Abstract
There is evidence that nephritis is dominated by a Th1 immune response in systemic lupus erythematosus. Since IL-18 promotes polarization of the immune response toward Th1, we investigated the role of this cytokine in lupus nephritis (LN). A total of 133 lupus patients and 44 healthy subjects were enrolled. Demographic and clinical characteristics with renal biopsy data were recorded. IL-18 along with IFN-γ and IL-4, two prototypical of Th1 and Th2 cytokines, were measured in serum by ELISA. Peripheral blood lymphocytes were analysed by flow cytometry for IFN-γ and IL-4. IL-18 expression was determined by immunohistochemistry in 13 renal biopsy specimens from patients with LN and 2 controls. Serum IL-18 was higher in lupus patients than in controls. Levels of IL-18 correlated with urinary microalbumin and were increased in patients with LN when compared to those without LN. IL-18 expression was also increased within the glomeruli of nephritic patients and was primarily detected within the mesangial matrix and in infiltrating mononuclear cells. Measurement of IFN-γ and IL-4 in either sera or peripheral blood lymphocytes showed high IFN-γ along with low IL-4 expression in LN patients compared to patients without nephritis. A positive correlation between serum IL-18 and IFN-γ levels was found. IL-18 may play a prominent role in the pathogenesis of LN by promoting a cytokine imbalance towards a Th1 immune response. Measurement of IL-18 may be helpful for the early identification of lupus patients with LN and may help gauge the response to treatment in patients with active LN undergoing treatment.
Keywords: SLE IL-18 lupus nephritis IFN-γ IL-4
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by a wide variety of immunologic abnormalities. Lupus nephritis (LN) is a major contributor to morbidity and mortality in patients with SLE and up to half of lupus patients develop LN during the course of their disease. LN is though to be initiated by glomerular deposition of immune complexes though other essential events such as those mediated by Fc receptor signalling [1], cytokine [2] and chemokine [3] production are required to trigger glomerular inflammation and promote tissue damage. However, the specific role of each of these pathogenic components is presently unclear.
Recent evidence suggest that a Th1 cytokine response with excess of IFN-γ production is essential for the development of nephritis in murine models of lupus [4–9] and also plays an important pathogenetic role in human LN [10,11]. IL-18 is a proinflammatory cytokine, predominantly released by antigen presenting cells such as macrophages and dendritic cells, which acts as a Th1 cytokine since it promotes both proliferation of Th1 lymphocytes and IFN-γ production by these cells [12]. MRL/lpr mice, which spontaneously develop a lupus–like syndrome with severe glomerulonephritis, show higher serum levels of IL-18 than wild type MRL mice [13]. Moreover, enhanced IL-18 renal expression has been found in nephritic MRL/lpr mice [14] whereas daily injections of IL-18 in these mice resulted in an increase of serum proinflammatory cytokines leading to accelerated proteinuria and aggravation of glomerulonephritis [13]. In further support of the relevance of this cytokine for LN, elevated IL-18 serum concentrations have been detected in SLE patients [15–19] although no correlation with LN has been reported. However, the in vitro production of IL-18 by peripheral mononuclear cells has been found to be up-regulated in lupus patients with altered renal function compared to those without evidence of renal disease [17]. Thus, the pathogenic role of IL-18 in LN remains uncertain. In an attempt to elucidate the role of IL-18 for the development of LN in SLE, we investigated its serum levels and glomerular expression. Moreover, we examined the peripheral Th1/Th2 (IFN-γ/IL-4) cytokine balance in lupus patients with and without nephritis.
MATERIALS AND METHODS
Study population
A total of 133 SLE patients (≥4 ACR criteria) [20] and 44 healthy individuals from the Division of Rheumatology of the University of Florida and from the Department of Internal Medicine and Oncology and the Division of Nephrology of the University of Bari were enrolled. Written informed consent was obtained from all subjects. Whole blood and serum samples were obtained. Sera were frozen at −80°C until use. Detailed demographic characteristics, drug history, laboratory parameters and physical examination data were recorded at the study visits. Subjects with chronic renal insufficiency, uncontrolled hypertension, chronic infections, malignancies and diabetes were excluded. Renal pathology data were available for 51 out of 61 patients with LN and class of nephritis was assessed according to the WHO classification [21]. Clinical ACR criteria supported the diagnosis of LN in those patients lacking a detailed biopsy description. Urinalysis with no evidence of proteinuria, red cells or casts was interpreted as absence of active nephritis. The characteristics of the study population are summarized in Table 1. The study was separately approved by the institutional review boards of both Universities.
