Abstract
Lipoarabinomannan (LAM) is a major structural carbohydrate antigen of the outer surface of Mycobacterium tuberculosis. High antibody titres against LAM are often seen in active tuberculosis (TB). The role of such LAM-specific antibodies in the immune response against TB is unknown. Here we have investigated a monoclonal antibody (MoAb) SMITB14 of IgG1 subclass and its corresponding F(ab′)2 fragment directed against LAM from M. tuberculosis strain H37Rv. MoAb SMITB14 was shown by immunofluorescence to bind to whole cells of the clinical isolate M. tuberculosis strain Harlingen as well as to M. tuberculosis H37Rv. The binding of MoAb SMITB14 to LAM was inhibited by arabinomannan (AM) and oligosaccharides (5·2 kDa) derived from LAM, showing that the MoAb binds specifically to the AM carbohydrate portion of LAM. In passive protection experiments BALB/c mice were infected intravenously with M. tuberculosis Harlingen. MoAb SMITB14 was added intravenously either prior to, or together with, the bacteria. The antibody proved to be protective against the M. tuberculosis infection in terms of a dose-dependent reduction in bacterial load in spleens and lungs, reduced weight loss and, most importantly, increased long-term survival.
Keywords: lipoarabinomannan, monoclonal antibody, passive protection, tuberculosis
INTRODUCTION
In many bacterial infections antibodies directed against bacterial carbohydrate surface antigens have been shown to be important in protective immunity. However, immunity against tuberculosis (TB) has been assumed to rely solely on cellular defence mechanisms, and antibody-mediated immunity has either been disregarded or even thought to facilitate the development of active TB. These assumptions were based mainly on the results of early serum therapy studies performed in the beginning of the 1900s and on serological investigations, of various clinical forms, of TB in humans and experimental animals, including passive protection experiments using immune sera (for a recent extensive review see [1]).
In these earlier studies the protective effect of antibodies against Mycobacterium tuberculosis and its target antigens were not, or only poorly, characterized. There is some early evidence that antibodies directed towards mycobacterial carbohydrate antigens such as lipoarabinomannan (LAM) may be beneficial [1], while there is less evidence of beneficial effects conferred by antibodies directed against protein antigens, including the components comprising purified protein derivative (PPD). Hence, Choucroun reported in 1949 a correlation between the presence of serum antibodies to M. tuberculosis carbohydrate antigens and acquired immunity to disease [2]. Later, studies by Sibert et al. associated protective effects with antibodies to mycobacterial polysaccharides but not to protein antigens [3,4]. Recently, Costello et al. found that children with disseminated TB had a significantly lowered IgG response to mycobacterial LAM and other mycobacterial antigens [5]. They concluded that a weak antibody response to LAM and other mycobacterial antigens before or in the early stages of infection increased the likelihood of dissemination.
LAM is a major structural carbohydrate component of the outer surface of M. tuberculosis, and frequently gives rise to high antibody responses in the infected host [6]. The role of such antibodies in the pathogenesis of TB is not known. By using monoclonal antibodies (MoAbs) it is possible to study antibodies with precisely defined epitope specificity, subclass and isotype for their possible beneficial or deleterious activity in TB.
We have prepared an array of MoAbs with specificity for epitopes located in the arabinomannan part of LAM. Here we describe the preparation of MoAbs and the effect of one of these MoAbs of IgG1 subclass and its corresponding F(ab′)2 fragment in passive protection experiments in BALB/c mice against experimental TB.
MATERIALS AND METHODS
Bacterial strains
M. tuberculosis H37Rv, obtained originally from the ATCC collection, was maintained at the Swedish Institute for Infectious Disease Control (SIIDC), Stockholm, Sweden. The clinical isolate M. tuberculosis strain Harlingen used for the experimental infections was kindly provided by Dr J. van Embden, RIVM, the Netherlands.
Materials and reagents
A mouse MoAb of IgG1 subclass against human haemoglobin (SMIHHG1) was obtained from the Department of Immunology, Karolinska Institute, Stockholm. Microtitre Maxisorb™ 96-well plates were from Nunc, Kampstrup, Denmark. Yeast mannan, methyl α-D-mannopyranoside, DL-arabinose, pepsin and alkaline phosphatase-conjugated goat antimouse IgG were from Sigma Chemicals, USA. If not stated otherwise, all other chemicals were of analytical purity.
Preparation of LAM
LAM from M. tuberculosis H37Rv was prepared as described previously [7]. Arabinomannan (AM) was obtained by mild alkaline hydrolysis of LAM as described previously [8]. Sodium periodate oxidation of delipidated purified LAM was done by treatment with 10 mm NaIO4 in acetate buffer pH 6·0 at + 4°C in the dark for 15 min.
