Abstract
To study the consequences of the interaction of respiratory syncytial virus (RSV) with dendritic cells in vitro, we established a model of the primary immune response using dendritic cells, autologous naive T cells and the superantigen toxic shock syndrome toxin 1 (TSST 1). About 10% of the naive T cells express the T cell receptor chain Vβ2. These cells were stimulated by TSST 1 and could be analysed by flow cytometry. Cultures infected with RSV produced significantly less interferon-γ compared to uninfected cultures. In a first set of experiments we evaluated whether this culture model using isolated CD4+ CD45RA+ T cells, in fact, reflects the primary immune response. In a prospective study, cells were isolated from 13 children at birth, at 1 year of age and at 4 years of age. RSV reduced interferon-γ production at all the age groups analysed and the results were stable over time within a given individual. In a second set of experiments, we asked whether clinical differences in the course of RSV infection are due to variations in the cellular immune response. At the age of 1 year (5–9 months after the RSV epidemic) dendritic cells and naive T cells were obtained from 27 children with a history of bronchiolitis, from 15 children with a benign course of RSV infection and from 26 controls without RSV infection. The frequency of interferon-γ-producing cells in RSV infected cultures was significantly lower (P < 0·001) in cultures from children with a history of RSV bronchiolitis compared to children with mild RSV infection. Cultures from children without infection displayed a wide range of results. Overall, interferon-γ generation in this group was still lower (P < 0·05) than in the group with mild RSV infection. Because we have ruled out that memory cells play a role in the experiments performed, the most likely explanation for our results is that a high generation of interferon-γ in the primary immune response protects from severe RSV mediated disease.
Keywords: bronchiolitis, dendritic cells, interferon type II, respiratory syncytial virus infections, T lymphocytes
INTRODUCTION
In infants and toddlers under the age of 2 years, the virus isolated most frequently during wheezing episodes is respiratory syncytial virus (RSV) [1,2]. RSV affects 70% of all children less than 12 months of age [3]. Infections go unnoticed in nearly two-thirds of the infants infected [3]. One-third suffers from mild lower respiratory tract disease [3] and a small percentage of children develop bronchiolitis and requires hospitalization. Peak rates of severe bronchiolitis occur in young infants aged 6 weeks to 6 months [4]. The majority of infants hospitalized for RSV bronchiolitis do not suffer from pre-existing disease as prematurity or congenital heart disease, which are known risk factors. However, it was found that lymphocytes isolated during RSV bronchiolitis display a depressed interferon-γ production compared to cells from non-infected controls [5–7]. Thus, it seems that the primary immune response to RSV is altered in these children.
It is difficult however, to delineate further the molecular mechanisms responsible for this finding. Access to specimens during an acute RSV infection is limited. Furthermore, the local immune response might not be represented by peripheral blood cells. It is now clear from animal models that RSV infection results in sustained increase in numbers of mature dendritic cells in the lung [8]. Dendritic cells are critical in inducing primary T cell responses [9] and interference with the function of these cells is known to constitute a very powerful mechanism for viruses to escape immune responses [10]. To study the consequences of the interaction of RSV with dendritic cells in vitro, we established a model of the primary immune response using dendritic cells, autologous naive T cells and the superantigen toxic-shock syndrome-toxin 1 (TSST 1) [11]. About 10% of the naïve T cells express the T cell-receptor chain Vβ2. These cells were stimulated by TSST 1 and could be analysed by flow cytometry. Cultures infected with RSV produced significantly less interferon-γ compared to uninfected cultures.
These original experiments were performed with cells derived from cord blood. In this study we evaluated whether cultures set up with monocyte derived dendritic cells and naive T cells isolated from the peripheral blood of older children give the same results. In addition, we assessed whether individual levels of interferon-γ generation persist during infancy and early childhood. Furthermore, we asked whether there is a difference between children with a history of RSV bronchiolitis, children with mild disease, and children without RSV infection.
PATIENTS AND METHODS
Ethics
The study was approved by the Research Ethics Committee of the Ruhr University Bochum. Before enrolment of the participants, an informed consent was obtained from the parents.
Participants
Children followed from birth to 4 years of age
Subjects who participated in this part of the study were enrolled at birth in the obstetric department of the Augusta Krankenanstalten Bochum. In addition to cord blood, blood samples were obtained at 1 and 4 years of age. None of the children had respiratory distress during the neonatal period and none of them had been hospitalized up to 4 years of life. During the first RSV epidemic, parents were contacted regularly. None of the children in this group had significant clinical signs of RSV mediated disease (see below). At birth, all children were considered naive for RSV. By serology (see below), at 1 year of age seven had contact with RSV and six not, whereas at age 4 years all were positive for RSV.