Table 1.
Characteristics of SLE patients and control subjects
| SLE with LN* | SLE without LN | Controls | |
|---|---|---|---|
| Number | 61 | 72 | 44 |
| Sex (%) | |||
| Female | 87 | 94 | 88 |
| Male | 13 | 6 | 12 |
| Age, years (mean ± SD) | 36·9 ± 11·9 | 42·1 ± 10·9 | 35·5 ± 14·6 |
| Race (%) | |||
| White | 52 | 44 | 55 |
| Black | 36 | 45 | 26 |
| Mixed | 10 | 8 | 8 |
| Asian | 2 | 3 | 11 |
| WHO Class of LN (n)** | |||
| I | 1 | ||
| II | 4 | ||
| III | 11 | ||
| IV | 20 | ||
| V | 15 | ||
| Treatment with prednisone | |||
| Daily dose, mg (mean ± SD) | 17·3 ± 23·6*** | 6·5 ± 11 | |
| Treatment with azathioprine | |||
| Daily dose, mg (mean ± SD) | 11·6 ± 46·1 | 6·7 ± 26·7 | |
| Treatment with hydroxychloroquine | |||
| Daily dose, mg (mean ± SD) | 134·1 ± 183·9*** | 166·2 ± 191·2 | |
Diagnosis of lupus nephritis (LN) was based both on biopsy (84%) and ACR criteria.
Numbers indicate the patients showing the defined class of LN by kidney biopsy evaluation.
P < 0·05 vs. SLE patients without LN (by Mann–Whitney test).
ELISAs
Serum samples were tested at 1/5 dilution for the 18 kD bioactive isoform of IL-18 using a human IL-18 ELISA kit (Medical & Biological Laboratories, Nagoya, Japan) according to the manufacturer's instructions. ELISAs for IFN-γ and IL-4 were performed using mouse mAb pairs (BD PharMingen, San Diego, CA, USA). Briefly, microtitre plates were incubated overnight with capture antibody (2 µg/ml) then washed, blocked and samples were analysed at 1/50 and 1/5 dilutions for IFN-γ and IL-4 ELISA, respectively. Known concentrations of purified recombinant human cytokines (BD PharMingen) served as standards. After overnight incubation, the plates were washed and biotinylated mouse anti-human cytokine-specific antibodies at 2 µg/ml were added. Streptavidin-alkaline phosphatase (Southern Biotechnology, Birmingham, AL, USA) was added at 1/1000 dilution and the reaction was developed with o-phenyldiamine chromogen solution (Sigma, St. Louis, MO, USA). Plates were red at 405 nm in a Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Each analysis was performed in triplicate and data were analysed by the Softmax software and expressed as mean values.
Flow cytometry
To phenotype the cellular profile of IFN-γ and IL-4 cytokines, aliquots of whole blood were stimulated with 25 ng/ml phorbol myristate acetate (Sigma) and cultured for 24 h at 37°C. Cultures were further incubated for 4 h with the GolgiStop solution (BD PharMingen) to block the cytokine secretion and enhance their staining signal. Non-adherent cells were harvested, depleted of erythrocytes by ACK lysing buffer (Biosource International, Camarillo, CA, USA), washed in phosphate-buffered saline, fixed and permeabilized by Cytofix/Cytoperm solution (BD PharMingen). Cells were then stained with fluorescein isothiocyanate (FITC)-conjugated IFNγ-specific mAb and phycoerythrin (PE)-conjugated IL-4 specific mAb (PharMingen) for 30 min at room temperature and analysed in a FACScan using CellQuest software (Becton-Dickinson, San Jose, CA, USA). Each analysis included 10 000 events and was performed by gating on lymphocyte subpopulation.