Preparation of anti-LAM monoclonal antibodies
The antigen for immunizations was prepared by mixing 1 mg of purified LAM from M. tuberculosis H37Rv with 4 mg of heat-killed M. tuberculosis H37Rv bacteria. The mixture was evaporated slowly to dryness under reduced pressure and resuspended in 5 ml phosphate buffered saline (PBS). Ten outbred female Naval Medical Research Institute (NMRI) mice (8–10 weeks, B & K, Sweden) were injected, intraperitoneum, with 200 µl of the antigen mixture emulsified in complete Freund's adjuvant (Difco Laboratory, USA) at a ratio of 1 : 1. The mice were then boosted twice, at 2-week intervals, with the same amount of antigen in incomplete Freund's adjuvant (Difco Laboratory). Three days prior to spleen cells harvest for hybridoma production, the mice were given 200 µl of the antigen intravenously in PBS without adjuvant. Spleen cells were fused with SP2/AG64G14 myeloma cells essentially following the method of Köhler and Milstein [9]. Hybridomas that secreted LAM-specific antibodies were identified by enzyme-linked immunosorbent assay (ELISA) and cloned three times by limiting dilutions. Individual colonies were then chosen for expansion. The MoAbs were purified, from culture supernatants, by affinity chromatography over protein G-Sepharose according to the supplier's instructions (GamMAbind Plus Sepharose, Pharmacia & Upjohn, Uppsala, Sweden). The clonal purity of the purified MoAbs was ascertained by isoelectric focusing using automated PhastSystem™ with PhastGel IEF 3–9 (Pharmacia & Upjohn, Uppsala, Sweden; DT File no. 210). After focusing, the gels were silver-stained as recommended by the manufacturer (ST file no. 100).
The isotypes of the MoAbs were identified by ELISA, using LAM as coating antigen and alkaline phosphatase-conjugated goat antimouse IgG subclass specific antibodies (Sigma Chemical Co, USA). One MoAb (SMITB14) of IgG1 subclass was chosen for further studies of its potential effect in passive protection experiments.
Quantification of MoAbs
The relative titres of the MoAbs were determined by ELISA. Wells of polystyrene microplates (Maxisorb, Nunc, Denmark) were coated with 100 µl of purified LAM (10 µg/ml) in 0·05 m carbonate buffer, pH 9·6, at room temperature overnight. The plates were washed three times with rinsing buffer (PBS containing 0·05% Tween), and then blocked with 0·5% casein for 1 h at 37°C. After washing, 100 µl of serial dilutions of each MoAb were added to the wells and incubated for 1 h at 37°C. After washing with rinsing buffer, 100 µl of alkaline phosphatase-conjugated goat, antimouse IgG (Sigma Chemical Co., diluted 1/2000 in PBS) was added to each well and the plates were incubated for a further 1 h at 37°C. After subsequent washings, the plates were developed at room temperature using p-nitrophenyl phosphate (Sigma Chemical Co.) as substrate and the colour reaction was measured by increase in absorbance at 405 nm using an ELISA reader (Dynatech, MR 5000).
Preparation of F(ab′)2 fragments
F(ab′)2 fragments from MoAb SMITB14 and from the isotype control MoAb SMIHHG1 were prepared as follows: MoAbs were dialysed for 6 h at room temperature against 0·1 m glycine-HCl buffer, pH 2·8, followed by a second dialysis against 0·1 m Na-acetate buffer, pH 4·5, overnight. F(ab′)2 fragments were prepared by treating the antibody with 1% pepsin (w/w) in the acetate buffer for 2·5 h at 37°C. The reaction was stopped by raising the pH to 8·0 with 0·1 m NaOH, and the resulting F(ab′)2 fragments were purified by ultrafiltration using an Omega ultrafiltration cell with a 50 kDa MW cut-off (Filtron, Northborough, USA). The purity of the obtained F(ab′)2 fragments was ascertained by sodium dodecyly suplohate-polyacrylamide gel electrophoresis (SDS-PAGE) (4–15% Phast Gels™ Pharmacia, Uppsala, Sweden) under reducing and nonreducing conditions.
Binding specificity of MoAb SMITB14
The ability of MoAb SMITB14 to detect whole mycobacterial cells was examined by immunofluorescence, as described previously [10]. Briefly, 20 µl of heat-killed bacteria in PBS (105/ml) was gently mixed with 20 µl of fluoroscein isothyocyanate (FITC)-labelled MoAb in PBS (0·2 mg/ml). Binding was detected using an immunofluorescence microscope.
The specificity of MoAb SMITB14 was established by inhibition ELISA. ELISA was performed as described above, but prior to the addition to the wells, the MoAb SMITB14 was preincubated for 30 min at room temperature with different inhibitors at varying concentrations.
Experimental infections
Female BALB/c mice (8–10 weeks, B&K, Stockholm, Sweden), were used throughout these studies. After delivery to the BSL3 laboratory at SIIDC the mice were acclimatized for 1 week in a BSL3 isolator (Elwyn E. Roberts Isolators Ltd, Shropshire, UK). Mice were housed at a maximum of five animals per cage.
M. tuberculosis Harlingen was cultured on solid Löwenstein Jensen medium for 4 weeks and then propagated in liquid Middlebrook 7H9 medium for another 3 weeks. Aliquots of bacilli were suspended in 7H9 medium with 10% glycerol at 107−108 bacilli/ml and frozen at −70°C. The viability of the frozen suspension was checked by plating the thawed bacteria on Middlebrook 7H11 agar supplemented with oleic acid albumin–dextrose–catalase (OADC; Difco).
Prior to infection, frozen ampoules of M. tuberculosis Harlingen were thawed, diluted in PBS to the appropriate concentration and shaken on a Vortex shaker for 10 s to disperse clumps of bacteria. The titre of the inoculum was reconfirmed by dilution plating of the bacterial stock. Mice were infected intravenously (i.v.) via the lateral tail vein.
