Abstract
Serum activity of the adenosine deaminase (ADA) isozyme, ADA2, has been reported to be elevated during various disease states. Macrophages have been suggested as the cellular source of extracellular ADA activity because they are one of the only cell types in which intracellular ADA2 activity has been measured, but extracellular secretion has never been demonstrated. Rat primary peritoneal macrophages (PPMs) and peripheral blood monocytes (PBMs) were harvested and incubated for 18 h in RPMI supplemented with horse serum. PPM and PBM lysates were assayed for intracellular ADA activity (ammonia production). In vitro and in vivo extracellular ADA activities were measured in media and rat serum, respectively. Activity of ADA1 was confirmed by selective inhibition with erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA). ADA2 activity was inhibited by 2′-deoxycoformcin only, and was increased at a low pH (6·5). Activity of both ADA isozymes was found in PPMs and PBMs, and their media. In a separate group of rats, peritonitis was induced by ip insertion of 400 mg/kg caecal slurry. PPMs were harvested 24 h later and incubated for 18 h. In PPMs from rats with peritonitis both isozymes were elevated by a similar proportion. In contrast, media from these PPMs had a lower ADA1 and a higher ADA2 activity compared to PPMs from nonseptic rats. This resulted in a greater proportion of ADA2 in media. The isozyme proportions in serum from septic rats more closely resembled that of the PPM media. The response of PBM was small relative to that of PPM. These results suggest that macrophages are a significant source of extracellular ADA isozymes, the activity of which increases during an inflammatory response. Because extracellular isozymes profiles differ from cellular concentrations, the data also suggest differential release of each isozyme from PPMs.
Keywords: isozymes, sepsis, peritonitis, EHNA, deoxycoformycin, inflammation, purine metabolism, nucleoside
INTRODUCTION
Adenosine deaminase (ADA) is important in acute and protracted inflammatory responses [1–3]. Recent work in our laboratory has demonstrated elevated ADA activity during inflammatory responses in macrophage-rich tissues, such as liver and spleen [3]. Specifically, a disproportionate increase in the enzyme activity attributable to the isozyme ADA2 has been found in pleural effusions of tuberculosis patients and serum of patients infected with HIV [4–7]. Thus, several investigators have suggested that increased ADA2 in the serum or pleural effusates may be a marker of specific disease states [4,8], and as such have suggested it as a diagnostic tool [7,9]. Preliminary work from our laboratory has shown significant increases in serum ADA2 in septic rats. Despite the implications of these findings, important basic information needed for further characterization and identification of ADA2, such as the cellular source, have not been forthcoming.
Two primary forms of ADA demonstrate enzymatic activity. There has been no consensus of nomenclature in this field, so for the purposes of this manuscript, discussion of and reference to ADA2 uses the nomenclature as outlined by Hirschhorn & Ratech [10] and other major laboratories [4,5,11–15]. ADA1 has been extensively characterized. It is critically important in lymphocyte proliferation and development. Congenital deficiency of ADA1 in humans results in severe combined immunodeficiency syndrome (SCIDS) (for review see [16]), and ADA knockout mice are not viable [17]. In contrast, almost nothing is known about the molecular characteristics of ADA2. The protein sequence, structure, and molecular regulation of this isozyme has not been explored, presumably because no specific physiologic or pathologic roles have been identified for it. As such, current methods of investigation that are commonly used, including the use of RNA or DNA sequence probes, or any form of immunodetection requiring antibodies to the molecule, are not available. The current state of art method for segregating the effects of ADA1 and ADA2 is the same as that used more than 10 years ago. ADA1 is completely inhibited by 100µm erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), while ADA2 is resistant to inhibition by EHNA [7,18,19].
A specific role for ADA2 has yet to be demonstrated. However, Gakis et al. [13] have hypothesized that relative changes in ADA1 and ADA2 could influence the concentrations of deoxyadenosine in monocytic cells, which could contribute to the innate defense response to pathogenic organisms. Identification of the cellular source of ADA2 would contribute to a better understanding of it's role in pathophysiology. The observation that ADA2 has only been demonstrated in monocytes and macrophages has led some to suggest these cells as the source of elevated serum or pleural effusion ADA2 [5,11,12]. However, the ability of these cells to release ADA into their environment has yet to be conclusively demonstrated. In this initial attempt to test the hypothesis that extracellular ADA2 can represent release from macrophages, and that these cells contribute to the alterations in serum ADA2 activity after an inflammatory challenge, we sought to determine if (1) ADA1 and 2 are released by macrophages in vitro, and (2) if the pattern of ADA1 and 2 release from septic animals differs from nonseptic, and (3) if sepsis-associated changes in macrophage ADA2 release can explain changes in serum of septic animals.
MATERIALS AND METHODS
Animals
The experiments reported herein were approved by the Animal Care and Use Committee of the University of Illinois, and were conducted according to the principles set forth in the ‘Guide for the Care and Use of Laboratory Animals’, Revised 1996. Male Sprague-Dawley rats (Charles River, MA, USA) weighing 300–350 g, were housed at constant temperature with 10/14-h periods of light and dark exposure, respectively. Animals were allowed access to standard rat chow and water ad libitum during an acclimation period of at least seven days prior to use in these experiments.