Immunohistochemistry
Immunohistochemical evaluation of IL-18 expression in LN was carried out on frozen renal biopsy sections from 13 lupus patients and 2 controls with isolated urinary alterations but normal kidney histology. Briefly, 4 µm sections of OCT-embedded tissue were mounted on poly L-lysine-coated slides, air dried and fixed in acetone for 10 min. After blocking with 2% horse serum at room temperature for 20 min, the slides were incubated overnight with a mouse mAb anti-IL-18 at 1 µg/ml (Medical & Biological Laboratories) specific for the 18 kD bioactive isoform of the cytokine. Binding of the secondary biotinylated horse antimouse IgG was detected by the Vectastain ABC system as an immunodetection kit according to the manufacturer's instructions (Vector, Burlingame, CA, USA). The endogenous peroxidase activity was quenched by incubation in a solution of methanol/0·3% H2O2 for 30 min. Finally, the diaminobenzidine tetrahydro-chloride solution (Vector) was used as chromogen and the slides were counterstained with haematoxylin. Negative controls were performed without primary antibody. Staining was evaluated by light microscopy. The number of IL-18+ cells within the glomeruli in each biopsy specimen was expressed as mean value/glomerular cross section (gcs).
Statistical analysis
The Mann–Whitney test was used to assess differences in serum cytokine levels, IFN-γ/IL-4 ratios and prednisone dosages. The statistical significance of the relationship between serum IL-18 levels, urinary microalbumin and serum IFN-γ titres was tested using Spearman's rank correlation analysis. P-values less than 0·05 were considered significant.
RESULTS
Serum elevations of IL-18 correlate with nephritis in SLE
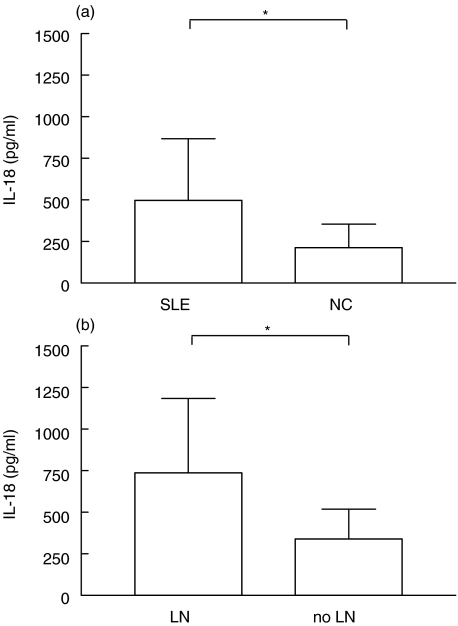
Figure 1 shows IL-18 serum levels in both SLE patients and normal controls (NC). As shown, the cytokine titres (sect. A) were significantly higher in patients and their mean value was twice that of controls (mean ± SD 498·7 ± 369 pg/ml versus 213·7 ± 142·5 pg/ml, P < 0·0001). Levels of IL-18 were positively correlated with urinary microalbumin (P < 0·05, r = 0·3234) and significantly increased in patients with LN (sect. B) compared to patients without LN (736·9 ± 447·8 pg/ml versus 340 ± 177·6 pg/ml, P < 0·0001). Age, sex and race did not influence IL-18 levels. In addition, no association of IL-18 levels with other organ involvement such as central nervous system, joint, lung or haematological disease was found thus suggesting that the high IL-18 serum titres within the SLE cohort was primarily attributable to the occurrence of LN.
Fig. 1.
Correlation of serum IL-18 levels with occurrence of nephritis in SLE. (a) ELISA measurement of the cytokine revealed higher values in SLE patients (n = 133) than in NC (n = 44). (b) Levels of IL-18 were significantly increased in LN patients (n = 61) as compared to patients without LN (n = 72). Bars represent the mean and SD in each group. *P < 0·0001 by Mann–Whitney test.
Since histological grading was available on most patients with LN, it was of interest to evaluate the relationship between serum IL-18 and WHO class of nephritis. The highest titres of serum IL-18 were detected in patients with diffuse proliferative and membranous glomerulonephritis (class IV and V, mean values 749·4 and 693·7 pg/ml, respectively), followed by those with focal segmental disease (class III, 534·5 pg/ml) and pure mesangial pathology (class II, 441·5 pg/ml). A single patient with trace amount of proteinuria and microscopic haematuria though in the presence of normal glomerular histology (class I) had a serum IL-18 concentration of 380 pg/ml, akin to the average of non-nephritic patients.