In the passive protection experiments the respective MoAbs and F(ab′)2 fragments were diluted in PBS and mixed with M. tuberculosis Harlingen at indicated concentrations. The mixture was incubated for 30 min with constant agitation at room temperature and 100 µl of the mixture was injected in the lateral tail vein. In some experiments MoAbs (100 µl) were administered i.v. 60 min prior to the injection of bacteria (100 µl). For intranasal (i.n.) infection experiments, the F(ab′)2 fragment of MoAb SMITB14 was mixed with freshly thawed M. tuberculosis Harlingen to which Lutrol® F127 was added (BASF Ludwigshafen, Germany; final concentration 0·12%) to give a single cell suspension. After 15 min incubation at room temperature 30 µl of such mixture was administered through both nostrils (3 × 5 µl per nostril) with the animal held in an upright position.
The mice were observed daily and weighed individually on the day of infection and then once a week. Mice were sacrificed at indicated times after infection, or when moribund, by cervical neck dislocation and lungs, spleens and livers were aseptically removed, weighed and homogenized in a Colworth Stomacher 80 homogenizer (A. J. Seward UAC House, Blackfriar Road, London) in 0·01% Tween 80-saline solution. Colony-forming units (CFU) were then determined by plating the appropriate dilutions of the homogenates on Middlebrook 7H11 agar supplemented with OADC, polymyxin B and amphotericin B.
Statistical analysis
Data were analysed using the unpaired two-tailed Student's t-test for CFU counts and Kaplan–Meier survival plots followed by the log-rank test for survival data.
RESULTS
Characterization of MoAb SMITB14
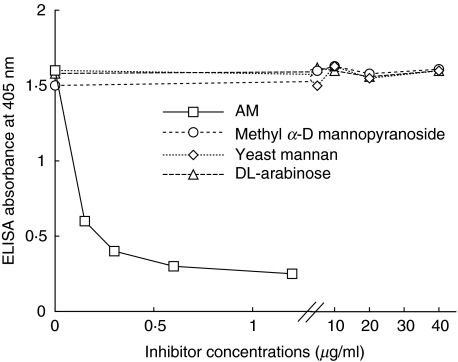
FITC-labelled MoAb SMITB14 efficiently bound to whole M. tuberculosis bacteria (H37Rv and Harlingen strains) as well as to the attenuated M. bovis Calmette–Guérin strain (BCG) but not to other non-mycobacterial species such as Escherichia coli strains of various O serotypes, Streptococcus pneumoniae isolates, strains of Salmonella, Yersinia enterocolitica and Y. pseudotuberculosis and Nocardia sp. (data not shown). The specificity of MoAb SMITB14 was analysed in more detail in ELISA inhibition experiments, using the following potential inhibitors: AM, yeast mannan, methyl α-D-mannopyranoside and DL-arabinose (Fig. 1). The binding of MoAb SMITB14 to LAM was inhibited by purified AM in a dose-dependent manner, while yeast mannan, methyl α-D-mannopyranoside and DL-arabinose at a concentration up to 40 µg/ml were not inhibitory. The inhibition by AM was abolished completely after sodium periodate oxidation, further confirming that specificity of MoAb SMITB14 binding resided in the carbohydrate portion of LAM (data not shown).
Fig. 1.
Inhibition of binding of MoAb SMITB14 to LAM (10 µg/ml as coating antigen) assayed by ELISA. The following putative inhibitors, arabinomannan (AM) at a concentration ranging from 0 to 1 µg/ml, mannan, methyl a-D-mannopyranoside and DL-arabinose at concentrations of 0–40 µg/ml were preincubated with the appropriate dilution of the MoAb.
Effect of MoAb SMITB14 on bacterial loads in organs of i.v.-infected mice
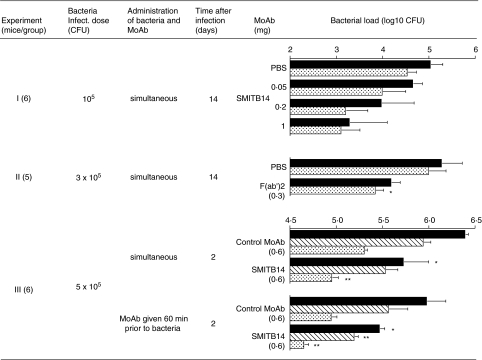
In a first experiment (Fig. 2, exp. I) BALB/c mice were challenged i.v. with M. tuberculosis Harlingen, either alone or in combination with MoAb SMITB14. After 14 days the mice were sacrificed and the CFU counts in lungs and spleens were determined. In mice given bacteria with MoAb SMITB14 there was a dose-dependent reduction in CFU counts in both lungs and spleens, compared to mice given bacteria alone. Thus, at the highest concentration of MoAb SMITB14 (1 mg per mouse) the difference in bacterial counts reached 1·8 log unit and 1·3 log unit for lungs and spleens, respectively. These differences were, however, non-significant due to the considerable variation between individual animals.
Fig. 2.
Bacterial counts in organs of BALB/c mice after i.v. infection with M. tuberculosis strain Harlingen. CFU counts were determined in different organs: black bars, lung; dotted bars, spleen; striated bars, liver. Mean log10 (CFU) ± s.e.m. from five to six mice per group are shown. Asterisks indicate statistically significant difference between control and specific MoAb group (*P < 0·05; **P < 0·01). Experiment I: mice were challenged i.v. with 105 CFU M. tuberculosis Harlingen, either alone, or in combination with indicated amounts of MoAb SMITB14. After 14 days the mice were sacrificed and the CFU counts in lungs and spleens were determined. Experiment II: mice were challenged i.v. with 3 × 105 CFU M. tuberculosis Harlingen alone or in combination with F(ab′)2 fragment of MoAb SMITB14 (0·3 mg) and sacrificed 14 days later and CFU counts in lungs and spleens were determined. Experiment III: one group of mice was given a mixture of bacteria and MoAb SMITB14 (5 × 105 CFU M. tuberculosis Harlingen and 0·6 mg MoAb) and another group of mice was inoculated with MoAb SMITB14 1 h prior to M. tuberculosis infection. Control groups consisted of mice that received an irrelevant MoAb of the same isotype as SMITB14 (SMIHHG1, IgG1, directed against human haemoglobin) at the same dose, either together with or prior to bacteria. All mice were sacrificed 2 days after infection and the CFU counts in lungs and spleens were determined.