Materials
EHNA, a selective inhibitor of ADA1, was obtained from Sigma (St. Louis, MO, USA). 2-deoxycoformycin (dCF; pentostatin) a potent inhibitor of both ADA1 and ADA2 was provided by Supergen, Inc. (Dublin, CA, USA). Horse Serum was purchased from Fisher Scientific (Hanover Park, IL, USA).
Collection of cells, serum and tissues
Rats were anaesthetized with 60 mg/kg pentobarbital IP and the ventral abdominal surface was shaved and cleaned with betadine and alcohol. A small incision was made through the cutaneous tissue layer along the midline and the skin was peeled away from the abdominal area. The peritoneal cavity was then flushed with 1 × PBS 1 mm EDTA three times in order to collect peritoneal macrophages. After each flush the PBS and suspended macrophages were collected from the peritoneum.
Blood was obtained prior to euthanasia by cardiac puncture. After allowing blood to clot at room temperature, samples were spun down and serum was collected. Prior to euthanasia samples of the duodenum were removed, and immediately frozen at −80 C for later assay.
Isolation of peritoneal macrophages
The macrophage cell suspension was spun at 1000 g for 10 min to pellet the cells. Following aspiration of the PBS cells were resuspended in red cell lysis buffer (155 mm NH4Cl, 10 mm NaHCO3, 0·1 mm EDTA) and incubated on ice for 10 min. Remaining cells were pelleted by centrifugation at 1000 g for 10 min. Red cell lysis buffer was aspirated and cells resuspended in RPMI 1640 media supplemented with 10% horse serum (HS) and a 5% penicillin, streptomycin, neomycin mixture (PSN). Cells were transferred to cell culture dishes and allowed to adhere at 37°C and 5% CO2. After a 1·5-h incubation nonadherent cells were removed by repeated washes with cold 1 × PBS. This resulted in a purified population of adherent macrophages. Macrophage specific staining with alpha-napthalacetate confimed > 98% enrichment for macrophages, and trypan blue staining confirmed > 95% viability. The adherent macrophages were scraped, counted and re-plated into 35 mm dishes with 1 ml RPMI + HS + PSN for incubation overnight.
Isolation of periipheral blood monocytes
Monocytes were isolated from peripheral blood by Ficoll (Amersham Biosciences, Piscataway, NJ, USA) separation, according to the manufacturer's specifications. After the first centrifugation, the layer enriched for monocytes and lymphocytes was plated and incubated for 1·5 h at 37°C and 5% CO2. Non-adherent cells were then discarded, plates washed, and 500µl new media added to the adherent monocytes for further incubation. Cells and media were collected 22·5 h later.
Induction of sepsis
Macrophages and monocytes were collected from a separate group of septic rats for study. Peritonitis was induced as previously described [1,3]. Briefly, 400 mg/kg·5 ml of a cecal slurry was introduced through a 0·25-cm vertical midline abdominal incision and diffusely distributed. All rats received 50 ml/kg of 0·9% normal saline intravenously 2-h after peritonitis induction for resuscitation over 20 min. Eighteen hours after the induction of sepsis, rats were anaesthetized in order to collect peritoneal macrophages, tissue, monocytes, and serum samples.
Adenosine deaminase activity
ADA activity was determined using a modification of the methods described by Vielh and Castellazzi [20]. Duodenal tissues were homogenized in 9 × vol/wt lysis buffer (50 mm KH2PO4, 0·125 mm EDTA, 0·5 mm MgCl2) with 0·5% Triton X, 20 µm leupeptin, and 0·03 µm pepstatin A. Tissues were then homogenized for three-10 second bursts using a Kika-Werk Ultra Turrax homogenizing probe. Samples were centrifuged at 4°C (20 000 g, 20 min) and supernatants were collected.
After an 18 h incubation period at 37°C and 5% CO2 macrophage media was collected and cells were collected in lysis buffer with 0·5% (v/v) Triton X 100. Macrophages were lysed by shaking horizontally at room temperature for 2 h and then sonicated for 10 min. Lysed cells were centrifuged at 4°C (20 000 g, 20 min) and the supernatant collected.
Five microliters of serum, cell lysates, or media were incubated for varying amounts of time with reaction mixture (3 mm deoxyadenosine, 0·1 m KH2PO4) at 37°C. The reaction was stopped with the addition of Solution I (50 mg/ml Phenol, 0·25 mg/ml Sodium Nitroprusside). The colourimetric reaction was initiated by Solution II (75 mg/ml Sodium Hydroxide, 0·89% Sodium Hypochlorite). Colour was allowed to fully develop by incubating the 96 well plate at 56°C for one hour. NaOH (0·5 m) was added and the OD was determined at 625 nm. One unit of ADA activity was defined as the amount of enzyme generating 1 nmol of NH3/h under the experimental conditions. ADA2 activity with deoxyadenosine was adjusted to account for the 0·25 deoxyadenosine:adenosine enzymatic use ratio [7,19].
Our original attempts to measure ADA activity in the media of cultured macrophages was unsuccessful due to extremely high levels of background ADA activity from fetal bovine serum used to supplement the media. This problem was overcome by supplementing the medium with horse serum rather than fetal bovine serum.
Statistics
All data demonstrated homogeneity of variance. Differences between means limited to two groups were compared by unpaired t-test. A two way anova was used to compare between groups (factor 1), and ADA sources (factor 2). Studies were designed to detect > 25% differences at power > 0·8, and differences were accepted at P < 0·05.