Glomerular expression of IL-18 is up-regulated in LN
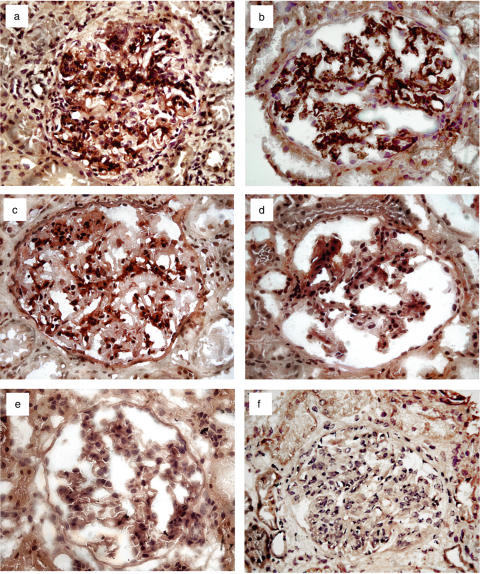
To evaluate the kidney IL-18 local production, we examined the in situ expression of the cytokine in renal biopsy specimens from SLE patients by immunohistochemistry. We detected a preponderance of IL-18 in glomeruli from SLE patients with both class IV (Fig. 2a) and V glomerulonephritis (Fig. 2b). Interestingly, renal tissue of class III LN patients (Fig. 2c) showed a weak glomerular IL-18 expression, whereas almost no evidence of this cytokine was found in glomeruli with class II nephritis. As expected, IL-18 was not detected in the SLE patient with class I nephritis (Fig. 2d) and in 2 control subjects (Fig. 2e) showing normal kidney histology.
Fig. 2.
Representative patterns of glomerular expression of IL-18 in LN. Prominent expression of IL-18 (dark brown) occurred in (a) all patients (n = 4) with class IV diffuse proliferative nephritis and (b) in those (n = 3) with diffuse membranous (class V) glomerulonephritis. (c) In contrast, a weak presence of the cytokine was documented in kidney specimens from patients (n = 3) with focal and segmental glomerulonephritis of class III. IL-18 was primarily detected within the mesangial matrix as well as in infiltrating mononuclear cells. (d) On the contrary, no significant evidence of IL-18 protein was observed in glomeruli from a single SLE patient (class I) with minimal proteinuria and without renal lesions. (e) Control bioptic specimens from normal kidneys (n = 2) showed no presence of the cytokine. (f) The pattern without the primary anti-IL-18 antibody is also shown. Original magnification × 40.
With regard to the location of glomerular cytokine expression, IL-18 was present within the mesangial matrix as well as in infiltrating mononuclear cells. As a result of the small number of available renal biopsies, correlative analyses between the amount of IL-18+ cells in glomeruli and the WHO histological grading of LN could not be addressed. However, the number of IL-18+ glomerular mononuclear cells/gcs was higher in class IV and class V biopsies (mean ± SD 21·5 ± 8 and 14·3 ± 2, respectively) than in patients with lesser glomerular lesions, including both class III and II LN (4·4 ± 3·8). IL-18+ cells were also detected in interstitial infiltrating mononuclear cells but their presence was rare. Finally, IL-18 detection within the nephritic kidneys was not confined to glomerular or interstitial infiltrating mononuclear cells but also occurred in some of the tubular epithelial cells (TECs) though its expression was weak and similar to that observed in normal kidneys. We consider tubular staining non specific since tubular brush border staining by immunoperoxidase was commonly detected also in control bioptic specimens processed without the primary anti-IL-18 antibody (Fig. 2f).
