Abstract
Natural killer (NK) cells are an important component of the immediate immune response to infections, including infection by intracellular bacteria. We have investigated recognition of Chlamydia trachomatis (CT) by NK cells and show that these cells are activated to produce interferon (IFN)-γ when peripheral blood mononuclear cells (PBMC) are stimulated with CT organisms. Furthermore, infection of epithelial cell lines with CT renders them susceptible to lysis by human NK cells. Susceptibility was observed 18–24 h following infection and required protein synthesis by the infecting chlamydiae, but not by the host cell; heat or UV inactivated chlamydiae did not induce susceptibility to NK cell lysis. CT infection was also shown to decrease the expression of classical and non-classical major histocompatibility complex (MHC) molecules on infected cells, thus allowing recognition by NK cells when combined with an activating signal. A candidate activating signal is MICA/B, which was shown to be expressed constitutively on epithelial cells.
Keywords: bacterial infection, human, NK cells
INTRODUCTION
Chlamydia trachomatis (CT) is an obligate intracellular bacterium that infects the genital tract and the eye and is a major cause of infertility in women and of blindness [1,2]. Only a proportion of women with lower genital tract infection develop salpingitis and pelvic inflammatory disease leading to infertility, and similarly the majority of children can resolve ocular infection without proceeding to the scarring of trachoma. Therefore, there is considerable interest in the control of CT infection by the immune system, and aspects of the immune response that predispose to clinical complications of infection. One component of the immune response to infection that has received relatively little attention with respect to CT infection is the natural killer (NK) cell.
NK cells have been shown to be important in host defences against viral infections such as human cytomegalovirus (CMV) [3], and they have also been implicated in immune responses to intracellular bacteria such as Salmonella and Listeria[4,5] and parasite infections [6]. Lysis of infected cells by NK cells forms part of the innate host defence against infection. The importance of their role in vivo in humans has been shown by studies of humans with NK cell deficiency, in whom a particular susceptibility to herpesvirus infections was identified [7].
NK cells are either activated or inhibited when they interact with a target cell, depending on the strength of the signals obtained through activating and inhibitory receptors [8]. The majority of the inhibitory signals are transmitted through the interactions of members of the killer cell immunoglobulin-like receptor (KIR) family of molecules with HLA-A, B and C [9], and the interaction of the C-type lectin CD94/NKG2A with HLA-E [10]. Activating signals are transmitted through the interactions between other members of the KIR family with HLA-A, B and C [11], NKG2D with MICA/B [12] and natural cytotoxicity receptors with ligands that have not yet been characterized fully [13]. There are additional receptors found on NK cells for which ligands and signalling function are as yet unidentified.
Several viruses [14,15] and some intracellular bacteria [16] have mechanisms to decrease expression of major histocompatibility complex (MHC) class I antigens in the host cell. While this decreases presentation of pathogen-derived antigenic peptides to CD8+ cytotoxic lymphocytes, it also decreases the ability of the host cell to interact with the inhibitory receptors of NK cells and thereby increases the cell's susceptibility to lysis by NK cells, leading to the subsequent destruction of the intracellular pathogen. From the point of view of the host, NK cells provide a way of dealing with pathogens that have the ability to interfere with recognition by the adaptive immune system. From the point of view of the pathogen, additional strategies other than down-regulation of MHC class I are required to avoid recognition by the innate immune system. These can include the expression of alternative ligands for NK cell receptors or suppression of expression of ligands, as has been shown recently for human CMV [17–22].
In murine models of CT infection of the genital tract, NK cells are recruited into the genital epithelium at early time-points after infection, and these cells produce interferon (IFN)-γ[23]. Furthermore, CT has been reported to decrease expression of MHC class I molecules [16], which might increase the susceptibility of CT-infected cells to lysis by NK cells, but this has not been investigated previously. Therefore we have undertaken a detailed study of the recognition of CT-infected cells by human NK cells, and also investigated the effects of CT infection on the expression of the different inhibitory and activating ligands recognized by NK cells.
MATERIALS AND METHODS
IFN-γ production by PBMC infected with CT
PBMC from healthy donors were isolated by density gradient centrifugation over Ficoll-Hypaque (Amersham Pharmacia Biotech, Amersham, UK), resuspended in culture medium (CM) (RPMI-1640 supplemented with 2·5 mm sodium pyruvate (Sigma, Haverhill, UK), 2·5 mm non-essential amino acids (Sigma) and 2·5 mm l-glutamine) containing 5% human AB serum, and plated into 2 ml wells. They were then infected with CT L2 elementary bodies (EBs) at multiplicity of infection (MOI) of 10 and incubated overnight. Golgi stop was added for the last 6 h of culture. Uninfected PBMC were used as a negative control. Cells were then analysed by flow cytometry (see below).
