 0·06);
Discordant responders marginally higher than controls and complete responders (P = 0·05 and P = 0·07, respectively).
To determine which T-cell subset contributed to the increased IFNγ, IL-5 and CCR5 mRNA in stimulated PBMC from discordant responders, we stimulated CD4- and CD8-depleted PBMC from three healthy controls and three discordant responders with PMA/calcium-ionophore. Median IFNγ and IL-5 mRNA production were higher in CD4-depleted PBMC than CD8-depleted PBMC from all three discordant responders (IFNγ: 68 versus 10 and IL-5: 38 versus 23). In contrast, median IFNγ mRNA was similar and IL-5 mRNA was higher in CD8-depleted PBMC from healthy controls (IFNγ: 12 versus 11 and IL-5: 17 versus 29). This suggests CD8 T-cells were the source of cytokine mRNA in discordant patients.
Serum sCD30 and NO2–/NO3– levels
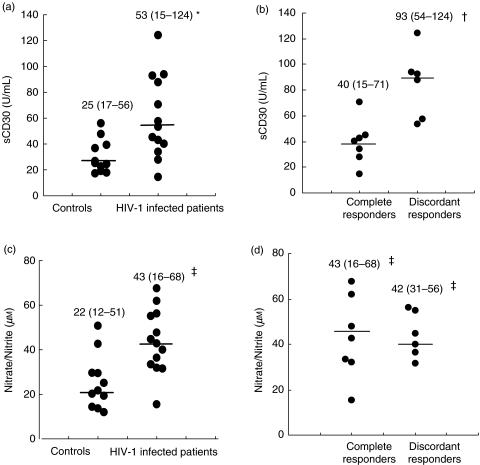
NO2–/NO3– levels were higher in all HIV-1 infected patients than controls (P = 0·003), with no difference between complete and discordant responders (Fig. 1c,d). Higher sCD30 levels were found in discordant responders compared to complete responders or controls (P = 0·004 and P = 0·0003, respectively) (Fig. 1b).
Fig. 1.
Serum sCD30 (a, b) and NO2–/NO3– (c, d) levels in controls and HIV-1 infected patients. Results are presented as median (range) and P-values are determined by Wilcoxon Rank Sum Test. Median values are shown as horizontal bars. *All HIV-1 infected patients significantly higher than controls (P = 0·007), †Discordant responders significantly higher than complete responders and controls (P = 0·004 and P = 0·0003, respectively), ‡All HIV-1 infected patients, complete responders and discordant responders significantly higher than controls (P = 0·003, P = 0·03 and P = 0·007, respectively).
There was a positive correlation between plasma HIV RNA levels and sCD30 (r = 0·78, P = 0·002, Spearman's Test). Serum NO2–/NO3– levels did not correlate with plasma HIV RNA levels or CD4 T-cells counts.
DISCUSSION
Immune dysfunction has not been adequately investigated in HIV-1 infected patients who were severely immunodeficient prior to HAART and the role of persistent HIV replication is unclear. Here, we used a sensitive technique to quantify mRNA for a range of critical inflammatory mediators directly ex vivo because prolonged culture selectively depresses lymphoproliferative and cytokine responses of viraemic patients, probably because the virus replicates in vitro[5]. We present a proof of concept study using small groups of patients matched for age, sex, pretreatment CD4 T-cell counts, treatment history and adherence to therapy. All patients had stable CD4 T-cell recovery after at least 25 months on HAART with different virological outcomes.
Our first striking finding was that spontaneous IFNγ mRNA levels were significantly lower in unfractionated PBMC and in purified CD4 and CD8 T-cells from patients when compared with healthy controls. This was not related to HIV viral load, as patients who maintained undetectable HIV RNA levels (complete responders) and patients with persistent viraemia (discordant responders) had similar IFNγ mRNA (Table 2). In an earlier study, higher IFNγ expression was seen after 48 weeks on HAART relative to HIV-1 negative controls [25]. A shorter median duration of HAART (48 weeks versus 200 weeks in the present study) and/or higher median baseline CD4 T-cell counts (93 versus 18 cells/µl) may explain the discrepancy between the two studies. Immune reconstitution may be unstable and decline after 2–4 years in patients who were originally very immunodeficient. Our group has shown that IFNγ responses to CMV in HIV-1 infected patients are diminished after 3 years of HAART [7] and longitudinal studies of other cytokines are warranted.