IL-18 increase correlates with predominance of Th1 immune response in LN
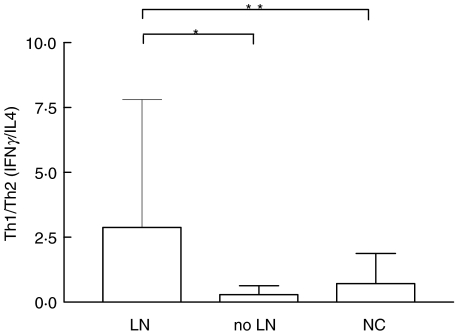
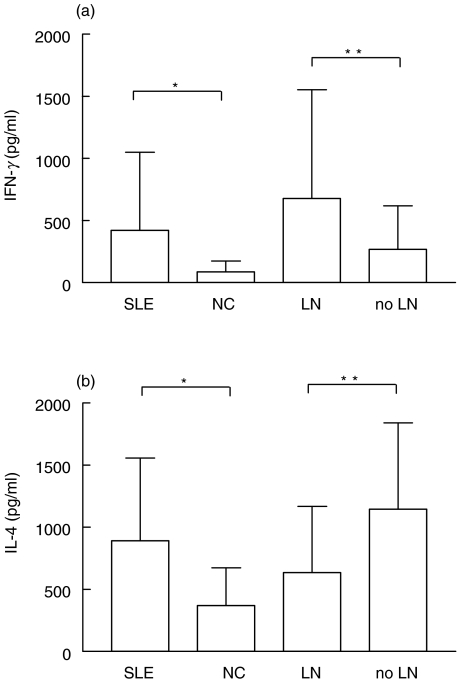
Figure 3 shows the mean levels of Th1/Th2 ratios in SLE patients in relation to LN as well as in controls. The Th1/Th2 ratios were calculated by dividing the levels of serum IFN-γ by those of IL-4. The mean ± SD Th1/Th2 ratio of patients with LN (2·9 ± 4·9) was significantly higher than those in both patients without renal disease (0·3 ± 0·3, P < 0·0001) and NC (0·7 ± 1·2, P < 0·05). The mean Th1/Th2 ratio of patients without LN was not significantly different from that of healthy controls. Among LN patients the highest mean levels of Th1/Th2 ratios were found in those with class IV and V glomerulonephritis (3·1 and 2·8, respectively) followed by those with less severe lesions (0·9). To evaluate the relative contribution of each cytokine to the presence of LN, serum levels of both IFN-γ and IL-4 were individually analysed. IFN-γ levels (Fig. 4a) were higher in SLE patients than in controls (mean ± SD 421 ± 630·5 pg/ml versus 87·7 ± 87·1 pg/ml, P < 0·0001) and correlated with the occurrence of nephritis (677·6 ± 874·5 pg/ml in patients with LN versus 267·9 ± 350·3 pg/ml in SLE patients without nephritis, P < 0·05). On the contrary, serum IL-4 levels (Fig. 4b) were higher in SLE patients than in controls (890·2 ± 667·6 pg/ml versus 352 ± 298·1 pg/ml, P < 0·0001) though not correlated with the renal disease. It is noteworthy that levels of IL-4 were significantly lower in patients with LN when compared to SLE patients with no renal involvement (634·4 ± 535·2 pg/ml versus 1146 ± 694·9 pg/ml, P < 0·05).
Fig. 3.
Th1 cytokine predominance in LN. Levels of serum IFN-γ and IL-4 were measured by ELISA. The Th1/Th2 (IFN-γ/IL-4) ratios were higher in LN patients (n = 61) than in patients without LN (n = 72) and in NC (n = 44). Bars represent the mean and SD. *P < 0·0001, **P < 0·05 by Mann–Whitney test.
Fig. 4.
Patients and controls were compared for serum IFN-γ and IL-4 levels by ELISA. (a) Concentrations of IFN-γ were higher in SLE group (n = 133) than in NC (n = 44); levels of this cytokine were higher in patients with LN (n = 61) as compared to patients without LN (n = 72). (b) IL-4 concentrations were higher in SLE patients than in NC; levels of this cytokine were lower in patients with LN with respect to patients without LN. Bars represent the mean and SD. *P < 0·0001, **P < 0·05 by Mann–Whitney test.
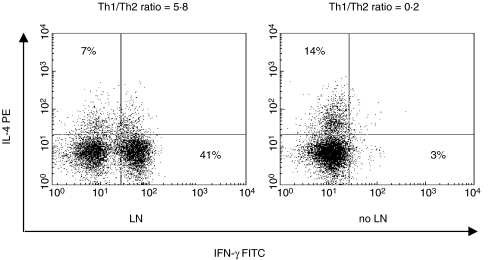
Further analyses assessed the phenotypic distribution of Th1+ and Th2+ cells in our patient population (Fig. 5). We found a predominance of IFN-γ expression with a relative lower expression of IL-4+ cells in patients with LN compared to non-nephritic patients (on average 40%versus 5% for IFN-γ and 6%versus 15% for IL-4). These results indicate that the cytokine balance is fundamentally skewed toward a Th1 phenotype in patients with LN and apparently related to the over-production of IL-18. In addition, we found that serum concentrations of IL-18 were positively correlated with those of IFN-γ (P < 0·05, r = 0·3429).