RESULTS
Separation of ADA1 and ADA2 activities
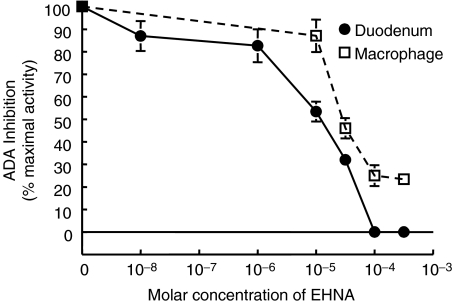
Eryththro-9-(2-hydroxy-3-nonyl) adenine (EHNA) has been used extensively as an inhibitor of ADA enzymatic activity [21]. EHNA preferentially inhibits ADA1, thus unmasking ADA2 activity which is resistant to EHNA. ADA2 activity has only been demonstrated in monocytes and macrophages [5,19], therefore an initial dose–response curve was performed using duodenal cell lysates, which express very high levels of ADA1 activity. Duodenal cell lysates were treated in vitro with EHNA concentrations ranging from 10−8 to 5 × 10−4m and ADA activity was subsequently determined. Figure 1 shows the inhibition of ADA activity by increasing concentrations of EHNA. There was a dose dependent decrease in the percent of maximal ADA activity between 10−6 and 10−4m EHNA. When EHNA concentrations between 10−4m and 5 × 10−4 were used ADA activity fell below detection.
Fig. 1.
ADA isozyme sensitivity to EHNA: Duodenal homogenates (•) and macrophage cell lysates (□) were treated in vitro with EHNA concentrations ranging from 10−8 to 5 × 10−4m and adenosine deaminase activity was subsequently determined. Inhibition of ADA activity by EHNA relative to basal activity is shown. There was a dose dependent decrease in ADA activity between 10−7 and 10−4m EHNA. At concentrations higher than 10−4m EHNA ADA activity was below detection limits in duodenal homogenates. In contrast, the inhibition of macrophge cell lysates ADA activity reached a nadir at around 20–30%. The activity that remained represents ADA2 activity.
Another dose–response curve was performed using macrophage cell lysates which are one of the few known sources of ADA2 activity. There was a significant inhibition of ADA activity between 10−5 and 10−4m EHNA. In contrast to the complete inhibition seen with the duodenal cell lysates, inhibition of ADA activity by EHNA reached a nadir at around 20–30% of maximal activity even when treated with higher concentrations of EHNA. The remaining ADA activity represented ADA2 activity. This was confirmed by loss of all activity (below detection) when the assay was performed in the presence of 50µm dCF. Therefore, in subsequent experiments 100µm EHNA was used to separate out the relative activities of ADA1 from ADA2.
While the optimum pH of ADA1 is neutral, optimal ADA2 activity is seen at a pH of 6·5. A separate set of ADA activity assays were carried out at pH 7·0 and 6·5. Table 1 shows that a change in pH from 7·0 to 6·5 caused a 4% decrease in ADA activity in duodenal samples. In contrast, a change in pH from 7·0 to 6·5 caused an 11·7% increase in macrophage ADA activity. These results further confirmed the presence of ADA2 in macrophages. All subsequent ADA activity assays were carried out at a pH of 6·5.
Table 1.
Effect of pH on ADA activity. Values are means ± SEM. Because the pH optimum for ADA1 and ADA2 are 7 and 6·5, respectively, ADA assays were carried out at different pH's in an effort to optimize the assay. A 4% decrease in activity was seen in duodenal samples when the pH was shifted from 7·0 to 6·5. In contrast there was an 11·7% increase in ADA activity in the macrophage lysates when pH was shifted from 7·0 to 6·5. It is important to keep in mind that ADA1 accounts for all of the ADA activity in the duodenal samples, but only 70–80% of the activity in the macrophage samples
| pH 7·0 | pH 6·5 | |
|---|---|---|
| Duodenum | 183·18 ± 0·48 | 176·33 ± 0·20 |
| Macrophages | 42·96 ± 0·03 | 48·0 ± 1·07 |
ADA isozyme activity in peritoneal macrophage lysates and media
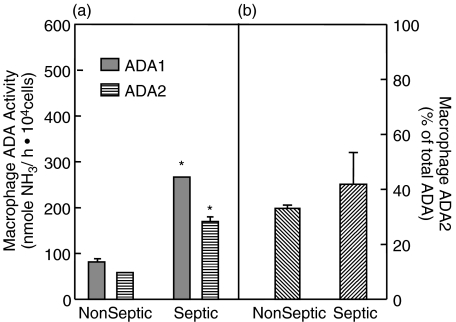
Both ADA1 and ADA2 were measurable in macrophage lysates (Fig. 2a); the total ADA activity was relatively low compared to other tissues [1]. ADA2 accounted for ∼35% of the total ADA activity (Fig. 2b).
Fig. 2.