Generation and maintenance of NK cell lines
PBMC from healthy donors were isolated by density gradient centrifugation over Ficoll-Hypaque. PBMC were incubated at 37°C for 60 min and the non-adherent cells collected. The non-adherent PBMC were resuspended in CM containing 10% human antibody serum. Cocultures were established between 1·5 × 106 non-adherent cells and 5 × 105 irradiated (3·7Gy) cells from the MHC class I-negative B lymphoblastoid cell line (LCL), 721·221, in 2 ml wells. These cultures were maintained at 37°C in 5% CO2 for 5–7 days. Following coculture, the NK cells were isolated by negative selection using the MACS protocol (Miltenyi Biotech, Germany). Hybridoma supernatants UCHT1 (anti-CD3), UCHM1 (anti-CD14), QS4120 (anti-CD4) and BU12 (anti-CD19) (all the kind gift of Professor Peter Beverley) were diluted in buffer [phosphate buffered saline (PBS), 0·5% bovine serum albumin (BSA; Sigma), and 2 mm ethylene diamine tetra-acetic acid (EDTA; BDH, Poole, UK)]. NK cells were collected using LD+ (>107 cells) or MS+ (<107 cells) MACS columns (Miltenyi Biotech, Germany) and cultured in NK culture medium supplemented with 30% conditioned media from the PBMC:721.221 cocultures and 100 IU/ml rhIL-2. The NK cell lines were periodically restimulated with irradiated PBMC and 721·221 cells, or PBMC and allogeneic LCL cells. Flow cytometry showed that the NK cell lines generated by these techniques were CD3-, CD94+.
Culture of target cell lines
HeLa-229 and SiHa, cervical epithelium derived cell lines (ATCC), were maintained in culture in RPMI supplemented with 5% fetal calf serum (FCS, Firstlink), 20 mm HEPES (Sigma) and 2·5 mm l-glutamine (RPMI–FCS). The cells were passaged using trypsin-EDTA (Sigma). 721·221 and K562 cells were maintained in suspension culture in RPMI–FCS.
Preparation of CT and infection of target cells
CT organisms were propagated and harvested from HeLa cells according to standard protocols [24]. Target cell infection protocols were optimized for each cell type. Epithelial cell lines (SiHa and HeLa) were infected with 5 IFU per cell and cultured for 60 min at 37°C. The medium containing organisms was then replaced with fresh medium and the cell cultured for 24 h prior to use in cytotoxicity assays. Non-adherent K562 cells were infected at 10 infection units (IFU) per cell with 30 min centrifugation (1400 g). Again fresh medium was added after centrifugation and the cells cultured for 24 h. The same procedure was used with heat or UV-inactivated EBs (see below) and mock-infected cells also received the same periods of incubation and/or centrifugation. The strains used were the type strain of Serovar L2 (L2/434/BU) and Serovar K (kind gift of Dr Jens Kuipers). CT organisms were inactivated by either exposure to UV-light or heat. For UV inactivation, the EBs were treated for 10 min at maximum dose setting [UV-Stratalinker 2400 (Stratagene, Amsterdam Holland)]. Heat inactivation was achieved by the heating of the EBs to over 70°C for 10 min.
In experiments where the effects of antibiotics were tested, rifampicin (1 µg/ml) and penicillin (100 µg/ml) were added at the time of infection and remained in the culture medium for the 24 h before the cells were tested in cytotoxicity assays. The effect of inhibition of host cell protein synthesis by cycloheximide (1 µg/ml) was also tested by adding the drug at the time of infection with CT.
Cytotoxicity assay
The Cytotox 96 (Promega, Southampton, UK) is a colourimetric cytotoxicity assay measuring lactate dehydrogenase (LDH) levels; the manufacturer's protocols were followed. All assays were performed in triplicate using phenol-red free RPMI (Gibco, Grant Island, NY, USA). The total time of incubation was 4 h unless indicated otherwise. LDH release was analysed colourimetrically using the Labsystems Original Multiskan MS Elisa Plate Reader (Labsystems, UK) at 492 nm in conjunction with genesis software, version 2·12 (Labsystems).