Infants studied at 1 year of age
For infants with RSV bronchiolitis, from November 1999 to April 2002, 27 children under 1 year of age and without concomitant chronic respiratory, cardiac or other disease were hospitalized at the Pediatric Department, St Josef Hospital Bochum Germany for RSV bronchiolitis. The diagnosis of RSV infection was made with the Abbott Testpack RSV from nasopharyngeal aspirates. The clinical diagnosis of acute bronchiolitis required the presence of tachypnoea (>55 breaths/min), wheeze, a prolonged expiratory phase and crackles on auscultation recorded at some time during the admission [12]. In addition, the presence of chest indrawing, poor drinking and the need for oxygen supplementation was recorded.
For infants with mild RSV infection and infants without RSV infection, 41 control children were selected from a cohort of children followed from birth. Families lived in the catchment area of the hospital. The mean difference in the birth dates between the RSV children and their matched controls was 23 days (range 7–45 days). None of them had respiratory distress during the neonatal period. One child had symptoms of atopic dermatitis before RSV infection. None of the controls had been hospitalized for respiratory symptoms between the neonatal period and their first examination. During the RSV epidemic, parents were contacted by telephone every second week and asked about the presence of wheezing, cough, tachypnoea, poor drinking, chest indrawing or oxygen supplementation. Infants were grouped as being uninfected with RSV or having had a mild RSV infection according to the result of the anti RSV IgG enzyme-linked immunosorbent assay (ELISA) (see below).
Study design
Children with RSV bronchiolitis as well as controls were re-examined at the mean ages of 1 year (5–9 months after the RSV epidemic) by one of the authors. The parents were interviewed concerning background factors for recurrent respiratory disease and respiratory symptoms during the RSV epidemic using a structured questionnaire.
Heparinized blood was taken to isolate naive T cells and culture dendritic cells. Samples were stored and a batch of one RSV patient and one or two controls were tested simultaneously in the same test. In addition, serum IgG antibodies against RSV were analysed using enzyme-linked immunosorbent assay (Genzyme Virotech, Rüsselsheim, Germany). Values of greater than 6 units were regarded as positive.
Serum IgG antibodies to RSV were found in all 27 children with bronchiolitis and in 15 of 41 controls (37%) (Table 1). All the 15 controls with anti RSV IgG antibodies had a history of a mild respiratory infection with rhinitis and cough but without respiratory distress (in eight cases combined with wheezing) not requiring hospitalization at the time of the RSV epidemic. The groups were similar for 10 of 11 background factors, including family history of atopy/asthma. There were significant differences in the number of siblings (Table 2).
Table 1.
Anti-RSV IgG at the age of 1 year and respiratory symptoms during the RSV epidemic*
| Uninfected (n = 26) | Mild RSV infection (n = 15) | RSV bronchiolitis (n = 27) | |
|---|---|---|---|
| Anti RSV IgG (units)* | 2·8 | 22·4 | 17·3 |
| (1–6) | (14–30) | (6–30) | |
| Wheezing | 0 | 8 | 25 |
| Cough | 10 | 12 | 27 |
| Tachypnoea (>55 breaths/min) | 0 | 0 | 27 |
| Poor drinking | 0 | 0 | 23 |
| Chest indrawing | 0 | 0 | 22 |
| Oxygen supplemetation | 0 | 0 | 20 |
Data are presented as n or median (range).
Table 2.
Evaluation of background factors at the age of 1 year*
| Background factor | Uninfected (n = 26) | Mild RSV infection (n = 15) | RSV bronchiolitis (n = 27) | P-value |
|---|---|---|---|---|
| Heredity for atopy | 9 | 8 | 9 | 0·4516 |
| Heredity for asthma | 3 | 2 | 7 | 0·3031 |
| Male sex | 17 | 8 | 13 | 0·4389 |
| Smoking by father | 19 | 10 | 13 | 0·1587 |
| by mother | 17 | 8 | 14 | 0·5715 |
| during pregnancy | 9 | 4 | 11 | 0·6555 |
| Indoor furred animals | 4 | 4 | 9 | 0·2416 |
| No. of siblings | 0·50 ± 0·51† | 0·75 ± 0·45 | 1·53 ± 1·40 | 0·0071 |
| Mean duration of pregnancy (weeks) | 39·9 ± 0·8 | 39·8 ± 0·9 | 39·3 ± 1·2 | 0·221 |
| Mean birth weight kg | 3·6 ± 0·3 | 3·6 ± 0·4 | 3·4 ± 0·6 | 0·2320 |
| Breast feeding (months) | 3·2 ± 2·7 | 2·5 ± 1·8 | 1·8 ± 2·0 | 0·2435 |
Data are presented as n or mean ± s.d.