Macrophage lysate ADA activity: Peritoneal macrophages were lysed after 24 h incubation in vitro. ADA activity was measured and reported as nmoles NH3/ h·104cells. (a) Both ADA1 ( ) and ADA2 (
) and ADA2 ( ) were measurable in macrophage lysates of rats, with ADA1 representing the predominant proportion of total ADA activity. Activity of both isozymes were significantly higher in macrophages from rats with peritonitis. (b) Activity attributable to ADA2 represented < 50% of the total activity, regardless of rat condition. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
) were measurable in macrophage lysates of rats, with ADA1 representing the predominant proportion of total ADA activity. Activity of both isozymes were significantly higher in macrophages from rats with peritonitis. (b) Activity attributable to ADA2 represented < 50% of the total activity, regardless of rat condition. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
As we have previously demonstrated in liver and spleen [1], total macrophage ADA activity was higher in septic rats relative to nonseptic rats. Both ADA1 and ADA2 activity were similarly higher in macrophages after sepsis induction compared to nonseptic rats (P < 0·001). Because both ADA1 and ADA2 activities were similarly elevated the relative ratio of ADA1 to ADA2 did not change significantly (Fig. 2b).
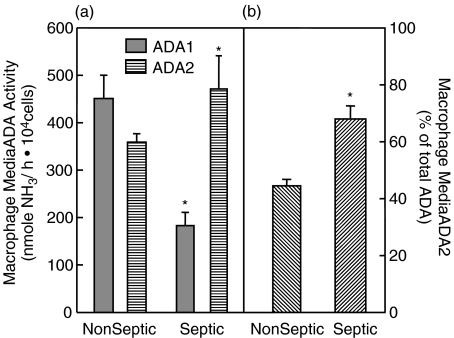
The media in which macrophages were incubated showed both ADA1 and ADA2 activity (Fig. 3). This activity varied directly with the incubation time indicating the accumulation of ADA1 and 2 activity in the media over time (data not shown). ADA1 accounted for the majority of total activity in the media in nonseptic rats (Fig. 3a). There were no significant differences in activity attributable to ADA2 compared to the macrophage lysates (Fig. 3b compared to Fig. 2b).
Fig. 3.
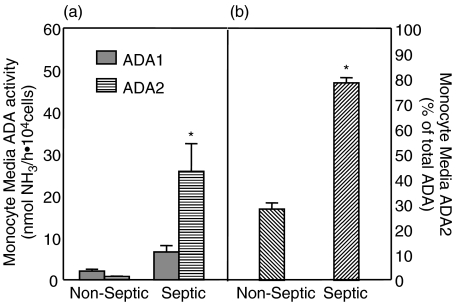
Macrophage media ADA activity: Primary peritoneal macrophages were incubated with RPMI media supplemented with 10% HS and 5% PSN for 24 h. Media was collected and subsequently assayed for ADA activity. (a) Both ADA1 ( ) and ADA2 (
) and ADA2 ( ) were measurable in macrophage media. In nonseptic rats, ADA1 represented the predominant proportion of total ADA activity. ADA1 activity in the media of septic macrophages was lower than in the media of naive macrophages. Conversely, ADA2 activity was higher in the media collected from septic macrophages. (b) Activity attributable to ADA2 was significantly higher in media of macrophages collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
) were measurable in macrophage media. In nonseptic rats, ADA1 represented the predominant proportion of total ADA activity. ADA1 activity in the media of septic macrophages was lower than in the media of naive macrophages. Conversely, ADA2 activity was higher in the media collected from septic macrophages. (b) Activity attributable to ADA2 was significantly higher in media of macrophages collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
The relative amounts of activity that could be attributed to each isozyme depended considerably upon the condition of the animal from which the cells came. ADA1 activity in the media of septic macrophages was significantly (P < 0·001) lower (183 ± 28 NH3/ h106 cells) than in the media of nonseptic rat macrophages (451 ± 49 NH3/ h106 cells). Conversely, ADA2 activity was higher (P = 0·013) in the media collected from septic macrophages (471 ± 70 NH3/ h106 cells) than in the media from naive macrophages (359 ± 18 NH3/ h106 cells)(Fig. 3a). As can be seen in Fig. 3b ADA2 became the dominant isozyme in the media of septic macrophages, and accounted for 68 ± 4·6% of total ADA activity (P = 0·008). This differed significantly from the profile seen in macrophage lysates from septic rats, suggesting that ADA isozymes are differentially released from macrophages, rather than simply in the proportion represented in those cells.
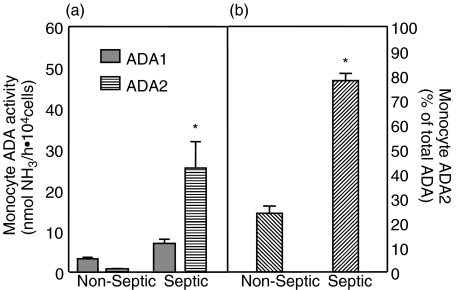
ADA isozyme activity in peripheral blood monocyte lysates and media
Both ADA1 and ADA2 were measurable in monocyte lysates (Fig. 4a) and media (Fig. 5a), but the activity was considerably less than that measured in macrophages. Particular attention should be directed to the differences in scales for macrophage versus monocyte figures. Monocytes had 50–200-fold lower ADA activity than did macrophages. In addition, the release of ADA activity from monocytes into their media was less robust than that seen in macrophages.
Fig. 4.