Flow cytometric analysis of NK cells and expression of NK cell ligands on CT-infected cells
Cells for staining were resuspended in Facs buffer (PBS supplemented with 0·1% BSA and 0·01% sodium azide). 1 × 105−1 × 106 cells were stained with 100 µl of fluorescently conjugated antibody. Negative controls consisted of a fluorescently labelled isotype matched antibody. The plates were kept in the dark on ice for 20–30 min. Cells were fixed with 100 µl of 4% paraformaldehyde (Sigma). The cells were either analysed using a FacsSorter (Becton Dickinson, Oxford, UK) or a FacsCalibur (Becton Dickinson).
Analysis of ligands on infected cells and staining for intracellular interferon gamma were carried out using a permeabilization and fixation protocol to identify infected and cytokine-producing cells. The above protocol was followed until the fixation step; cells were then permeabilized using Perm/Fix reagent (Pharmingen/Becton Dickinson) for 30 min and washed in perm/wash buffer. Anti-IFN-γ–FITC (BD Biosciences/Pharmingen) or anti-CT LPS antibody (Dako, Ely, UK) reconstituted in perm/wash buffer was added for 30 min, and the cells were then analysed as above. Blocking with 10% mouse serum (Sigma) was used when dual staining with an unconjugated antibody followed by a conjugated antibody. Antibodies used were: anti-CD94-PE (BD Biosciences/Pharmingen), anti-CD3-RPE-Cy5 (Dako), anti-MHC class I (W6/32: kind gift of Dr Hidde Ploegh), anti-β2m (BM-63: Sigma), anti-HLA-C/E (DT9: Dr V. M. Braud), anti-CD58 (Serotec, Kidlington, UK), anti-CD71 (Serotec), anti-MICA (6D4: Dr T. P. Spies) and negative controls (Dako).
RESULTS
NK cells in PBMC produce IFN-γ in response to CT organisms
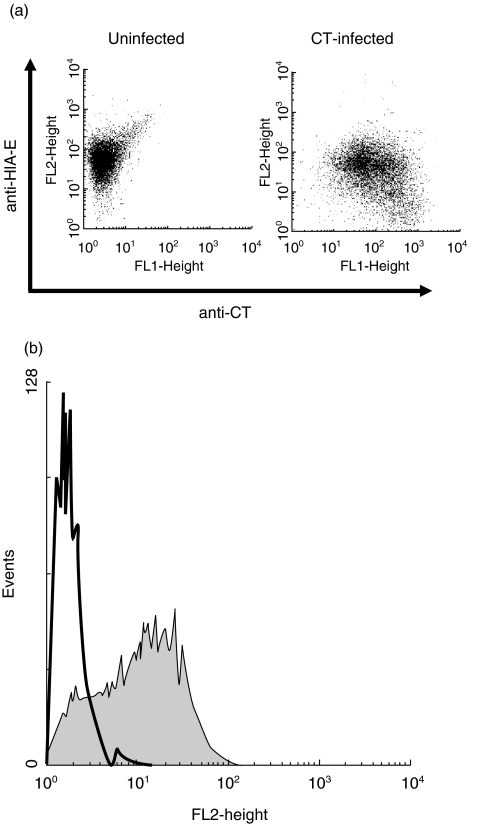
PBMC were cocultured with CT EBs and production of IFN-γ was measured in supernatants. As early as 14 h after the cultures were initiated, high levels (33 ng/ml) of IFN-γ were detected in supernatants (data not shown). To determine which cells were responsible for the production of the cytokine, we performed an intracellular flow cytometric analysis. In PBMC cocultured for 20·5 h with CT, a significant population of CD3- cells stained positively for IFN-γ. Further analysis revealed they were CD94+ (Fig. 1) and CD56+, markers typical of NK cells. Experiments with PBMC also showed consistent but low levels of lysis of CT-infected cells, consistent with the activity of NK cells within PBMC (data not shown). In view of these results, we sought to characterize NK cell recognition of CT-infected cells more fully using in vitro-generated NK cell lines.