Fig. 5.
Flow-cytometry distribution of circulating Th1 (IFNγ+) and Th2 (IL-4+) in SLE patients divided in relation to the presence of nephritis. The analysis revealed an expansion of the IFNγ+ cell population in LN patients with respect to their control. Unstained cells served to adjust for autofluorescence and arrange quadrants. Percent of positive cells and relative Th1/Th2 ratios are indicated. The results are representative each of 5 patients.
DISCUSSION
Contrary to the predominant paradigm that SLE is a Th2 cytokine driven disease [22], recent studies have shown that Th1 cytokines may play an important role in the induction and progression of renal disease in lupus [10]. Results from the present study indicate that the increased expression of IL-18, a cytokine involved together with IL-12 in the priming of cells to produce IFN-γ and other Th1 cytokines [23], is strikingly associated with the presence of nephritis in lupus patients. Therefore, IL-18 is potentially a crucial pathogenic mediator of this important complication of SLE.
The role of IL-18 in LN is presently debated. Wong et al. [17] recently reported the positive correlation between plasma elevations of IL-18 and disease activity in SLE patients with renal involvement. In contrast, no significant association with specific renal manifestations or disease activity has been found by others [18]. Our results demonstrate that the serum concentration of the bioactive IL-18 isoform was significantly increased in SLE patients compared to controls, and that this elevation was associated with LN. It has been suggested that serum levels of cytokines may not reflect actual inflammation owing to their functional state as complexed molecules bound to soluble receptors [24]. Moreover concern about the interference of other proteins including the rheumatoid factors on the quantitative assays used to determine cytokine levels in serum has been raised [25]. To overcome these potential problems in our evaluation, we demonstrated in situ expression of IL-18 in biopsy specimens from patients with LN. Excess IL-18 within nephritic glomeruli, provides evidence for the potential pathogenic role of this cytokine in LN. It is noteworthy that IL-18 expression within the kidneys, as well as its serum levels, showed a trend toward a direct correlation with the severity of the histopathologic pattern of LN indicating that increased IL-18 production may be directly related to the severity of renal disease in SLE. In support of this hypothesis, Faust et al. [14] have recently reported that the up-regulation of IL-18 is restricted to nephritic kidneys of lupus-prone MRL/lpr mice and parallels the severity of the disease. Our findings suggest that the major site of IL-18 expression in LN is the glomerulus. Although the presence of the cytokine within the mesangial matrix does not allow us to differentiate between its basal expression and de novo synthesis, the finding of cytoplasmic staining for IL-18 in glomerular mononuclear cells strongly indicates that glomerular expression of IL-18 is derived, at least in part, from its local production. In particular, we suspect that the primary source of IL-18 is provided by activated glomerular infiltrating macrophages [11]. These cells are well known producers of the cytokine in inflammed sites [26] and have been reported to be increased in LN [27]. Based on these results, we speculate that, besides other sources of IL-18, such as lymphoid tissue and spleen, local IL-18 production within the kidney contributes to the elevated circulating levels of IL-18 observed in LN.
Data from our study provide evidence that both serum Th1 (IFN-γ) and Th2 (IL-4) cytokines can be elevated in SLE patients thus emphasizing that SLE is a complex disease that may be supported by the activation of different cytokine systems at different time-points during disease development [19,28,29]. Predominance of Th1+ cells by immunohistochemical analysis has been reported in kidneys of SLE patients with diffuse proliferative glomerulonephritis and the increased IFN-γ/IL-4 ratio in peripheral blood has been proposed as a promoter of renal tissue damage [11]. IL-18 is capable of inducing a Th1 response in T cells either in synergy or independently of IL-12 through the production of IFN-γ[30–33]. Moreover, Fantuzzi et al. [34] reported that IL-12-induced IFN-γ production is dependent of IL-18. In our study increased IL-18 levels were associated with excessive IFN-γ production and reduced IL-4 in patients with LN compared to SLE patients without overt renal disease. This may suggest that over-production of IL-18 contributes to both induction of a Th1-type immune response and IFN-γ up-regulation resulting in autoimmune renal injury in LN. Shift toward a Th2 cytokine profile may theoretically prevent the onset of renal damage in SLE.