IFNγ, IL-5 and CCR5 mRNA production were higher in PMA/calcium-ionophore-stimulated PBMC from discordant responders compared with complete responders. A small study of CD4 and CD8-depleted PBMC suggested that the increased mRNA after mitogenic stimulation observed in discordant responders originates from CD8 T-cells. Moreover the CD4:CD8 ratio was significantly lower than in complete responders (data not shown). This observation is consistent with persistent HIV replication activating CD8 T-cells, as demonstrated by increased expression of CD38 and HLA-DR [26].
Serum nitric oxide and sCD30 levels were assessed as markers of a T1 or T2 environment and of immune activation [20,21]. NO2–/NO3– levels were significantly higher in all HIV-1 infected patients than healthy controls, regardless of virological outcome during HAART. Levels of sCD30 were significantly higher in discordant responders than complete responders or healthy controls. Levels were also proportional to HIV viral load, supporting our previous finding [27]. CD30 can be expressed by both activated CD4 and CD8 T-cells but the majority of CD30+ T-cells from healthy donors were CD8+ T-cells [28]. Moreover, higher numbers of CD8+ T-cell clones from HIV-1 infected patients express CD30 and release more sCD30 into cell culture supernatants than clones from healthy individuals [29]. Therefore, higher serum sCD30 levels in discordant responders may indicate ongoing activation of CD8 T-cells, rather than a T2 cytokine environment.
In conclusion, spontaneous IFNγ mRNA was low in PBMC and in CD4 and CD8 T-cells of all immune reconstituted HIV-1 infected patients despite at least 25 months of HAART. This was not affected by HAART-induced suppression of viral loads. However immune reconstituted patients with persistent HIV viraemia displayed higher than normal expression of IFNγ and IL-5 mRNA after mitogenic stimulation and increased sCD30 levels in serum. These data suggest a persistent immune deficit in immune reconstituted patients after prolonged HAART and associate a discordant response to treatment with immune activation predominantly affecting CD8 T-cells.
Acknowledgments
We thank Ms Niamh Keane for collecting and storing PBMC and sera, Ms Anothai Pocathikorn for primer sequences for β-actin and the patients and control donors for blood samples. This work was supported by the National Centre for HIV Research and Clinical Epidemiology and a Medical School Grant from Merck, Sharp and Dohme. This is publication number 2003–07 of the Department of Clinical Immunology and Biochemical Genetics, Royal Perth Hospital, Western Australia.
REFERENCES
- 1.Mathez D, Bagnearelli P, Gorin I, et al. Reduction in viral load and increases in T lymphocyte numbers in treatment-naïve patients with advanced HIV-1 infection treated with ritonavir, zidovudine, zalcitabine triple therapy. Antiviral Ther. 1997;2:175–3. [PubMed] [Google Scholar]
- 2.Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 3.Mezzaroma I, Carlesimo M, Pinter E, et al. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. AIDS. 1999;13:1187–93. doi: 10.1097/00002030-199907090-00006. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 5.Keane N, Price P, Stone SF, John M, Murray RJ, French MA. Assessment of immune function by lymphoproliferation underestimates lymphocyte functional capacity in HIV patients treated with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2000;16:1991–6. doi: 10.1089/088922200750054729. [DOI] [PubMed] [Google Scholar]
- 6.Sieg SF, Mitchem JB, Bazdar DA, Lederman MM. Close link between CD4+ and CD8+ T cell proliferation defects in patients with human immunodeficiency virus disease and relationship to extended periods of CD4+ lymphopenia. J Infect Dis. 2002;185:1401–8. doi: 10.1086/340509. [DOI] [PubMed] [Google Scholar]
- 7.Keane NM, Price P, Lee S, et al. Restoration of CD4 T-cell responses to cytomegalovirus is short-lived in severely immunodeficient HIV patients responding to HAART. HIV Med. 2004 doi: 10.1111/j.1468-1293.2004.00245.x. (in press) [DOI] [PubMed] [Google Scholar]