P = 0·0022 for uninfected versus bronchiolitis.
Cell culture
Generation of dendritic cells from cord blood
Haematopoietic stem cells were isolated using anti-CD34 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and MiniMACS separation columns (Miltenyi Biotec). Cells were cultured at 37°C in a humidified atmosphere in the presence of 5% CO2 for 12 days with rh-granulocyte-macrophage stimulating factor (GM-CSF) (100 ng/ml, Novartis, Nürnberg, Germany), rh-tumour necrosis factor (TNF)-α (2·5 ng/ml; Peprotech, Rocky Hill, NJ, USA) and rh stem cell factor (SCF) (100 ng/ml; Peprotech) in RPMI-1640 (Biochrom, Berlin, Germany) 10% fetal calf serum (FCS) (Biochrom). Dendritic cells (DCs) were purified by using anti-CD1a microbeads (Miltenyi Biotec) and an AutoMACS separation device (Miltenyi Biotec) [13]. Cell aliquots were resuspended in cell freezing medium (10% dimethyl sulphoxide in 45% FCS, 45% RPMI; Biochrom) at 10 × 106/ml to 30 × 106/ml in rubber-gasketed microscrew cap tubes (Sarstedt, Nümbrecht, Germany). The vials were transferred to a liquid nitrogen freezing chamber.
Generation of dendritic cells from monocytes
Peripheral blood monocytes were purified using anti-CD14 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and cultured at 37°C in a humidified atmosphere in the presence of 5% CO2 in RPMI-1640 supplemented with 10% FCS (Biochrom), 50 ng/ml GM-CSF and 1000 U/ml IL-4 for 6–7 days [14]. Cell aliquots were resuspended in cell freezing medium (10% dimethyl sulphoxide in 45% FCS, 45% RPMI; Biochrom) at 10 × 106/ml to 30 × 106/ml and frozen (see above).
Isolation of naive T cells
Naive autologous CD4+CD45RA+ T cells were obtained from the effluent of stem cell or monocyte separations by negative sorting with anti-HLA-DR, anti-CD8, anti-CD56, anti-CD45RO magnetic beads (Miltenyi Biotec) using an AutoMACS separation device (Miltenyi Biotec) and frozen (see above).
Preparation of RSV and infection with RSV
A viral stock (RSV long strain) was prepared by infection of HEp2 cells as described previously [13]. Cell lines and virus preparations were tested for mycoplasma by polymerase chain reaction (PCR) with a mycoplasma detection kit as described in the manufacturer's manual (American Type Culture Collection, Manassas, VA, USA). Cell suspensions were incubated for 2 h with 1 ml of virus at a multiplicity of infection (MOI) of 10. The inoculum was removed and replaced by culture medium containing 10% FCS.
Co-culture and flow cytometry
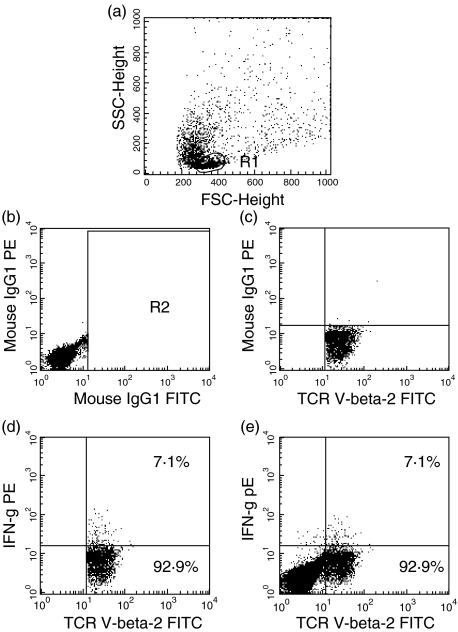
Because of the small amount of blood that could be obtained, not all tests were performed in every child. The number of mononuclear cells required for a set of co-culture experiments was 6 × 106. Therefore, the number of tests performed in every child depended on the number of mononuclear cells obtained. Cells were thawed and DCs were either left unstimulated, infected with RSV or prestimulated with poly IC 10 µg/ml (Sigma, Taufkirchen, Germany) for 24 h. Co-cultures of 5 × 103 DCs and 5 × 104 autologous naive T cells/well in 96-well flat-bottomed microtitre plates (Nunc, Wiesbaden, Germany) were incubated with 10 ng/ml toxic shock syndrome toxin (TSST 1) (Toxin Technology, Sarasota, FL, USA) and analysed for cytokine production by intracellular staining after 3 days of incubation. Cytokine secretion was inhibited by brefeldin 1 µm (Sigma, Taufkirchen, Germany) for 16 h. In a separate set of experiments, after 3 days of culture with TSST 1 cells were stimulated with 10 ng/ml phorbol myristate acetate (PMA) and 1 µm ionomycin for 5 h and cytokine