The use of high doses of prednisone in the patients with LN raises the concern that Th1 cytokine predominance along with a relative Th2 defect may be attributed to steroid therapy. However, despite steroid treatment, all LN patients showed active renal manifestations, suggesting that the cytokine alterations were likely related to disease itself rather than secondary to therapy. This hypothesis was further confirmed by the immunohistochemistry results demonstrating increased IL-18 expression in the kidneys of patients with LN. Indeed, none of the 13 patients studied was treated with corticosteroids and/or immunosuppressive agents at the time of biopsy. In further support of a lack of interference of steroid therapy with our results there is evidence that corticosteroids may interfere with the T cell differentiation by inhibition of Th1 development and enhancement of Th2 response [35,36].
In murine models of LN, IFN-γ plays a crucial role in provoking kidney damage by promoting the formation of pathogenic complement-fixing antibodies [37] as well as by increasing major histocompatibility complex class II antigen espression [4]. Recently it has been shown that IFN-γ may contribute to the pathogenesis of diffuse proliferative glomerulonephritis by both up-regulation of CD40 and activation of the cellular immune response in human lupus [38]. Moreover, IFN-γ is known to enhance integrin expression including both intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), on glomerular cells [39], which in turn may facilitate influx of inflammatory cells. Indeed, IL-18 itself has the potential to induce the expression of adhesion molecules and chemokines in an IFN-γ-independent way [40,41]. Aside from the costimulatory functions on Th1 cytokines, IL-18 also induces the production of other proinflammatory cytokines that have been implicated in the pathogenesis of renal disease in SLE, such as tumour necrosis factor-α (TNF-α) and nitric oxide [42,43]. Further pathophysiological effects caused by IL-18 may include the induction of apoptosis in TECs [14]. Although, IL-18 receptors have not been yet detected on renal cells, it is conceivable that the local production of the cytokine is devoted to activate specific cells within this microenvironment. However, future studies are needed to demonstrate the local functional role of IL-18.
In conclusion, our findings suggest that IL-18 may play a crucial role in triggering inflammation in LN by promoting a cytokine imbalance toward a Th1 type response. Lupus patients with nephritis usually have an unfavourable prognosis and many eventually progress to renal failure despite aggressive treatments. Therefore, prognostic markers and more effective therapeutic regimens are needed. Our data show that elevated circulating concentrations of IL-18 reflect high local expression in LN patients, in particular in those with either proliferative or membranous glomerulonephritis. Thus, elevation of this cytokine could be useful as a predictor of the severity and progression of renal disease in SLE. Therapeutic strategies devoted to down-regulate IL-18 may be helpful in the treatment of LN. In this context, a recent study has shown that IL-18 cDNA vaccination induces the production of autoantibodies to IL-18 conferring protection from development and progression of nephritis in lupus-prone MRL/lpr mice [44].
Acknowledgments
We gratefully thank Vincenzo Gesualdo and Massimo Dell’Aquila for excellent technical support. H.B. Richards is supported by The National Institutes of Health (grant NIDDK K08 DK02890-02 and NCRR MO1 RR00082), the State of Florida and the Lupus Research Institute NYC.