Peripheral blood monocyte lysate ADA activity: Peripheral blood monocytes were lysed after 24 h incubation in vitro. ADA activity was measured and reported as nmoles NH3/ h·104cells. (a) Both ADA1 ( ) and ADA2 (
) and ADA2 ( ) were measurable in monocyte lysates of rats, with ADA1 representing the predominant proportion of total ADA activity. However, cell concentrations were 50–200-fold lower than that of macrophages (note axis scale difference compared to Fig. 2). Activity of only ADA2 was significantly higher in monocytes from rats with peritonitis. (b) Activity attributable to ADA2 was significantly higher in monocytes collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
) were measurable in monocyte lysates of rats, with ADA1 representing the predominant proportion of total ADA activity. However, cell concentrations were 50–200-fold lower than that of macrophages (note axis scale difference compared to Fig. 2). Activity of only ADA2 was significantly higher in monocytes from rats with peritonitis. (b) Activity attributable to ADA2 was significantly higher in monocytes collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
Fig. 5.
Peripheral blood monocyte media ADA activity: Peripheral blood monocytes were incubated with RPMI media supplemented with 10% HS and 5% PSN for 24 h. Media was collected and subsequently assayed for ADA activity. (a) Both ADA1 ( ) and ADA2 (
) and ADA2 ( ) were measurable in monocyte media. In nonseptic rats, ADA1 represented the predominant proportion of total ADA activity. Only ADA2 activity was higher in the media collected from septic monocytes. (b) Activity attributable to ADA2 was significantly higher in media of monocytes collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
) were measurable in monocyte media. In nonseptic rats, ADA1 represented the predominant proportion of total ADA activity. Only ADA2 activity was higher in the media collected from septic monocytes. (b) Activity attributable to ADA2 was significantly higher in media of monocytes collected from rats with peritonitis. *Significantly different relative to nonseptic naive values. P < 0·05. Samples sizes (n) were 4 for nonseptic (each sample representing the pooled cells from 2 to 3 rats) and 6 for septic groups, each sample representing macrophages from a single septic rat.
Total monocyte ADA activity was higher in septic rats relative to nonseptic rats, but unlike the response seen in macrophages, almost all of the increase could be attributed to ADA2 (P < 0·001). As a result, the relative amount of ADA2 (Fig. 4b) rose significantly (P < 0·001). These changes in activity were mirrored in the media from the monocytes (Fig. 5; P < 0·001), and again contrast the response of peritoneal macrophages.
ADA isozyme activity in serum
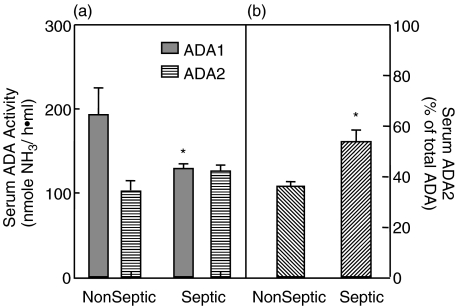
Rat serum ADA activity was readily measurable; ADA1 and ADA2 isozyme concentrations, as well as the relative abundance of ADA2, are shown in Fig. 6. There was nearly 2-times as much ADA1 as ADA2 based on enzyme activity in nonseptic animals. A different profile was seen in septic rats. As can be seen in Fig. 4a ADA1 activity was significantly (P = 0·014) lower in septic animals when compared to the serum of nonseptic rats. In comparison, there was no significant difference in serum ADA2 activities between nonseptic and septic animals. Thus, the proportion of total ADA activity represented by ADA2 was significantly higher (P = 0·007) in septic rats (Fig. 4b).
Fig. 6.
Serum ADA activity: ADA activity is reported as nmol ammonia/ h·ml serum. (a) Both ADA1 ( ) and ADA2 (
) and ADA2 ( ) were measurable in serum of rats, with ADA1 representing the predominant proportion of total ADA activity in nonseptic rats. In rats with peritonitis, serum ADA1 and ADA2 activity levels were comparable. (b) The proportion of total ADA activity represented by ADA2 activity in the serum is represented. ADA2 became the predominant isozyme in serum collected from septic rats as compared to nonseptic rats. *Significantly different relative to nonseptic naive values. P < 0·05. n = 9 and 12 for nonseptic and septic groups, respectively.
) were measurable in serum of rats, with ADA1 representing the predominant proportion of total ADA activity in nonseptic rats. In rats with peritonitis, serum ADA1 and ADA2 activity levels were comparable. (b) The proportion of total ADA activity represented by ADA2 activity in the serum is represented. ADA2 became the predominant isozyme in serum collected from septic rats as compared to nonseptic rats. *Significantly different relative to nonseptic naive values. P < 0·05. n = 9 and 12 for nonseptic and septic groups, respectively.
DISCUSSION
Our data supports the concept that macrophages are a significant source of serum ADA2 activity. ADA2 activity represented a significant portion of total ADA activity in peritoneal macrophage lysates. Furthermore, the accumulation of ADA activity in media incubated with macrophages or monocytes suggests these cells are capable of releasing ADA activity into their surrounding environment, a phenomenon heretofore suggested, but never demonstrated. The differences in the relative abundance of ADA1 and ADA2 in the media and macrophage cell lysates indicates that media ADA activity cannot be entirely explained by simple cell death and lysis. Finally the change in relative ADA isozyme activity profiles in the macrophages obtained from septic animals is starkly different from the change in relative isozyme proportions seen in the media. This suggests differential release of each isozyme. Taken together these data support the hypothesis that the release of ADA activity by the macrophages into their surroundings contributes to the relative elevation of serum ADA2 activity seen following an inflammatory response. In confirming this, and in the context of previous work from our lab demonstrating beneficial effects of ADA inhibition [1–3], we hope these data spur interest in investigating the physiological role of ADA2 in the inflammatory response.