production was inhibited by 2·5 µm monensin. The cytokines were measured in fixed and permeabilized cells as described previously [15]. The permeabilized cells were incubated with fluoroscein isothiocyanate (FITC)-labelled antibodies against T cell-receptor (TCR)-Vβ2 chain (Beckman Coulter, Unterschleissheim, Germany), and phycoerythrin (PE)-labelled anti-interferon-γ or anti-IL-4 (Pharmingen, Heidelberg, Germany) for 20 min at 4°C (Medac, Hamburg, Germany). After washing with saponin buffer the cells were resuspended in 200 µl Hanks's balanced salt solution (HBSS) for flow cytometric analysis with a FACScan® flow cytometer (Becton Dickinson, Mountain View, USA). Gating was performed as outlined in Fig. 1.
Fig. 1.
Consecutive steps in the analysis of single cytokine production in TCR-Vβ2+ cells. (a) The complete lymphocyte population was identified on the basis of morphological characteristics. (b) Cells were labelled with fluorescein isothiocyanate (FITC) and phycoerythrin (PE) isotype controls. A gate (R2) excluding the FITC isotype control labelled cells was set. (c) Cells were labelled with FITC anti-TCR-Vβ2 and PE isotype control. Quadrants discriminating PE positive and negative cells were set. (d) Cells were labelled with FITC anti-TCR-Vβ2 and PE anti-interferon-γ. Interferon-γ-producing cells were discriminated from nonproducing cells within the TCR-Vβ2+ cells and percentages were determined. (e) Percentages determined in part (d) were given with the whole data set.
Statistics
Comparisons of results with and without RSV infection were performed by Wilcoxon signed rank test. Correlations between measurements at different time points were calculated by Spearman's rank test. For comparisons between groups, χ2-test was used for discrete variables. Kruskal–Wallis test and Dunn's multiple comparison test as post-test were used for continuous variables using the statview 5·0 program package (SAS Institute, Cary, NC). A value of P < 0·05 was regarded as significant.
RESULTS
RSV mediated inhibition in cord blood correlates with inhibition of interferon-γ production at 1 year and 4 years of age
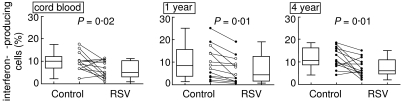
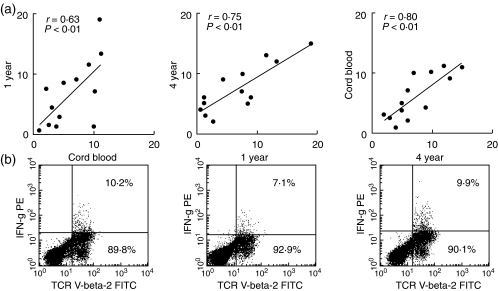
Thirteen children were followed prospectively from birth and underwent follow-up examinations at a mean age of 1 year and 4 years. Dendritic cells were derived from myelopoietic stem cells at birth and blood monocytes at 1 year and 4 years. The dendritic cells were infected with RSV and co-cultured with naive T cells stimulated with TSST 1 for 3 days. Interferon-γ generation was assessed by intracellular staining in Vβ2+ T cells, which are stimulated preferentially by the superantigen TSST 1. As shown in Fig. 2, RSV infection of dendritic cells resulted in a reduced rate of interferon-γ production in co-cultured T cells irrespective of the age at which the blood was obtained. In addition, the rate of interferon-γ-producing cells did not change significantly with age. At 1 year of age, RSV reduces interferon-γ generation in cultures from children with (P < 0·01) and without (P < 0·03) previous RSV infection. In addition, we asked whether individual levels of interferon-γ generation persist during infancy and early childhood. As shown in Fig. 3a, we found a significant correlation between the frequencies of interferon-γ-producing cells detected in RSV-infected cultures from cord blood and from peripheral obtained at 1 year as well as 4 years. In addition, a significant correlation between results was obtained with cells from 1 year and 4 years of age.