These results encouraged us to perform a second experiment (Fig. 2, exp. II). Mice were challenged i.v. with M. tuberculosis Harlingen alone or in combination with F(ab′)2 fragment of MoAb SMITB14 and sacrificed 14 days later. The CFU counts were significantly (P = 0·02) lower in the spleens of mice given the F(ab′)2 fragments and also lower in the lungs.
In yet another experiment (Fig. 2, exp. III) we attempted to determine whether a similar protective effect could be achieved by systemic administration of MoAb prior to challenge with M. tuberculosis . Therefore, one group of mice was given a mixture of bacteria and MoAb SMITB14 and another group of mice was inoculated with MoAb SMITB14 1 h prior to M. tuberculosis infection. Control groups consisted of mice that received an irrelevant MoAb of the matched isotype (SMIHHG1, IgG1) either together with, or prior to, bacteria. All mice were sacrificed 2 days after infection. In both groups of mice which received MoAb SMITB14 there was significant reduction of bacterial loads in lungs, livers and spleens compared to the mice which received isotype control MoAb (Fig. 2, exp. III). This difference was of a similar order of magnitude in mice injected with bacteria–MoAb mixture and mice injected with MoAb prior to bacteria. Furthermore, the organs of mice that received MoAb SMITB14 before infection showed lower bacterial loads, relative to the mice that were given the same MoAb and bacteria together.
In order to confirm that the reduction in CFU counts observed in organs of challenged mice was not due to a direct killing or aggregation of bacteria by MoAb SMITB14, the aliquots of the inoculates used for infections consisting of bacteria alone, or mixtures of bacteria and MoAb SMITB14 or its F(ab′)2 fragment, were plated on Middlebrook agar. There was no reduction in CFU counts in the presence of the MoAb or the F(ab′)2 fragment, as compared to bacteria alone or bacteria mixed with the isotype control MoAb (data not shown).
Effect of MoAb SMITB14 on body weight and survival of i.v.-infected mice
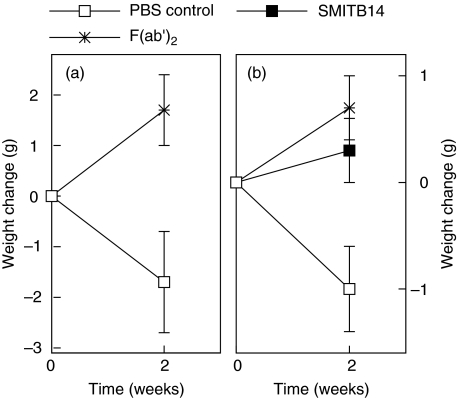
Fourteen days postchallenge there was a significant (P = 0·02) reduction in weight in the group of mice given M. tuberculosis alone compared to the mice given M. tuberculosis in combination with F(ab′)2 fragment (Fig. 3a, exp. I). A similar observation was made when the experiment was repeated using a somewhat higher natio of SMITB14 or its F(ab′)2 fragment to bacteria (Fig. 3b). Mice receiving bacteria together with MoAb or F(ab′)2 fragment suffered significantly less weight loss (P = 0·024 and P = 0·006, respectively) than mice infected with bacteria alone (Fig. 3b).
Fig. 3.
Change in body weight of BALB/c mice 2 weeks after intravenous infection with M. tuberculosis Harlingen administered alone, with MoAb SMITB14 or the corresponding F(ab′)2 fragment. (a) Harlingen, 3 × 105 CFU; F(ab′)2, 0·4 mg. (b) Harlingen, 105 CFU; SMITB14, 0·4 mg, and F(ab′)2, 0·45 mg. Mean weight ± s.e.m. of five mice per group is shown. For details see Materials and methods.