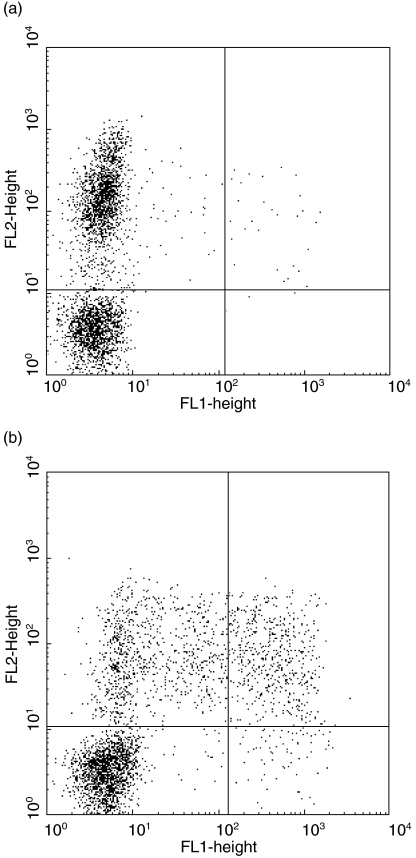
Fig. 1.
PBMC NK cells produce IFN-γ in response to CT EBs. Staining PBMC for expression of CD94-PE (y-axis) and intracellular IFN-γ-FITC (x-axis) after 20·5 h culture in: (a) medium alone, or (b) with CT EBs (10 organisms per cell). Golgi stop was added during the last 6 h of culture. IFN-γ+ CD94+ (NK) cells are readily visible in panel (b) (17%, top R quadrant; population gated on viable CD3- cells).
CT-infection does not protect K562 cells from lysis by NK cells
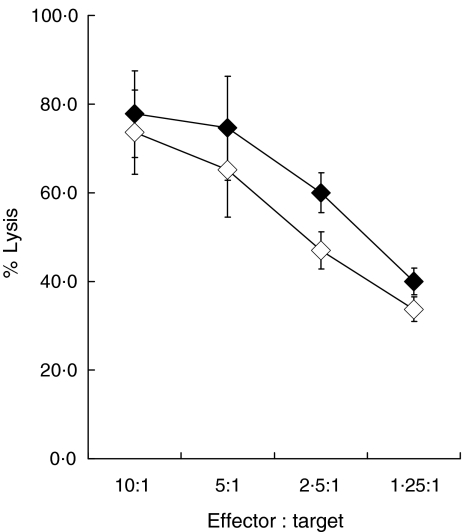
Before testing whether NK cells were able to recognize and lyse CT-infected cells, it was important to determine whether CT infection had any effect on lysis of cell lines such as K562, which are normally highly susceptible to NK lysis. This was relevant because it has been reported that CT-infected cells are resistant to the induction of apoptosis by molecules such as perforin and granzyme A, major components of the lytic granules released by NK cells [25]. Therefore NK cell lines were generated from the PBMC of healthy individuals and tested using CT infected and non-infected K562 cells. Infection of K562 was confirmed by intracellular staining with an anti-CT LPS antibody as described in Materials and Methods. As shown in Fig. 2, CT infection had no effect on the lysis of K562 by the NK cells; thus if CT infection confers resistance to perforin and granzyme another NK cell effector molecule, such as granulysin, may be responsible for the lysis of infected cells [26].
Fig. 2.
Infection by CT does not affect lysis of K562 by NK cells. Lysis of CT-infected (closed diamonds) and mock-infected (open diamonds) K562 cells by an NK cell line at effector : target ratios from 10 : 1 to 1·25 : 1.
CT-infection of transformed epithelial cell lines leads to an increase in susceptibility to lysis by NK cells
When SiHa cells, derived from cervical epithelial cells, were infected with CT, a significant increase in the level of lysis by NK cells was seen; Fig. 3a shows results from a representative experiment but similar results were obtained with NK cell lines from three different normal donors and from several independently derived lines from the same donor; the cytotoxicity experiments were performed 22–28 h following infection by CT. The same results were obtained when using CT of the L2 serovar (as shown in Fig. 3a) or serovar K (data not shown). Lysis was not due to an increased fragility of CT-infected cells; in parallel experiments with HLA-restricted CD8+ T cells no lysis of CT-infected cells was seen if they were not HLA-matched with the effector cells (data not shown). Furthermore, susceptibility to lysis required an interaction between viable organisms and the host cells as no increase in lysis was seen when cells were exposed to CT inactivated by UV irradiation; a similar lack of lysis was seen when using heat inactivated organisms. In the latter case lysis at E : T 20 : 1 was as follows: mock-infected 22%, CT-infected 30%, treated with heat-killed CT 21% (P = 0·03 for the difference in lysis between mock-infected and CT-infected cells). The same susceptibility to NK cell-mediated lysis following infection with viable CT was seen with HeLa cells (i.e. a second cervical epithelium-derived cell line, data not shown).