Fig. 2.
RSV depresses interferon-γ production in samples obtained at birth, at 1 year and at 4 years of age. Stem cell- or monocyte-derived dendritic cells were either infected with RSV or mock infected. DCs were cultured with peripheral blood naive T cells and TSST 1 for 3 days. The expression of interferon-γ in TSST 1 reactive Vβ2+ cells was measured by flow cytometry. The diagrams shows the results without (control) and with RSV infection in dot-plot as well as box and whiskers. The box delineates the median and the upper and lower quartile, whereas the whiskers delineate the range. The open dots represent values obtained from children without clinical contact with RSV and the closed circles represent values from children after contact with RSV as indicated by a positive serology.
Fig. 3.
RSV depressed interferon-γ frequencies at birth correlate with low frequencies at 1 year of age and 4 years of age. Correlations are demonstrated by diagrams in (a). (b) Original data from one typical example of measurements with cells obtained at the three time-points. Flow cytometric measurements were evaluated as described in Fig. 1.
Interferon-γ generation is reduced in patients with a history of bronchiolitis but not after mild RSV infection
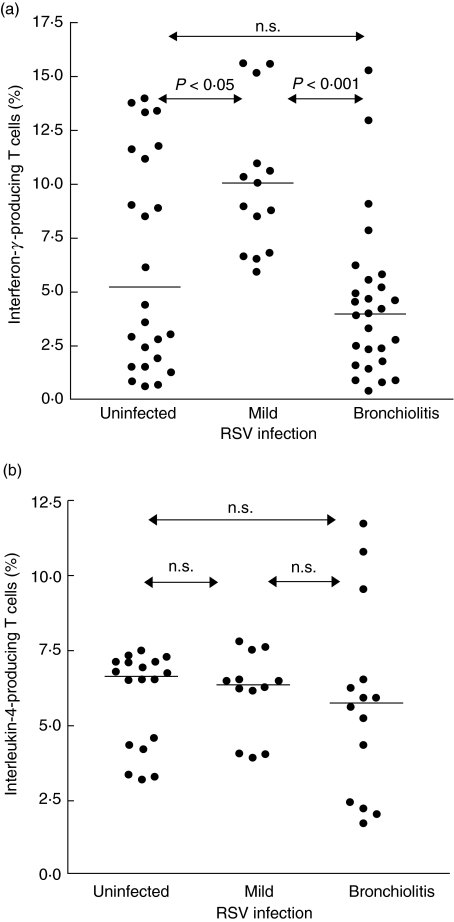
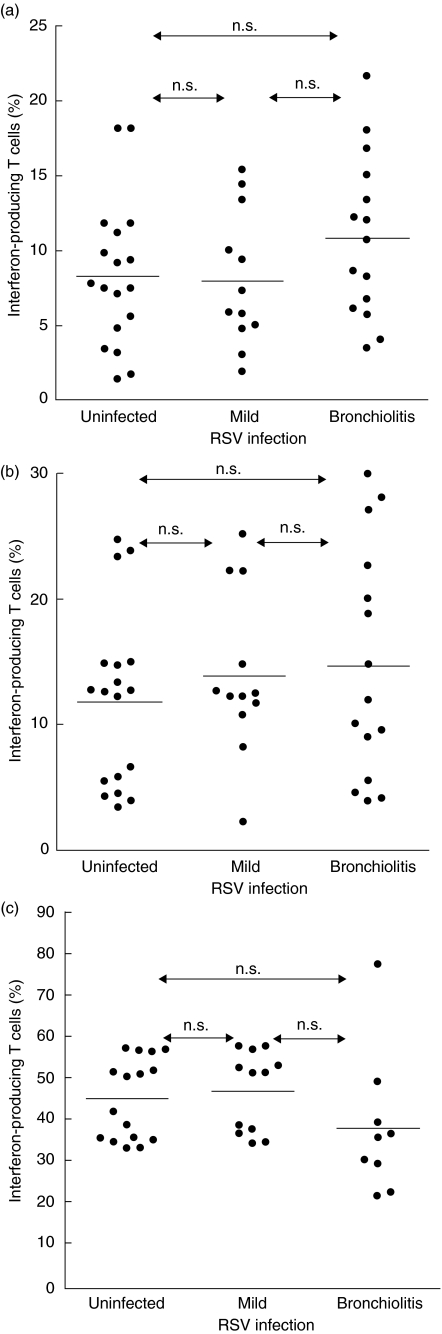
In the next set of experiments, we asked whether there were differences between children with a history of RSV bronchiolitis and children with a benign course of RSV infection. The frequency of interferon-γ-producing cells was significantly lower (P < 0·001) in cultures from children with a history of bronchiolitis compared to children with mild RSV infection. Cultures from children without infection displayed a wide range of results. Overall, interferon-γ generation in this group was still lower (P < 0·05) than in the group with mild RSV infection. (Fig. 4a). In contrast, there was no difference with regard to interleukin (IL)-4 generation between the three groups (Fig. 4b).