REFERENCES
- 1.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–4. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 2.Kelley VR, Wuthrich RP. Cytokines in the pathogenesis of systemic lupus erythematosus. Semin Nephrol. 1999;19:57–66. [PubMed] [Google Scholar]
- 3.Perez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlondorff D. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369–82. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 4.Ozmen L, Roman D, Fountoulakis M, Schmid G, Ryffel B, Garotta G. Experimental therapy of systemic lupus erythematosus. the treatment of NZB/W mice with mouse soluble interferon-gamma receptor inhibits the onset of glomerulonephritis. Eur J Immunol. 1995;25:6–12. doi: 10.1002/eji.1830250103. [DOI] [PubMed] [Google Scholar]
- 5.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL- Fas (lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 6.Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160:3713–8. [PubMed] [Google Scholar]
- 7.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-gamma is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–80. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas (lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–25. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 9.Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in Il−12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 10.Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1644–8. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 13.Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, Komai-Koma M, Wei X, Liew FY. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–47. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- 14.Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–95. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- 15.Wong CK, Li EK, Ho CY, Lam CW. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology. 2000;39:1078–81. doi: 10.1093/rheumatology/39.10.1078. [DOI] [PubMed] [Google Scholar]
- 16.Shibatomi K, Ida H, Yamasaki S, et al. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 2001;44:884–92. doi: 10.1002/1529-0131(200104)44:4<884::AID-ANR145>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Ho CY, Li EK, Tam LS, Lam CW. Elevated production of interleukin-18 is associated with renal disease in patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;130:345–51. doi: 10.1046/j.1365-2249.2002.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokano Y, Suzuki J, Amano H, Nozawa K, Morimoto S, Hashimoto H. Increased levels of interleukin-18 in patients with systemic lupus erythematosus: [comment on the article by Shibatomiet al] Arthritis Rheum. 2002;46:1410–2. doi: 10.1002/art.10253. [DOI] [PubMed] [Google Scholar]
- 19.Amerio P, Frezzolini A, Abeni D, Teofoli P, Girardelli CR, De Pita O, Puddu P. Increased IL-18 in patients with systemic lupus erythematosus: relations with Th-1, Th-2, pro-inflammatory cytokines and disease activity. IL-18 is a marker of disease activity but does not correlate with pro-inflammatory cytokines. Clin Exp Rheumatol. 2002;20:535–8. [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Appel GB, Silva FG, Pirani CL, Meltzer JI, Estes D. Renal involvement in systemic lupud erythematosus (SLE). a study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 1978;57:371–410. doi: 10.1097/00005792-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunol Rev. 1995;144:157–93. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–90. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz DA, Stohl W, Gray JD. T lymphocytes, natural killer cells, cytokines, and immune regulation. In: Wallace DJ, Han BH, editors. Dubois’ Lupus Erythematosus. Baltimore: Williams & Wilkins; 1997. pp. 155–94. [Google Scholar]
- 25.Kolb G, Allner R. A new method for rapid and simple adsorption of rheumatoid factors from serum. J Clin Chem Clin Biochem. 1986;24:379–86. [PubMed] [Google Scholar]
- 26.McInnes IB, Gracie JA, Leung BP, Wei XQ, Liew FY. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–5. doi: 10.1016/s0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- 27.Hill GS, Delahousse M, Nochy D, Remy P, Mignon F, Mery JP, Bariety J. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59:304–16. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 28.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN-gamma) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–95. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Interleukin-18. Methods. 1999;19:121–32. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuki T, Micallef MJ, Kohno K, Tanimoto T, Ikeda M, Kurimoto M. Interleukin 18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res. 1997;17:3253–8. [PubMed] [Google Scholar]
- 32.Shapiro L, Puren AJ, Barton HA, et al. Interleukin 18 stimulates HIV type 1 in monocytic cells. Proc Natl Acad Sci USA. 1998;95:12550–5. doi: 10.1073/pnas.95.21.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantuzzi G, Puren AJ, Harding MW, Livingston DJ, Dinarello CA. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1beta-converting enzyme (caspase-1) -deficient mice. Blood. 1998;91:2118–25. [PubMed] [Google Scholar]
- 34.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–7. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–95. [PubMed] [Google Scholar]
- 36.Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–94. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 37.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhm WS, Na K, Song GW, Jung SS, Lee T, Park MH, Yoo DH. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology. 2003;42:935–8. doi: 10.1093/rheumatology/keg255. [DOI] [PubMed] [Google Scholar]
- 39.Wuthrich RP. Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int. 1992;42:903–14. doi: 10.1038/ki.1992.367. [DOI] [PubMed] [Google Scholar]
- 40.Kohka H, Yoshino T, Iwagaki H, et al. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J Leukoc Biol. 1998;64:519–27. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- 41.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–4. [PubMed] [Google Scholar]
- 43.Belmont HM, Levartovsky D, Goel A, Amin A, Giorno R, Rediske J, Skovron ML, Abramson SB. Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1810–6. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 44.Bossu P, Neumann D, Del Giudice E, et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc Natl Acad Sci USA. 2003;100:14181–6. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]