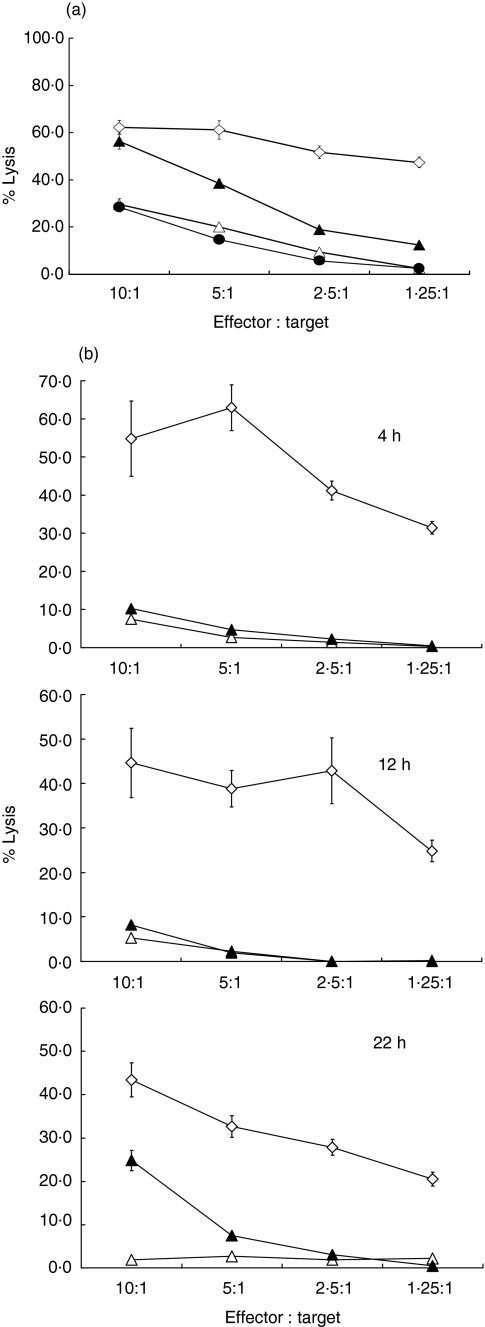
Fig. 3.
(a)Infection by viable CT renders epithelial cells susceptible to lysis by NK cells. Lysis of SiHa cells by NK cell lines cells following infection with live CT (closed triangles), UV inactivated CT (closed circles) or after mock infection (open triangles). Lysis of K562 (open diamonds) is shown for comparison. The difference between lysis of CT-infected and mock-infected cells is significant (P < 0·001 at effector : target ratios of 10 : 1). Significance was tested using Student's two-tailed t-test. (b) Time-course for the induction of susceptibility to lysis by NK cells following infection with CT. Lysis of SiHa cells by polyclonal NK cell lines at 4, 12 and 22 h following infection with CT (closed triangles) at effector : target ratios from 10 : 1 to 1·25 : 1. Lysis of mock-infected SiHa cells (open triangles) and of K562 (open diamonds) is also shown.
Time-course studies showed that the increase in susceptibility to NK cell-mediated lysis was first apparent in CT-infected cells at 18 and 24 h postinfection; the experiment shown in Fig. 3b is an example where significant lysis was first seen at 22 h postinfection.
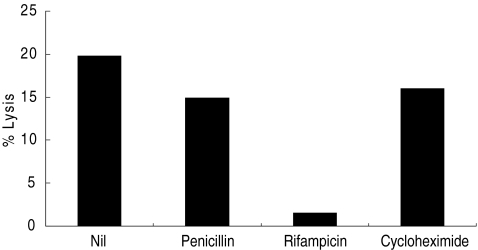
It was also shown that bacterial, but not host cell, protein synthesis was required to confer susceptibility to NK cell lysis, because treatment at the time of infection with rifampicin (which blocks bacterial protein synthesis) decreased lysis of infected cells, whereas cycloheximide (which blocks host cell protein synthesis) had no effect (Fig. 4). Interestingly, penicillin G, which interferes with the maturation of reticulate bodies into mature infectious elementary bodies, had no effect on the susceptibility to lysis of CT-infected SiHa cells, suggesting that not all stages of the CT replication cycle are required to confer susceptibility to NK cells.
Fig. 4.