Fig. 4.
RSV modulated cytokine production in patients with a history of RSV bronchiolitis and controls. Blood samples were obtained at 1 year of age. Monocyte derived dendritic cells were infected with RSV (MOI 10) and cultured with peripheral blood naive T cells and TSST 1 for 3 days. The expression of interferon-γ (a) and interleukin-4 (b) in TSST 1 reactive Vβ2+ cells was measured by flow cytometry as described in Methods. The infants were grouped according to their history of RSV infection. Horizontal lines denote the medians. Significant differences between the groups are indicated.
Interferon-γ generation in response to other stimuli
To assess whether the observed variations were confined to cultures treated with RSV, RSV infection was omitted in parallel cultures; in addition cultures were set up with poly IC to stimulate dendritic cells or restimulated with phorbolester/ionomycin, potent inductors of interferon-γ generation, at the end of the culture. Under these conditions, there were no differences in interferon-γ generation between the three groups of children (Fig. 5).
Fig. 5.
RSV modulated cytokine production. Blood samples were obtained at 1 year of age. Monocyte-derived DCs were cultured with peripheral blood naive T cells and TSST 1 for 3 days. The expression of interferon-γ in TSST 1 reactive Vβ2+ cells was measured by flow cytometry as described in Methods. The infants were grouped according to their history of RSV infection. Horizontal lines denote the medians. (a) Results of untreated cells; (b) results obtained with poly IC stimulation and (c) results after restimulation of the cells with PMA/ionomycin before cytokine measurement.
DISCUSSION
Respiratory syncytial virus is the most prominent pathogen causing airway infection in the first year of life. Interferon-γ is essential for limiting RSV infections. In mice, the resolution of RSV infection coincides with interferon-γ production [16] and prophylactic intranasal interferon-γ gene transfer decreases RSV replication and infection [17]. In human epithelial cells the interferon-γ-mediated inhibition of RSV infection involves the 2′−5′ oligoadenylate synthetase/RNase L pathway [18]. Because the most severe infections occur between 6 weeks and 6 months of age [4], RSV seems to interfere with the primary immune reaction. Recently, we were able to show that RSV infection decreases the rate of interferon-γ-producing T cells in an in vitro model for the primary immune reaction [11].
The aim of this study was to elucidate whether this phenomenon could explain the occurrence of severe RSV infections. In a first set of experiments we had to examine whether our culture protocol, which originally makes use of cord blood cells, could also be applied to cells obtained from the peripheral blood during early childhood. Thirteen children were followed from birth to the fourth year of life. Cultures were set up with monocyte-derived dendritic cells, naive T cells and TSST 1 as stimulus mimicking antigen. Cultures with cells from the first as well as the fourth year of life showed a decrease in the rate of interferon-γ producing cells, as was observed with cord blood cells. Moreover, when comparing individual levels of interferon-γ production, the rates appear to be highly constant irrespective of serological proven previous RSV infection. Thus, it is most probable that the results reflect the influence of RSV on the primary immune response of the individuals studied. This notion is backed by another line of evidence. T cells used in the experiment were CD4+ CD45RA+ cells and thus bear the well-known markers of naive T cells [19]. In recent years the existence of CD45RA+ memory cells has been reported [20]. However, these cells were found in patients [20] and animals [21] after haematopoietic stem cell transplantation and were found in adult recipients without a considerable thymic output of naive cells. In our experiments we studied the peripheral blood of healthy children up to 4 years of age who had presumably had a high thymic output of naive cells. Thus, it is also conceivable from this point of view that not memory but naive cells and thus a model of the primary immune response was studied.
As the antigen used in the cultures was a superantigen, it is most probable that the effect of RSV is unspecific. In fact, in a previous report [13] we were able to demonstrate that RSV infection of dendritic cells induced the release of prostaglandin E2, IL-6, IL-10 and IL-11, whereas parainfluenza virus induced only IL-6 and influenza induced predominantly IL-12p75. Only CD86 but not MHC II and CD83 were up-regulated in response to RSV infection. Thus, RSV induces only a partial maturation of infected dendritic cells [11].