The relative elevation in ADA2 we observed is consistent with what others have seen in various disease states including tuberculosis, human immunodeficiency virus, and leukaemia [4,5,8,22]. Our data suggests a proportional increase in relative ADA2 activity during protracted sepsis as well. Monocytes are one of the only tissues in which ADA2 activity has been demonstrated [5,19]. Consequently, many have speculated that monocytes and/or tissue resident macrophages are a source of extracellular ADA activity [5,23]. However, until now there has been no evidence demonstrating ADA2 release from macrophages or monocytes. Our findings provide support for this hypothesis directly for the first time. Although the mechanism controlling release of ADA from macrophages is not elucidated by our findings, the data cannot be explained simply by lysing of dying cells. If that was the case one would expect the proportionate quantities of ADA1 to ADA2 to be similar in the macrophages and the media. The change in ADA1 and 2 activity in the cells in response to sepsis is completely different from the change in isozyme expression in media (Figs 2 and 3). Furthermore the changes in isozyme expression in the media in response to sepsis is similar to what is seen in the serum. In both the media (Fig. 3b) and the serum (Fig. 4b) ADA2 becomes the dominant isozyme expressed in sepsis. This suggests that the macrophages are not only able to release ADA activity into their surroundings, but that there is differential release of each isozyme.
Our data confirms previous suggestions that monocytes are a likely source of serum ADA2, as they had previously been shown to be one of the few cell types that expressed this isozyme. However, the ADA2 activity found in and from macrophages was quantitatively much greater than that from monocytes, normalized to cell numbers. This raises the possibility that tissue resident macrophages activated during an inflammatory challenge may be a more abundant source of ADA2 than are circulating monocytes. Expression of ADA2 activity as a percentage of the total ADA activity has been commonly used by other investigators [4,5,8,22]. Our calculations of the percentage of total ADA attributable to ADA2 for each sample also suggest greater similarity between macrophage media and serum. We cannot know the absolute number of monocytes or macrophages contributing to serum ADA isozyme concentrations, so the relative contributions of macrophages and monocytes to serum ADA2 must remain speculation. It is clear, however, that tissue-resident macrophages can be an abundant source of serum ADA2.
The isozyme-specific changes between septic and nonseptic rats in macrophage or monocyte cell-normalized activity in the media are not the same as the changes in volume-normalized serum activities for each isozyme. Two primary factors influence this. Septic rats retain nearly all of the fluids administered, significantly increasing total extracellular volume. The nonseptic rats void this volume. There is no reason to believe that ADA2 would be selectively compartmentalized within the serum in vivo. Thus, the relative ADA activity appearing in the serum is distributed in a larger volume (total extracellular volume) in the septic rats. In this regard, it is important to note the small increase, rather than a decrease, in ADA2 concentration in serum, at a time associated with significant increases in volume of distribution. The second factor influencing this is the potential sources of ADA1. We are unaware of any work demonstrating the release of ADA1 from nonmonocytic cells, but in contrast to ADA2, nearly all tissues express ADA1.
Our data contrasts the interpretation of Shibagaki et al. [4]. These authors demonstrated that in the macrophage cell line U937 ADA2 activity did not contribute to more than 8% of total cellular ADA activity. They interpreted this to mean that macrophages were not the source of extracellular ADA2 activity, in which ADA2 represented a much higher proportion [4]. Some of the differences between our work and that of Shibagaki et al. regarding relative abundance of ADA2 in macrophages may have to do with our use of primary peritoneal macrophages. Extensive characterization of tissue specific macrophage populations have found evidence of functional heterogeneity [24,25]. In addition, despite the usefulness of cell lines, many instances have been reported where they do not display the same characteristics as the cell from which they are derived. However, another difference is likely due to the presumption that macrophages must release ADA isozymes into their surrounding in the same proportion as they exist intracellularly. Our data clearly demonstrate that the media from macrophages of animals mounting an inflammatory response demonstrate much higher relative ADA2 compared to cellular levels. This suggests differential regulation of extracellular ADA isozymes concentrations as well.