Effects of inhibitors of bacterial and host cell metabolism on induction of susceptibility to lysis by NK cells. The increase in lysis of CT-infected versus mock infected SiHa cells (Δ percentage lysis) by NK cells, untreated after infection (nil) or following treatment with penicillin G, rifampicin, or cycloheximide. Results using an effector : target ratio of 20 : 1 are shown. Differences between levels of lysis of CT-infected and mock-infected cells are significant for untreated (P < 0·002), penicillin-treated (P < 0·005) and cycloheximide-treated (P < 0·02) cells, but not for rifampicin-treated cells (P = 0·34). Significance was tested using Student's two-tailed t-test.
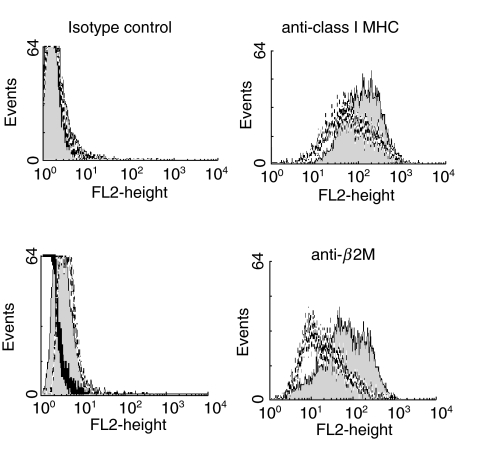
In view of the susceptibility to NK cell-mediated lysis demonstrated by CT infected SiHa cells, we investigated expression of ligands for NK cell receptors. The level of expression of MHC class I antigens was assessed using W6/32, which recognizes the β2m-bound form of MHC class I molecules. Zhong et al. [16] have shown previously that levels of expression of MHC class I antigens are reduced on HeLa cells following CT infection. We confirmed that this was also true for CT-infected SiHa cells using MoAb specific for MHC class I heterodimers or for β2m (Fig. 5); in contrast expression of CD71 (the transferrin receptor) was not affected by CT infection (data not shown).
Fig. 5.
CT infection decreases MHC class I antigen expression by epithelial cells. Expression of class I HLA antigens as detected by w6/32 (upper panel and a MoAb against β2m (lower panel) by CT-infected SiHa cells (dotted lines) or mock-infected SiHa cells (filled histograms). Staining with isotype match controls for each MoAb is also shown (left panels). Dead cells, which were more abundant in the CT-infected population, were excluded from analysis by gating on forward scatter.
W6/32 recognizes all classical and several non-classical MHC class I molecules (HLA-E and HLA-G). However, HLA-A and HLA-B comprise the majority of the W6/32-reactive cell surface molecules. This means that alterations in the level of staining with W6/32 at least reflect changes in HLA-A and HLA-B expression, while changes in HLA-C and HLA-E may not be detected readily. Therefore levels of expression of HLA-C and HLA-E were assessed using the DT9 antibody; as shown in Fig. 6a, the level of surface staining with DT9 on SiHa cells was also reduced following CT infection, with the lowest levels of expression being found in the cells staining most brightly for intracellular chlamydiae. This could reflect a reduction in expression of HLA-C or HLA-E, or of both. HLA-E levels are determined by the levels of expression of other MHC class I molecules, as these provide the leader peptides required for HLA-E expression [27]. Thus, as HLA-A -B and -C levels are reduced it is possible that HLA-E levels will also be reduced. Alternatively HLA-E may be down-regulated by CT, similarly to classical MHC class I molecules [16].
Fig. 6.
(a) CT infection decreases HLA-E and/or HLA-C expression by epithelial cells. Two-colour analysis of expression of HLA-E (y-axis), as detected by MoAb DT9 and of intracellular CT antigens (x-axis) by mock-infected SiHa cells or SiHa infected with live CT. (b) MICA/B is expressed constitutively by epithelial cells. Expression of MICA/B by SiHa cells (filled histogram). Staining using an isotype control MoAb is also shown (black line).
Finally, we examined expression of the MHC class I-related molecule MICA/B, which is a ligand for one of the activating NK cell receptors, NKG2D, using the 6D4 antibody. As shown in Fig. 6b, SiHa cells constitutively express MICA/B with no effect of CT infection on expression.
DISCUSSION
The data presented here show for the first time that human NK cells are able to respond to the important human pathogen, Chlamydia trachomatis, producing IFN-γ when PBMC are cocultured with CT EBs, and able to kill CT-infected epithelial tumour cell lines. The mechanism responsible for rapid production of IFN-γ is under investigation; while uptake of CT EBs by dendritic cells produces IL-12, this alone was not sufficient to elicit IFN-γ production by NK cells, although addition of IL-18 or supernatants from CT-infected epithelial cells to DC-derived IL-12 resulted in large amounts of IFN-γ (Hook et al., manuscript in preparation). In PBMC it is possible that NK cells interact with EBs directly or receive additional stimulation from cells which have taken up the EBs.