- 8.Imami N, Pires A, Hardy G, Wilson J, Gazzard B, Gotch F. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J Virol. 2002;76:9011–23. doi: 10.1128/JVI.76.18.9011-9023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 10.Imami N, Antonopoulos C, Hardy GA, Gazzard B, Gotch FM. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: impact of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 1999;15:1499–508. doi: 10.1089/088922299309784. [DOI] [PubMed] [Google Scholar]
- 11.Taoufik Y, Peguillet I, de Goer MG, et al. Effect of highly active antiretroviral therapy on expression of interleukin-10 and interleukin-12 in HIV-infected patients. J AIDS. 2001;26:303–4. doi: 10.1097/00126334-200104010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann D, Pantaleo G, Sundre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART) Swiss HIV Cohort Study Lancet. 1998;351:723–4. doi: 10.1016/s0140-6736(98)24010-4. [DOI] [PubMed] [Google Scholar]
- 13.Piketty C, Castiel P, Belec L, et al. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS. 1998;12:745–50. doi: 10.1097/00002030-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Renaud M, Katlama C, Mallet A, et al. Determinants of paradoxical CD4 cell regeneration after protease inhibitor-containing antiretroviral regimen. AIDS. 1999;13:669–76. doi: 10.1097/00002030-199904160-00007. [DOI] [PubMed] [Google Scholar]
- 15.D’Ettorre G, Forcina G, Andreotti M, et al. Discordant response to antiretroviral therapy: HIV isolation, genotypic mutations, T-cell proliferation and cytokine production. AIDS. 2002;16:1877–85. doi: 10.1097/00002030-200209270-00004. [DOI] [PubMed] [Google Scholar]
- 16.Sloand EM, Kumar PN, Kim S, Chaudhuri A, Weichold FF, Young NS. Human immunodeficiency virus type 1 protease inhibitor modulates activation of peripheral blood CD4+ T cells and decreases their susceptibility to apoptosis in vitro and in vivo. Blood. 1999;94:1021–7. [PubMed] [Google Scholar]
- 17.Deeks S, Hoh R, Grant RM, et al. CD4+ T cell kinetics and activation in human immunodeficiency-virus infected patients who remain viremic despite long-term treatment with protease inhibitor-based therapy. J Infect Dis. 2002;185:315–23. doi: 10.1086/338467. [DOI] [PubMed] [Google Scholar]
- 18.Stoddart CA, Liegler TJ, Mammano F, et al. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat Med. 2001;7:712–8. doi: 10.1038/89090. [DOI] [PubMed] [Google Scholar]
- 19.Cosmi L, Annunziato F, Iwasaki M, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Van’t Hof RJ, Ralston RH. Nitric oxide and bone. Immunology. 2001;103:255–61. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4 T cells producing Th2-type cytokines. FASEB J. 1995;9:81–6. [PubMed] [Google Scholar]
- 22.Yamamoto S, Hamasaki Y, Ishii E, Ichimaru T, Miyazaki S. Unbalanced production of interleukin-5 and interleukin-2 in children with atopic dermatitis. Ann Allergy Asthma Immunol. 1997;78:517–23. doi: 10.1016/S1081-1206(10)63241-3. [DOI] [PubMed] [Google Scholar]
- 23.Naif HM, Li S, Alali M, et al. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–6. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, MacQuillan GC, Keane NM, et al. Immunological markers predicting outcome in patients with hepatitis C treated with interferon-α and ribavirin. Immunol Cell Biol. 2002;80:391–7. doi: 10.1046/j.1440-1711.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, Michelet C, Peguillet I, et al. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after 1 year of highly active antiretroviral therapy. AIDS. 1999;13:185–94. doi: 10.1097/00002030-199902040-00006. [DOI] [PubMed] [Google Scholar]
- 26.Weiss L, Burgard M, Cahen YD, et al. Immunological and virological features of HIV-infected patients with increasing CD4 cell numbers despite virological failure during protease inhibitor-based therapy. HIV Med. 2002;3:12–20. doi: 10.1046/j.1464-2662.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 27.Keane NM, Price P, Lee S, Stone SF, French MA. An evaluation of serum soluble CD30 levels and serum CD26 (DPPIV) enzyme activity as markers of type 2 and type 1 cytokines in HIV patients receiving highly active antiretroviral therapy. Clin Exp Immunol. 2001;26:111–6. doi: 10.1046/j.1365-2249.2001.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996;157:3229–34. [PubMed] [Google Scholar]
- 29.Manetti R, Annunziato F, Biagiotti R, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180:2407–11. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]