ADA1 is a monomeric protein between 30 and 40 kD that is able to form a dimer and/or bind to larger molecular weight proteins to form large MW complexes possessing ADA1 enzyme activity. The enzyme kinetics of these forms are identical due to the kinetics of the root ADA1 monomer. The Km values of ADA1 for adenosine and deoxyadenosine are similar (50–70 µm), activity is optimal at neutral pH, and enzyme activity is fully inhibited by 100 µm erythro-9-(2-hydroxynon-3-yl)adenine (EHNA) [18]. Some tissue-associated variant proteins thus characterized have been studied extensively [14,15,26–30]. Multiple mutations of the ADA1 monomer have been identified, and the mutation or deficiency of these proteins is associated with severe combined immunodeficiency (SCID). Significant confusion is caused by the nomenclature used to identify the alleles of this isozyme, some of which have been referred to as ADA2 [27]. With regard to our investigation, ADA2 refers to the ADA isozyme that has been shown to exist only as a monomer of ∼100 kD. The Km values of this isozyme for adenosine and deoxyadenosine are reported in mm ranges, but differ substantially from each other, with deaminating acivity ratio for adenosine/deoxyadenosine of 0·25 [11,13,31]. In addition, ADA2 enzyme activity is optimal at a lower pH (6·5) and it is resistant to inhibition by EHNA [18]. Finally, ADA2 is unaffected in congenital ADA deficiency. Because the kinetics of this isozyme differ significantly from those that incorporate ADA1 isozyme catalytic activity, it has been suggested that these isozymes are encoded by entirely different genes [11], and may not represent true isozymes per se. But, for the purposes of this manuscript, and until molecular studies have shown otherwise, we will continue to use the ‘isozyme’ terminology and nomenclature in differentiating ADA1 and ADA2 based on their distinctive enzyme kinetics, as suggested by Hirschhorn and Ratech [10]. Several investigators have suggested that increased ADA2 in the serum or pleural effusates may be a marker of specific disease states [4,8], and as such have suggested it as a diagnostic tool [7,9]. Measurement of high ADA activity in pleural effusion has proven to be surprisingly accurate in the diagnosis of tuberculous [6,9,32]. ADA2 activity, which has been found exclusively in nonlymphoid cells, has also been used to distinguish between lymphoid and nonlymphoid types of leukaemia [8]. Future work to validate this phenomenon in patients with sepsis is needed. The data presented in this paper further underscore the need to address the question of the importance of ADA2 as a dynamic response molecule during the inflammatory response.
To the best of our knowledge the information currently available about ADA2 is limited to protein size and enzyme kinetics. Our hope is that this paper will provide the impetus for further investigation into the molecular aspects of the ADA2 protein. The production of ADA2 specific antibodies, as well as sequencing of the protein for the generation of probes, will allow for further investigation into the physiologic and pathophysiologic role of the ADA2 isozyme.
Acknowledgments
This work was supported by grants from the NIH Institute of General Medical Sciences (R41 G65593), Heart, Lung, and Blood T32 HL007692, and the Department of Veterans Affairs (Merit Review).
REFERENCES
- 1.Adanin S, Yalovetskiy IV, Nardulli BA, Sam ADII, Jonjev ZS, Law WR. Inhibiting adenosine deaminae modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol. 2002;282:R1324–R1332. doi: 10.1152/ajpregu.00373.2001. [DOI] [PubMed] [Google Scholar]
- 2.Cohen ES, Law WR, Easington CR, et al. Adenosine deaminase inhibition attenuates microvascular dysfunction and improves survival in sepsis. Am J Resp Crit Care Med. 2002;166:16–20. doi: 10.1164/rccm.200109-014OC. [DOI] [PubMed] [Google Scholar]
- 3.Law WR, Conlon BA, Valli VE. Therapeutic Potential for Transient Inhibition of Adenosine Deaminase in Systemic Inflammatory Response Syndrome. Crit Care Med. 2003;31:1475–81. doi: 10.1097/01.CCM.0000063259.09580.D8. [DOI] [PubMed] [Google Scholar]
- 4.Shibagaki T, Hasegawa Y, Saito H, Yamori S, Shimokata K. Adenosine deaminase isozymes in tuberculous pleural effusion. J Laboratory Clin Med. 1996;127:348–52. doi: 10.1016/s0022-2143(96)90182-1. [DOI] [PubMed] [Google Scholar]
- 5.Gakis C, Calia G, Naitana A, Pirino D, Serru G. Serum adenosine deaminase activity in HIV positive subjects. A hypothesis on the significance of ADA2. Panminerva Med. 1989;31:107–13. [PubMed] [Google Scholar]
- 6.Gorguner M, Cerci M, Gorguner I. Determination of adenosine deaminase activity and its isoenzymes for diagnosis of pleural effusions. Respirology. 2000;5:321–4. [PubMed] [Google Scholar]
- 7.Ungerer JP, Oosthuizen HM, Retief JH, Bissbort SH. Significance of adenosine deaminase activity and its isoenzymes in tuberculous effusions. Chest. 1994;106:33. doi: 10.1378/chest.106.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Ratech H, Martiniuk F, Borer WZ, Rappaport H. Differential expression of adenosine deaminase isozymes in acute leukemia. Blood. 1988;72:1627–32. [PubMed] [Google Scholar]