In exploring the mechanism underlying the susceptibility to NK cell lysis conferred by CT, it was found that CT infection resulted in a reduction in the level of surface expression of MHC class I molecules, as assessed by levels of staining with antibodies specific for β2m (BM63), or heterodimers (W6/32) or HLA-C/E (DT9). Because the activity of NK cells is inhibited by receptors which recognize MHC class I antigens, reduced expression of these surface molecules could confer susceptibility to lysis by NK cells [28].
Zhong et al. [16] also showed that MHC class I antigen expression, at both protein and mRNA levels, fell following infection with the L2 strain of CT. The same group showed initially that CT infection of cells abolished the IFN-γ-dependent increase in class II MHC expression [29], and reported later that IFN-γ induced up-regulation of class I was also reduced dramatically by CT infection [16]. These effects required chlamydial but not host cell protein synthesis, and were independent of the completion of the CT replication cycle; the same was found to be true of the induction of susceptibility to NK cell-mediated lysis by CT (Fig. 4). The S100 cytosolic fraction of CT-infected cells degrades the transcription factor RFX-5 [16], which is responsible for controlling synthesis of class I and class II MHC antigens, and also of β2M. More recently, the CT protein responsible for the destruction of RFX-5 has been identified [30] and is encoded by the open reading frame CT858. Thus, by degrading a crucial component of the transcription machinery required for maintenance of successful antigen presentation, CT is effectively able to prevent its own presentation to the adaptive immune system, but may thereby attract the attention of NK cells as an arm of innate immunity.
The decreased DT9 staining reflects a down-regulation of HLA-C and/or HLA-E which are the main ligands of inhibitory NK cell receptors KIR and CD94/NKG2A, respectively [10]. This down-regulation of HLA-C and HLA-E cell surface expression could involve several mechanisms: first, RFX-5 may also act on the HLA-C and HLA-E gene so that their expression falls following CT infection in the same way as that of HLA-A and -B antigens. Secondly, as expression of HLA-E is dependent on provision of leader peptides from other MHC class I molecules [27], CT infection which results in a fall in classical MHC class I expression will also limit the availability of the leader peptides needed for HLA-E expression. Finally, a peptide derived from human heat shock protein 60 has been shown to bind to HLA-E but prevent its interaction with CD94/NKG2A [31]. Human hsp60 may be induced in response to intracellular infection, or CT hsp60 which is a prominent antigen expressed by CT is likely to possess the same HLA-E inhibitory properties [31].
Chlamydiae are not unique in modulating MHC antigen expression as a way of avoiding recognition by the adaptive immune system. Human cytomegalovirus (hCMV) interferes with MHC class I antigen expression by the action of US2, 3, 6 and 11 gene products. US3 impairs processing of MHC class I so that the chains are retained in the perinuclear region of the cell [32], while US6 interferes with the loading of antigenic peptides by inhibiting TAP activity [33]. US2 and US11 bind and redirect MHC class I chains to the cytosol for proteasome degradation (discussed in Greijer et al. [34]). While these mechanisms to reduce MHC class I expression prevent T cell recognition of hCMV-infected cells, they could render the infected cells susceptible to lysis by NK cells. Indeed, this was observed when laboratory strains of hCMV were used to infect target cells [35–37]. However, clinical isolates of hCMV also avoid susceptibility to NK cells [38,39], and recent evidence points to additional avoidance mechanisms, such as UL142, which encodes an MHC class I-like molecule (M.W. personal communication). We observed increased susceptibility to NK cell lysis using both L2 and serovar K-infected cells, but it is possible that both these strains, which have been passaged for a number of years, might have lost the ability to counteract NK cells in the same way as laboratory strains of hCMV.
NK cells normally require a positive signal to activating NK cell receptors as well as the withdrawal of the inhibitory signals consequent to MHC class I down-regulation. We suggest that MICA/B, present constitutively on transformed epithelial cells, might represent such a positive signal. In natural infection CT infects epithelial cells of the genitourinary tract, and although there are no data on the expression of MICA/B by these cells, MICA/B is expressed in the gastrointestinal tract [40,41], particularly in response to stress, including bacterial infection [42]. It will now be important to examine susceptibility to NK cells of non-transformed genital tract-derived epithelial cells, including cells which maintain polarization.