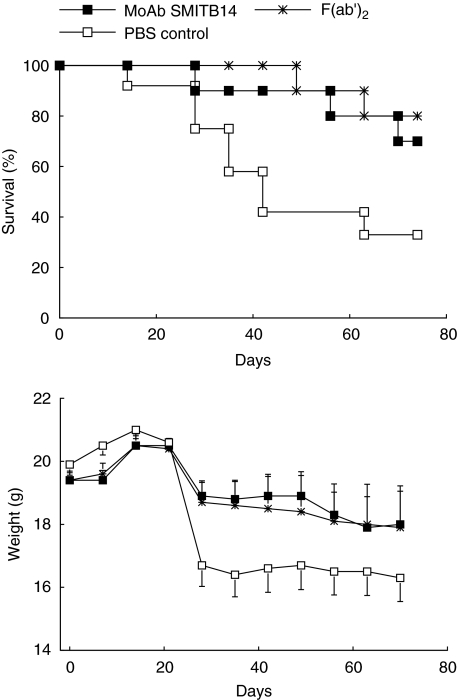
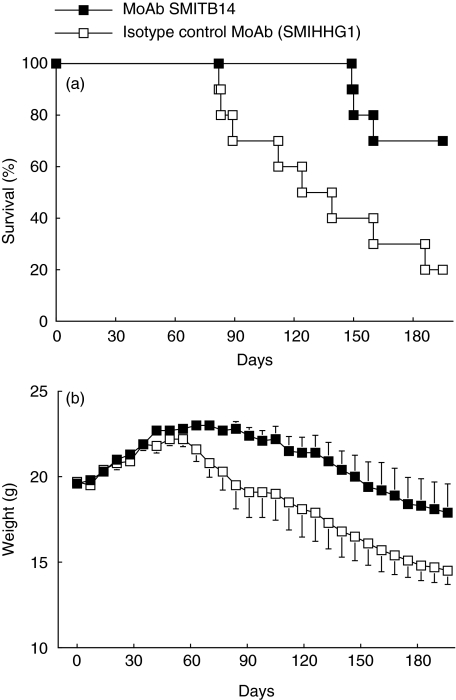
The possible effect of passive immunization with MoAb SMITB14 on long-term survival of mice was studied next. Mice were challenged i.v. with M. tuberculosis Harlingen alone, or M. tuberculosis Harlingen in combination with either MoAb SMITB14 or its F(ab′)2 fragment. The mice were monitored for 70 days, their individual body weights were determined on the day of infection and then weekly after infection (Fig. 4). Over the entire observation period there was less weight loss and reduced mortality in groups of mice challenged with bacteria together with MoAb or F(ab′)2 fragment compared to mice challenged with bacteria alone (P = 0·034 and P = 0·008 by log rank test, respectively). At the end of the observation period, seven of 10 mice in the MoAb group and 8/10 mice in the F(ab′)2 group had survived, compared to 4/12 mice in the control group.
Fig. 4.
Survival (upper panel) and body weight (lower panel) of BALB/c mice infected intravenously with 105 CFU M. tuberculosis Harlingen alone or in combination with MoAb SMITB14 or its F(ab′)2 fragment (0·25 mg per mouse). For details see Materials and methods. (n = 10–12 mice per group).
In another experiment (data not shown) mice were given i.v. M. tuberculosis Harlingen together with MoAb SMITB14 or its F(ab′)2 fragment while control groups of mice received respective amounts of isotype control MoAb (SMIHHG1). In this experiment the survival was monitored over an extended period of 180 days. The mice challenged with M. tuberculosis in the presence of either MoAb SMITB14 or its F(ab′)2 fragment survived significantly longer than their counterparts that received irrelevant MoAb (P = 0·017 and P = 0·0003, respectively). At the end of the experiment, three of 10 mice still remained alive and apparently healthy in the group given MoAb SMITB14 while all mice that received isotype control MoAb were dead.
The next experiment was performed in order to determine whether systemic administration of MoAb SMITB14 prior to infection would protect mice from weight loss and death (Fig. 5). Mice were inoculated i.v. with MoAb SMITB14, followed 60 min later by i.v. challenge with M. tuberculosis Harlingen. Control mice received isotype control MoAb SMIHHG1 prior to challenge infection. Also in this experimental setting mice were protected in terms both of prolonged survival (Fig. 5a, P = 0·013) and reduced weight loss (Fig. 5b) over the study period of 190 days.
Fig. 5.
Survival (a) and body weights (b) of BALB/c mice infected intravenously with 105 CFU M. tuberculosis Harlingen given 60 min after passive transfer of 0·1 mg of MoAb SMITB14. MoAb SMIHHG1 directed against human haemoglobin was used as isotype control antibody. (n = 10 mice per group).
Effect of MoAb SMITB14 on bacterial burden in lungs of intranasally infected mice
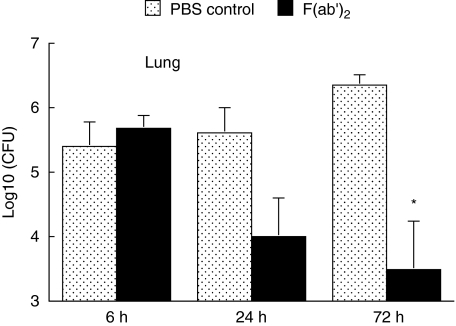
One of the major questions regarding antimycobacterial immunity is whether relevant immune mechanisms, including antibodies, operate at the site of infection i.e. on the surface of the lung mucosa. In order to address this question an experiment was performed where F(ab′)2 fragments from MoAb SMITB14 and M. tuberculosis Harlingen were administered simultaneously i.n. to BALB/c mice. Mice were sacrificed 6, 24 and 72 h later, lungs were isolated and bacterial loads were determined (Fig. 6). Early after infection (6 h) bacterial counts in lungs from mice that received M. tuberculosis together with F(ab′)2 fragment were similar to those found in mice infected with M. tuberculosis alone. At 24 h, however, there was considerable decrease in bacterial loads in lungs of mice from the F(ab′)2 group relative to the control group. The reduction in number of bacteria further progressed and at 72 h the difference between the F(ab′)2 group and the control group became significant (P < 0·01).
Fig. 6.
Recovery of mycobacteria from lungs of BALB/c mice at different times after intranasal infection with 105 CFU M. tuberculosis Harlingen alone, or in combination with, 0·5 mg of F(ab′)2 fragment from MoAb SMITB14. Mean log10 (CFU) ± SEM from nine mice per group are shown. Asterisk indicates statistically significant difference between control and F(ab′)2 group (P < 0·003).