- 9.Valdes L, Pose A, San Jose E. Marti, nez Vazquez JM. Tuberculous pleural effusions. Eur J Intern Med. 2003;14:77–88. doi: 10.1016/S0953-6205(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 10.Hirschhorn R, Ratech H. Isozymes of adenosine deaminase. Isozymes Curr Top Biol Medicalres. 1980;4:131–57. [PubMed] [Google Scholar]
- 11.Gakis C. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2: diagnostic and biological role. Eur Respir J. 1996;9:632–3. doi: 10.1183/09031936.96.09040632. [DOI] [PubMed] [Google Scholar]
- 12.Gakis C. Adenosine deaminase in pleural effusions. Chest. 1995;107:1772–3. doi: 10.1378/chest.107.6.1772-a. [DOI] [PubMed] [Google Scholar]
- 13.Gakis C, Cappio-Borlino A, Pulina G. Enzymes (isoenzyme system) as homeostatic mechanisms the isoenzyme (ADA2) of adenosine deaminase of human monocytes-macrophages as a regulator of the 2′deoxyadenosine. Biochem Mol Biol Int. 1998;46:487–94. doi: 10.1080/15216549800204012. [DOI] [PubMed] [Google Scholar]
- 14.Akeson AL, Wiginton DA, Dusing MR, States JC, Hutton JJ. Mutant human adenosine deaminase alleles and their expression by transfection into fibroblasts. J Biol Chem. 1988;263:16291–6. [PubMed] [Google Scholar]
- 15.Dusing MR, Brickner AG, Thomas MB, Wiginton DA. Regulation of duodenal specific expression of the human adenosine deaminase gene. J Biol Chem. 1997;272:26634–42. doi: 10.1074/jbc.272.42.26634. [DOI] [PubMed] [Google Scholar]
- 16.Hirschhorn R. Adenosine deaminase deficiency: molecular basis and recent developments. Clin Immunol Immunopathol. 1995;76:S219–S227. doi: 10.1016/s0090-1229(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 17.Wakamiya M, Blackburn MR, Jurecic R, et al. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc Natl Acad Sci USA. 1995;92:3673–7. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cristalli G, Costanzi S, Lambertucci C, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Rev. 2001;21:105–28. doi: 10.1002/1098-1128(200103)21:2<105::aid-med1002>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Ungerer JP, Oosthuizen HM, Bissbort SH, Vermaak WJ. Serum adenosine deaminase: isoenzymes and diagnostic application. Clin Chem. 1992;38:1322–6. [PubMed] [Google Scholar]
- 20.Vielh P, Castellazzi M. A colorimetric assay for serial determination of adenosine deaminase activity in small lymphocyte populations. J Immunol Meth. 1984;73:313–20. doi: 10.1016/0022-1759(84)90406-x. [DOI] [PubMed] [Google Scholar]
- 21.Cristalli G, Eleuteri A, Franchetti P, Grifantini M, Vittori S, Lupidi G. Adenosine deaminase inhibitors: synthesis and structure-activity relationships of imidazole analogues of erythro-9-(2-hydroxy-3-nonyl) adenine. J Medicalchem. 1991;34:1187–92. doi: 10.1021/jm00107a044. [DOI] [PubMed] [Google Scholar]
- 22.Ratech H, Hirschhorn R. Serum adenosine deaminase in normals and in a patient with adenosine deaminase deficient-severe combined immunodeficiency. Clin Chim Acta. 1981;115:341–7. doi: 10.1016/0009-8981(81)90247-3. [DOI] [PubMed] [Google Scholar]
- 23.Gakis C, Calia GM, Naitana AG, Ortu AR, Contu A. Serum and pleural adenosine deaminase activity. Correct interpretation of the findings. Chest. 1991;99:1555–6. doi: 10.1378/chest.99.6.1555. [DOI] [PubMed] [Google Scholar]
- 24.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–70. [PubMed] [Google Scholar]
- 25.Laskin DL, Sirak AA, Pilaro AM, Laskin JD. Functional and biochemical properties of rat Kupffer cells and peritoneal macrophages. J Leukoc Biol. 1988;44:71–8. doi: 10.1002/jlb.44.2.71. [DOI] [PubMed] [Google Scholar]
- 26.Danton MJ, Coleman MS. Isolation of mutant adenosine deaminase by coformycin affinity chromatography. Anal Biochem. 1986;159:233–9. doi: 10.1016/0003-2697(86)90333-7. [DOI] [PubMed] [Google Scholar]
- 27.Hirschhorn R, Ellenbogen A. Genetic heterogeneity in adenosine deaminase (ADA) deficiency: five different mutations in five new patients with partial ADA deficiency. Am J Hum Genet. 1986;38:13–25. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PC, Fisher JR, Ma PF. Comparative and immunochemical studies of bovine adenosine deaminases. Comp Biochem Physiol B. 1971;40:1071–7. doi: 10.1016/0305-0491(71)90051-4. [DOI] [PubMed] [Google Scholar]
- 29.Bhat SG, Mishra S, Mei Y, et al. Cisplatin up-regulates the adenosine A (1) receptor in the rat kidney. Eur J Pharmacol. 2002;442:251–64. doi: 10.1016/s0014-2999(02)01510-8. [DOI] [PubMed] [Google Scholar]
- 30.Ben Shooshan I, Parola AH. The CP-I subunit of adenosine deaminase complexing protein from calf kidney is identical to human, mouse, and rat dipeptidyl peptidase IV. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:289–92. doi: 10.1016/s0305-0491(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 31.Niedzwicki JG, Abernethy DR. Structure-activity relationship of ligands of human plasma adenosine deaminase2. Biochem Pharmacol. 1991;41:1615–24. doi: 10.1016/0006-2952(91)90162-x. [DOI] [PubMed] [Google Scholar]
- 32.Andreasyan NA, Hairapetian HL, Sargisova YG, Mardanyan SS, Badalyan LT, Khanoyan AS. Activity of adenosine deaminase and its isoforms in pleural fluid in tuberculous pleuritis. Med Sci Monit. 2002;8:CR708–12. [PubMed] [Google Scholar]