Acknowledgments
We thank Dr Thomas Spies, Seattle, for the generous gift of MoAb 6D4. This work was funded by grants from MRC UK and the Arthritis Research Campaign.
REFERENCES
- 1.Schachter J. Chlamydial infections. N Engl J Med. 1980;298:428–35. doi: 10.1056/NEJM197802232980805. 490–5; 540–9. [DOI] [PubMed] [Google Scholar]
- 2.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia − intracellular biology, pathogenesis and immunity. Washington: ASM Press; 1999. p. 139. [Google Scholar]
- 3.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 4.Griggs ND, Smith RA. Natural killer cell activity against uninfected and Salmonella typhimurium-infected murine fibroblast L929 cells. Nat Immun. 1994;13:42–8. [PubMed] [Google Scholar]
- 5.Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 6.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL. Natural killer cell receptors and MHC class I interactions. Curr Opin Immunol. 1997;9:126–31. doi: 10.1016/s0952-7915(97)80169-0. [DOI] [PubMed] [Google Scholar]
- 10.Braud VM, Allan DSJ, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 11.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 12.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 13.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–34. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 14.Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunol Today. 2000;21:447–55. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 16.Zhong GM, Liu L, Fan T, Fan PY, Ji HZ. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in Chlamydia-infected cells. J Exp Med. 2000;191:1525–34. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulbrecht M, Martinozzi S, Grzeschik M, et al. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J Immunol. 2000;164:5019–22. doi: 10.4049/jimmunol.164.10.5019. [DOI] [PubMed] [Google Scholar]
- 18.Cosman D, Fanger N, Borges L, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–82. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 19.Tomasec P, Braud VM, Rickards C, et al. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031–3. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 20.Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 21.Braud VM, Tomasec P, Wilkinson GW. Viral evasion of natural killer cells during human cytomegalovirus infection. Curr Top Microbiol Immunol. 2002;269:117–29. doi: 10.1007/978-3-642-59421-2_8. [DOI] [PubMed] [Google Scholar]
- 22.Dunn C, Chalupny NJ, Sutherland CL, et al. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med. 2003;197:1427–39. doi: 10.1084/jem.20022059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng CTK, Rank RG. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–75. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–90. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan T, Lu H, Hu H, et al. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–96. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol. 1998;161:1758–64. [PubMed] [Google Scholar]
- 27.Braud VM, Allan DSJ, Wilson D, McMichael AJ. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr Biol. 1998;8:1–10. doi: 10.1016/s0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 28.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 29.Zhong GM, Fan T, Liu L. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999;189:1931–7. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong GM, Fan PY, Ji HZ, Dong F, Huang YQ. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001;193:935–42. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaelsson J, deMatos CT, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–14. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–33. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn K, Gruhler A, Galocha B, et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–21. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 34.Greijer AE, Verschuuren EA, Dekkers CA, et al. Expression dynamics of human cytomegalovirus immune evasion genes US3, US6, and US11 in the blood of lung transplant recipients. J Infect Dis. 2001;184:247–55. doi: 10.1086/322039. [DOI] [PubMed] [Google Scholar]
- 35.Borysiewicz LK, Rodgers B, Morris S, Graham S, Sissons JG. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985;134:2695–701. [PubMed] [Google Scholar]
- 36.Wang EC, McSharry B, Retiere C, et al. UL40-mediated NK evasion during productive infection with human cytomegalovirus. Proc Natl Acad Sci USA. 2002;99:7570–5. doi: 10.1073/pnas.112680099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–12. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher JM, Prentice HG, Grundy JE. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J Immunol. 1998;161:2365–74. [PubMed] [Google Scholar]
- 39.Cerboni C, Mousavi-Jazi M, Linde A, et al. Human cytomegalovirus strain-dependent changes in NK cell recognition of infected fibroblasts. J Immunol. 2000;164:4775–82. doi: 10.4049/jimmunol.164.9.4775. [DOI] [PubMed] [Google Scholar]
- 40.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gamma delta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 41.Griffith E, Ramsburg E, Hayday A. Recognition by human gut gamma delta cells of stress inducible major histocompatibility molecules on enterocytes. Gut. 1998;43:166–7. doi: 10.1136/gut.43.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tieng V, Le Bouguenec C, du Merle L, et al. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci USA. 2002;99:2977–82. doi: 10.1073/pnas.032668099. [DOI] [PMC free article] [PubMed] [Google Scholar]