DISCUSSION
In tuberculosis, the current consensus is that only the cellular arm of the immune system is of importance for the effective control of the infection/disease. However, as in all infections, and also in natural M. tuberculosis infection and disease, the immune response of the host involves both the cellular and humoral immunological arms. Nevertheless, the possible beneficial or detrimental effects of elicited antibodies have largely been ignored. Therefore, in this study we have carried out a number of passive protection experiments in the M. tuberculosis infection model in mice to evaluate the possible beneficial/adverse effects mediated by a MoAb (SMITB14) with specificity for the polysaccharide portion of LAM, which is one of the major surface antigens of tubercle bacteria. In this model we found that the MoAb SMITB14 and its F(ab′)2 fragment significantly protected BALB/c mice against experimental M. tuberculosis infection. This protection was evidenced by a reduction in CFU counts in spleen and lung, by inhibition of the weight loss and, most importantly, by prolonged long-term survival (Figs 2–6). A control MoAb (specific for human haemoglobin) of the same IgG1 subclass and its F(ab′)2 fragment did not protect against M. tuberculosis challenge (Figs 2 and 5).
Prominent clinical features of TB in humans are fever and weight loss (wasting). In the mouse model, wasting is, in our experience, a good marker for the final outcome of acute TB. In particular, a negative weight development at the critical time period 2–4 weeks after challenge usually indicates that the mice will succumb early to the infection. Fever and weight loss have been attributed, at least in part, to the increased production of proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin (IL)-1α and IL-1β. These cytokines have been shown previously to be induced by stimulation of mononuclear cells with LAM [11–14]. Because the mice given MoAb SMITB14 or the corresponding F(ab′)2 together with M. tuberculosis did not lose as much weight as the mice given the control MoAb it is tempting to speculate that the anti-AM MoAb may inhibit the induction of proinflammatory cytokines by either the blockage of a crucial domain of LAM on the bacterial surface or by scavenging the released LAM. Similar effects have been reported previously in terms of the ability of O-antigen specific antibody to down-regulate the fever response induced by O-antigen homologous endotoxin in rabbits [15].
LAM is also known to have other biological effects on several components of the innate immune system, which are realized through interactions with a wide variety of host cells. Hence, LAM isolated from both M. tuberculosis and M. leprae has been reported to suppress T cell proliferation [16–18] and to interfere with gamma interferon-mediated activation of macrophages [19,20]. Other reported effects include inhibition of protein kinases [19], inhibition of the synthesis of mRNA encoding IL-2, IL-5, and granulocyte-macrophage colony stimulating factor (GM-CSF) in human T cells [21] and activation of the complement cascade [22]. MoAb SMITB14 may interfere with one or more of these potentially pathogenic effects of LAM, and this could conceivably contribute to the protection seen with this antibody.
The possibility that, in the presence of MoAb SMITB14, bacterial aggregates were formed which, by virtue of their size, would have their infectious potential diminished seems unlikely because: (1) plating of a mixture of M. tuberculosis-specific MoAb yielded CFU counts identical to those obtained with M. tuberculosis alone or M. tuberculosis-isotype control MoAb mixture; (2) specific MoAb passively transferred, prior to M. tuberculosis challenge, was as protective as MoAb preincubated with bacteria; and (3) the amounts of bacteria recovered very early (6 h) from lungs of intranasally infected mice were similar, irrespective of the presence of MoAb.
Structurally, LAM consists of a mannan polysaccharide backbone core, substituted with terminal oligoarabinosyl side chains. The mannan core polysaccharide backbone is in turn attached to a phosphatidyl inositol lipid moiety [23,24]. LAM is a biologically polymorphic structure, and two major variants have been reported; one with the arabinomannan core substituted by arabinofuranosyl containing termini (AraLAM) and another where these termini are mannose-capped (ManLAM). The LAM used for generation of MoAb SMITB14 was prepared from M. tuberculosis H37Rv and was, by gel electrophoresis and Western blot using anti-Erdman LAM MoAbs, identical to the LAM prepared from M. tuberculosis Erdman [8], both strains reported to be heavily mannosylated. Western blot of MoAb SMITB14 against LAM showed abolition of binding after NaIO4 treatment of LAM. In ELISA, the binding of MoAb SMITB14 to LAM was inhibited by AM in a dose-dependent fashion, showing that MoAb SMITB14 binds to the AM portion of LAM. MoAb SMITB14 also bound to the AM oligosaccharides conjugated to various types of proteins [25], demonstrating further the specificity of MoAb SMITB14 for the AM portion of LAM. The binding was not inhibited by yeast mannan, methyl α-D-mannopyranosides or DL-arabinose, indicating that the specificity of MoAb SMITB14 is directed to epitopes located outside of the mannose-capped region of LAM.
Antibodies could participate in protective immunity by many different mechanisms. The finding that MoAb SMITB14 binds to whole bacteria indicated that this antibody might be involved in blocking of a cellular invasion. This was corroborated by the findings that both i.v. and i.n. administration of this MoAb resulted in very early reduction of bacterial loads.