In addition, it has been shown that the non-structural proteins of RSV might be responsible for suppression of the interferon-γ synthesis in RSV-infected cultures. Human [22] as well as bovine [23] RSV non-structural proteins were recently demonstrated to suppress interferon-α/β production and interferon-α/β are known to be essential factors regulating interferon-γ synthesis [24]. These results are in line with our previous finding that the interferon-γ reducing effect in co-cultured naive T cells was observed only with viable RS virus [11]. The non-structural proteins are only expressed during viral replication.
In contrast to naive cells, it has been reported in the literature that memory cells from children at 7 years of age respond to inactivated virus with a considerable interferon-γ generation [25]. Because interferon-γ is an important mediator in limiting the replication of RSV [17] as well as in fully activating dendritic cells [26], interferon-γ production by memory cells might be important for limiting RSV infection to local inflammation as is observed in older children and adults.
In the second set of experiments we characterized children with either a history of severe RSV bronchiolitis, a mild RSV infection or no infection at the age of 1 year. The most interesting results were obtained in cultures set up with RSV-infected dendritic cells. The group without infection comprised children with a wide range of interferon-γ production. The children who suffered from severe bronchiolitis displayed remarkably low interferon-γ levels. In contrast, the group with mild infection showed significantly high interferon-γ levels compared to the two other groups. Because we have ruled out that memory cells play a role in the experiments performed, the most likely explanation for our results is that a high generation of interferon-γ protects from severe RSV-mediated disease. Our finding is even more interesting as differences were found neither in IL-4 generation in response to RSV nor in interferon-γ generation in response to other stimuli such as poly IC mimicking double-strained RNA or PMA/ionomycin as a potent T cell stimulus. These findings point to the fact that low interferon-γ generation is due to the interaction with virus and not due to a low capacity to produce interferon-γ as such. Comparable results have been described for other viruses. When exposed to rhinovirus, peripheral mononuclear blood cells from asthmatic subjects produced significantly lower levels of interferon-γ than normal subjects [27,28].
For RSV, interferon-γ generation has been examined before in peripheral blood [7,29] or nasopharyngeal aspirates [30] from children with ongoing infections. Children requiring oxygen [7] or mechanical ventilation [29,30] display lower interferon-γ levels compared to children with a milder course of the disease. However, it remains unclear from these studies whether the observed differences are inherent to the immune system or are due to different viral doses [31], treatment or stress [32] accompanying the illness. All these influences were eliminated in our in vitro system.
Interestingly, in the group without infection, rates of interferon-γ-producing cells encompass a wide range of values. Low rates were found in children with severe bronchiolitis, as well as high rates as found in children with mild RSV infection. It is conceivable that reaction to RSV infection is determined by genetic factors and that those children who tend to a low interferon-γ generation and in addition are infected at an early age will develop severe bronchiolitis whereas the others will only suffer from mild disease. This speculation is even more interesting, as gene polymorphisms associated with a severe course of RSV infection have been reported for TGF-β [33], IL-4, IL-4 receptor-alpha [34,35] and IL-10 [36]. Furthermore, an as-yet unidentified factor interacting with RSV non-structural proteins [22,23] might exhibit genetic polymorphism associated with severe disease.
Further understanding of the genetic basis of the interaction of RSV and the host immune cells is desperately needed. This may, on one hand, facilitate the identification of an ideal active vaccine that protects the few children at risk but does no harm to the others [37]. On the other hand, the expensive passive immunization already available [38] might be restricted to those genetically at risk to develop severe RSV mediated disease.
Acknowledgments
This work was supported by grants from the Bundesministerium für Bildung und Forschung (01GC9801), the Deutsche Forschungsgemeinschaft (DFG Scha 385/4–1) and from Abbott Laboratories.