The fate of tubercle bacilli within a host phagocyte depends on the pathway by which the bacteria reach the intracellular milieu of the phagocytic cell. Different macrophage opsonic and non-opsonic receptors have been reported to mediate this uptake [26,27]. It has been suggested that receptors specific for surface carbohydrate epitopes may allow mycobacteria to bypass the bactericidal activity of macrophages [28]. LAM is known to mediate M. tuberculosis uptake by phagocytic cells, such as macrophages and dendritic cells and has been reported to utilize at least two receptors, CD14 and the murine mannose receptor, for entry into host phagocytes [29,30]. MoAb SMITB14 may prevent the uptake of M. tuberculosis through receptor(s) that facilitate bacterial survival and growth. It could be hypothesized that the MoAb SMITB14 binding to the surface of the invading tubercle bacilli leads to their uptake by particular cell populations that are specially effective in either direct killing or mediating proper cytokine/cellular signals to other effector cells with killing abilities.
Mice given MoAb SMITB14 lived significantly longer than control mice. Also, mice given F(ab′)2 fragment of MoAb SMITB14 survived longer than mice given control F(ab′)2 fragment. Protection afforded by the F(ab′)2 fragments indicates lack of involvement of the Fc phagocytic receptor [31]. It can also be speculated that anti-LAM antibodies and their F(ab′)2 fragments, by binding to LAM, could interfere with LAM-mediated inhibition of phagosome-lysosome fusion [32]. Recently, Teitelbaum et al. reported that a murine MoAb of IgG3 subclass (MoAb 9d8), also reactive with arabinomannan, partially protected C57BL/6 and BALB/c mice from death after respiratory challenge with a clinical isolate of Mtb [33]. In the same study, another MoAb of IgM subclass with LAM specificity did not affect the course of infection in mice. The protective IgG3 MoAb used by Teitelbaum et al. did not reduce the bacillary load in the lungs of infected animals, but it changed the distribution of the mycobacteria within the lung granulomas. In our studies we did not examine the distribution of the bacteria in lungs but in contrast to these investigators we noticed a significant reduction in bacterial load in the lungs. This latter finding might, at least, in part be due to our use of the i.v. challenge route, while Teitelbaum et al. [33] used the intratracheal route.
Studies of M. tuberculosis infections in gene-deleted mice, incapable of making B cells, are often regarded as providing definitive evidence of the lack of role of antibodies in antimycobacterial defence. However, the results of three studies published so far on M. tuberculosis infections in B cell-deficient mice seem contradictory and do not allow for the dismissal of a role for antibody. Vordermeier et al. reported that i.v. challenge resulted in significantly higher bacterial counts in organs of B cell-deficient mice, compared to the wild strain, but no difference in survival ensued [34], Johnson et al. reported no difference in bacterial loads or early lung pathology upon aerosol challenge (survival was not recorded) [35], while Bosio et al. reported that aerosol challenge resulted in similar lung bacterial loads but considerably different pathology in B cell-deficient and wild-type mice [36]. Furthermore, the observation by Bosio et al. [36], that the supplementation of the deficient mice with passively transferred whole immune serum did not lead to the reversal of observed changes, should be treated with caution. It is conceivable that the presence of the whole repertoire of antibodies, such as those present during ‘natural’ immune response [37] or in passively transferred whole serum, may result in neutralization of the effect of beneficial antibodies by disadvantageous ones. Thus, it has been shown in other systems, that the fine specificity and isotype [33,38], and the amount of an individual MoAb [39] may decide whether or not it is advantageous for the host. In addition, a paradoxical loss of efficacy for certain antibodies, passively transferred at high doses, has been described [39]. Considering the variety of polyclonal antibodies raised, in response to a multitude of mycobacterial antigens in the course of infection, it may be argued that antibodies of a particular isotype against a specific antigen/epitope could be of clinical interest, e.g. in preparing a new effective vaccine against TB.
To what extent the humoral immune response plays a role in innate and acquired immunity to active TB in man still remains an enigma. There has been no extensive research on the potential association between antibody deficiency and the risk of acquiring either infection or active TB, although sporadic cases have been reported. Accordingly, a somewhat higher incidence of TB has been reported in patients with hypogammaglobulinaemia [40].
The findings presented here that passively transferred antibodies against AM resulted in significant protection against TB have encouraged us to study the possible protective effects of LAM oligosaccharide-based protein conjugates as putative vaccine candidates. Indeed, these conjugate vaccines − when administered in proper adjuvants − proved to elicit not only cell-mediated immune responses but also high anti-LAM IgG antibody titres [8] and protected animals efficiently against TB [25]. We suggest that the antibodies elicited by these new vaccine candidates, at least in part, mediated this protection.
In summary, the observations reported in the present study indicate further that certain antibodies may be protective in experimental TB and justify the need for continued studies to clarify the role of humoral responses in the natural course of TB as well as in vaccine-induced immunity.
Acknowledgments
The study was supported by grants from the European Community (contracts TS-CT94-0001 and BMH4-CT97-2671), the Swedish Medical Research Council (grant K99–06X), King Oscar II Jubilee Foundation and the Swedish Heart–Lung Association.
REFERENCES
- 1.Glatman-Freedman A, Casadeval A. Serum therapy for tuberculosis revisited. Reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–32. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choucroun N. Precipitin test for carbohydrate antibodies in human tuberculosis. Am Rev Tuberc. 1949;59:710–2. doi: 10.1164/art.1949.59.6.710. [DOI] [PubMed] [Google Scholar]
- 3.Seibert FB, Miller EE, Buseman U, Seibert MV, Soto-Figueroa E, Fry L. The significance of antibodies to tuberculoprotein and polysaccharide in resistance to tuberculosis. Am Rev Tuberc Pulm Dis. 1956;73:547–62. doi: 10.1164/artpd.1956.73.4.547. [DOI] [PubMed] [Google Scholar]
- 4.Seibert FB, Seibert MV. Relationship between immunity and circulating antibodies, complement and tuberculopolysaccharide in tuberculosis. J Infect Dis. 1967;101:109–18. doi: 10.1093/infdis/101.2.109. [DOI] [PubMed] [Google Scholar]
- 5.Costello AM, Kumar A, Narayan V, et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg. 1992;86:686–92. doi: 10.1016/0035-9203(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 6.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–51. [PubMed] [Google Scholar]
- 7.Hamasur B, Källenius G, Svenson SB. A new rapid and simple method for large-scale purification of mycobacterial lipoarabinomannan. FEMS Immunol Med Microbiol. 1999;24:11–9. doi: 10.1111/j.1574-695X.1999.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamasur B, Källenius G, Svenson SB. Synthesis and immunologic characterisation of Mycobacterium tuberculosis lipoarabinomannan specific oligosaccharide-protein conjugates. Vaccine. 1999;17:2853–61. doi: 10.1016/s0264-410x(99)00124-3. [DOI] [PubMed] [Google Scholar]
- 9.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 10.Hamasur B, Bruchfeld J, Haile M, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial arabinomannan in urine. J Microbiol Meth. 2001;45:41–52. doi: 10.1016/s0167-7012(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PF, Fong S-J, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1999;145:149–54. [PubMed] [Google Scholar]
- 12.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992;60:1441–6. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juffermans NP, Verbon A, Belisle JT, et al. Mycobacterial lipoarabinomannan induces an inflammatory response in the mouse lung: a role for interleukin-1. Am J Respir Crit Care Med. 2000;162:486–9. doi: 10.1164/ajrccm.162.2.9911009. [DOI] [PubMed] [Google Scholar]
- 14.Moreno C, Taverne J, Mehlert A, et al. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989;76:240–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Lindberg AA, Greisman SE, Svenson SB. Induction of endotoxin tolerance with nonpyrogenic O-antigenic oligosaccharide-protein conjugates. Infect Immun. 1983;41:888–95. doi: 10.1128/iai.41.3.888-895.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan G, Gandhi RR, Weinstein DE, et al. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987;138:3028–34. [PubMed] [Google Scholar]
- 17.Molloy A, Gaudernack G, Levis WR, Cohn ZA, Kaplan G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:973–7. doi: 10.1073/pnas.87.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno C, Mehlert A, Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988;74:206–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Chan J, Fan X, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–61. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibley LD, Adams LB, Krahenbuhl JL. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin Exp Immunol. 1990;80:141–8. doi: 10.1111/j.1365-2249.1990.tb06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chujor CSN, Kuhn B, Schwerer B, Bernheimer H, Levis WR, Bevec D. Specific inhibition of mRNA accumulation for lymphokines in human T cell line Jurkat by mycobacterial lipoarabinomannan antigen. Clin Exp Immunol. 1992;87:398–403. doi: 10.1111/j.1365-2249.1992.tb03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetland G, Wiker HG, Hogåsen K, Hamasur B, Svenson SB, Harboe M. Involvement of antilipoarabinomannan antibodies in classical complement activation in tuberculosis. Clin Diagn Laboratory Immunol. 1998;5:211–8. doi: 10.1128/cdli.5.2.211-218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee D, Bozic CM, McNeil M, Brennan PJ. Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J Biol Chem. 1991;266:9652–60. [PubMed] [Google Scholar]
- 24.Hunter SW, Brennan PJ. Evidence for the presence of a phosphatidyl inositol anchor on lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;267:9272–9. [PubMed] [Google Scholar]
- 25.Hamasur B, Haile M, Pawlowski A, et al. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–93. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 26.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–21. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 27.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–80. [PubMed] [Google Scholar]
- 28.Ehlers MRW, Daffe M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 1998;6:328–35. doi: 10.1016/s0966-842x(98)01301-8. [DOI] [PubMed] [Google Scholar]
- 29.Prigozy TI, Sieling PA, Clemens D, et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immmunity. 1997;6:187–97. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 30.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–75. [PubMed] [Google Scholar]
- 31.Sedlacek HH, Gronski P, Hofstaetter T, Kanzy EJ, Schorlemmer HU, Seiler FR. The biological properties of immunoglobulin G and its split products [F(ab′)2 and Fab] Klin Wochenschr. 1983;61:723–36. doi: 10.1007/BF01497399. [DOI] [PubMed] [Google Scholar]
- 32.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci USA. 2003;100:5437–42. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teitelbaum R, Glatman-Freedman A, Chen B, et al. A MoAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–93. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vordermeier HMN, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–6. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CM, Cooper AM, Frank AA, Bonorino CB, Wysoki LJ, Orme IM. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tuber Lung Dis. 1997;78:257–61. doi: 10.1016/s0962-8479(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 36.Bosio CM, Gardner D, Elkins K. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164:6417–25. doi: 10.4049/jimmunol.164.12.6417. [DOI] [PubMed] [Google Scholar]
- 37.Navoa JAD, Laal S, Pirofski L-A, et al. Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clin Diagn Lab Immunol. 2003;10:88–94. doi: 10.1128/CDLI.10.1.88-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun. 2003;71:4225–8. doi: 10.1128/IAI.71.8.4225-4228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG1. J Immunol. 2003;170:3621–30. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 40.Anonymous. Hypogammaglobulinaemia in the United Kingdom. Lancet. 1969;1:163–8. [PubMed] [Google Scholar]