REFERENCES
- 1.Johnston SL. The role of viral and atypical bacterial pathogens in asthma pathogenesis. Pediatr Pulmonol Suppl. 1999;18:141–3. [PubMed] [Google Scholar]
- 2.McIntosh K, Ellis EF, Hoffman LS, Lybass TG, Eller JJ, Fulginiti VA. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr. 1973;82:578–90. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 4.Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354:847–52. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 5.Renzi PM, Turgeon JP, Marcotte JE, et al. Reduced interferon-gamma production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159:1417–22. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]
- 6.Roman M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 7.Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–8. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 8.Beyer M, Bartz H, Horner K, Doths S, Koerner-Rettberg C, Schwarze J. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol. 2004;113:127–33. doi: 10.1016/j.jaci.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–7. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 10.Klagge I, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–33. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 11.Bartz H, Türkel Ö, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naïve T cells. Immunology. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer U, Hoffjan S, Bittscheid J, et al. Respiratory syncytial virus bronchiolitis and risk of wheeze and allergic sensitization in the first year of life. Eur Respir J. 2002;20:1277–83. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 13.Bartz H, Büning-Pfaue F, Türkel Ö, Schauer U. Respiratory syncytial virus (RSV) induces prostaglandin E2 (PGE2), IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129:438–45. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Meth. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Matsuse H, Behera AK, Kumar M, Rabb H, Lockey RF, Mohapatra SS. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J Immunol. 2000;164:6583–92. doi: 10.4049/jimmunol.164.12.6583. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. Intranasal IFN-gamma gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine. 1999;18:558–67. doi: 10.1016/s0264-410x(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 18.Behera AK, Kumar M, Lockey RF, Mohapatra SS. 2′−5′ oligoadenylate synthetase plays a critical role in interferon- gamma inhibition of respiratory syncytial virus infection of human epithelial cells. J Biol Chem. 2002;277:25601–8. doi: 10.1074/jbc.M200211200. [DOI] [PubMed] [Google Scholar]
- 19.Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 20.Fallen PR, Duarte RF, McGreavey L, et al. Identification non-naive CD4+CD45RA+ T cell subsets in adult allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32:609–16. doi: 10.1038/sj.bmt.1704185. [DOI] [PubMed] [Google Scholar]
- 21.Bell EB, Sparshott SM, Bunce C. CD4+ T cell memory, CD45R subsets and the persistence of antigen − a unifying concept. Immunol Today. 1998;19:60–4. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 22.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and gamma interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J Virol. 2004;78:4363–9. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valarcher JF, Furze J, Wyld S, Cook R, Conzelmann KK, Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J Virol. 2003;77:8426–39. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parronchi P, Mohapatra S, Sampognaro S, et al. Effects of interferon-alpha on cytokine profile, T cell receptor repertoire and peptide reactivity of human allergen-specific T cells. Eur J Immunol. 1996;26:697–703. doi: 10.1002/eji.1830260328. [DOI] [PubMed] [Google Scholar]
- 25.Pala P, Bjarnason R, Sigurbergsson F, Metcalfe C, Sigurs N, Openshaw PJ. Enhanced IL-4 responses in children with a history of respiratory syncytial virus bronchiolitis in infancy. Eur Respir J. 2002;20:376–82. doi: 10.1183/09031936.02.00249902. [DOI] [PubMed] [Google Scholar]
- 26.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–32. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091–4. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 29.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–9. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 30.Bont L, Heijnen CJ, Kavelaars A, et al. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis. 2001;184:355–8. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 31.Hall CB, Douglas RG, Jr, Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–83. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 33.Hoffjan S, Ostrovnaja I, Nicolae D, et al. Genetic variation in immunoregulatory pathways and atopic phenotypes in infancy. J Allergy Clin Immunol. 2004;113:511–18. doi: 10.1016/j.jaci.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Hoebee B, Rietveld E, Bont L, et al. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J Infect Dis. 2003;187:2–11. doi: 10.1086/345859. [DOI] [PubMed] [Google Scholar]
- 35.Choi EH, Lee HJ, Yoo T, Chanock SJ. A common haplotype of interleukin-4 gene IL4 is associated with severe respiratory syncytial virus disease in Korean children. J Infect Dis. 2002;186:1207–11. doi: 10.1086/344310. [DOI] [PubMed] [Google Scholar]
- 36.Hoebee B, Bont L, Rietveld E, et al. Influence of promoter variants of interleukin-10, interleukin-9, and tumor necrosis factor-alpha genes on respiratory syncytial virus bronchiolitis. J Infect Dis. 2004;189:239–47. doi: 10.1086/380908. [DOI] [PubMed] [Google Scholar]
- 37.Polack FP, Karron RA. The future of respiratory syncytial virus vaccine development. Pediatr Infect Dis J. 2004;23(Suppl. 1):S65–73. doi: 10.1097/01.inf.0000108194.71892.95. [DOI] [PubMed] [Google Scholar]
- 38.Handforth J, Sharland M, Friedland JS. Prevention of respiratory syncytial virus infection in infants. Br Med J. 2004;328:1026–7. doi: 10.1136/bmj.328.7447.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]